Abstract
Cartilage provides the template for endochondral ossification and is crucial for determining the length and width of the skeleton. Transgenic mice with targeted expression of recombinant cartilage-derived morphogenetic protein-1 (CDMP-1), a member of the bone morphogenetic protein family, were created to investigate the role of CDMP-1 in skeletal formation. The mice exhibited chondrodysplasia with expanded cartilage, which consists of the enlarged hypertrophic zone and the reduced proliferating chondrocyte zone. Histologically, CDMP-1 increased the number of chondroprogenitor cells and accelerated chondrocyte differentiation to hypertrophy. Expression of CDMP-1 in the notochord inhibited vertebral body formation by blocking migration of sclerotome cells to the notochord. These results indicate that CDMP-1 antagonizes the ventralization signals from the notochord. Our study suggests a molecular mechanism by which CDMP-1 regulates the formation, growth, and differentiation of the skeletal elements.
Keywords: bone morphogenetic protein family, cartilage, ectopic expression, skeletal abnormalities, transgenic mice
Cartilage is a highly specialized tissue and serves as the template for the development of most bones. Cartilage development is initiated by mesenchymal cell condensation to form primordial cartilage followed by chondrocyte maturation processes. These include resting, proliferative, prehypertrophic, and hypertrophic chondrocytes. As a final step in endochondral bone formation, the hypertrophic cartilage is invaded by blood vessels and osteoprogenitor cells, and the calcified cartilage is subsequently replaced by bone. Thus, spatial and temporal regulation of chondrocyte differentiation is essential in determining the length and width of skeletal components.
Bone morphogenetic proteins (BMPs)1 were originally identified as proteins capable of inducing ectopic endochondral bone formation in subcutaneous implants (Urist et al., 1979; Sampath and Reddi, 1981). Subsequent molecular cloning revealed that the BMP family consists of a large number of related molecules which belong to the TGF-β superfamily (Wozney et al., 1988; reviewed in Hogan, 1996). Although BMPs were initially found in the bone matrix, it is now clear that they are expressed in a variety of tissues each with unique tissue specificity. BMPs have diverse biological activities including promoting cell proliferation, differentiation, and apoptosis and regulating development of various organs and tissue repair (reviewed in Hogan, 1996). Among the BMP family, Bmp2, Bmp4, and Bmp7 are expressed in perichondrium (Macias et al., 1997; Zou et al., 1997) and Bmp6 in hypertrophic chondrocytes (Lyons et al., 1989; Vortkamp et al., 1996). All are thought to play a role in skeletogenesis during development. However, the cellular and molecular mechanisms of their action on cartilage and bone formation are not well understood.
Growth and differentiation factor 5 (GDF5) is a relatively new member of the BMP family. Like other BMPs, implantation of recombinant GDF5 can induce ectopic cartilage formation in muscular tissues (Hötten et al., 1996). Null mutations of GDF5 reduce the size of skeletal components in the limb of brachypodism (bp) mice (Storm et al., 1994). The human homologue of GDF5, termed cartilage-derived morphogenetic protein-1 (CDMP-1), was independently identified by degenerate PCR amplification of mRNA from articular cartilage (Chang et al., 1994). Mutations of the CDMP-1 gene have been identified in patients with acromesomelic chondrodysplasia Hunter-Thompson type and Grebe type which are characterized by short limbs, especially the distal part of the limbs and by the absence of several phalangeal joints (Thomas et al., 1996, 1997). In addition, autosomal dominant brachydactyly type C is caused by mutation in the CDMP-1 gene (Polinkovsky et al., 1997). Expression of GDF5/CDMP-1 is restricted to the primordial cartilage of appendicular skeleton (Chang et al., 1994; Storm et al., 1994; Storm and Kingsley, 1996). Little expression of GDF5/CDMP-1 is found in the axial skeleton such as vertebrae and rib. This restricted spatial expression pattern of the Gdf5/CDMP1 gene accounts for the unique chondrodysplasia phenotype with few abnormalities in the axial skeleton in mice and humans. These results suggest that GDF5/CDMP-1 plays a crucial role in the patterning of the appendicular skeleton, longitudinal bone growth, and chondrogenesis.
Cartilage consists of a large extracellular matrix maintained by chondrocytes. Type II collagen, the major component of cartilage, forms fibrils. Type XI collagen, a minor collagen, regulates formation of the collagen fibrils. We previously identified the promoter and first intron enhancer sequences responsible for the cartilage- and notochord-specific expression of the α2 type XI collagen gene (Col11a2) (Tsumaki et al., 1996; Tsumaki et al., 1998). The α2(XI) collagen promoter is capable of directing expression in cartilage but not in the notochord. Inclusion of the first intron in the α2(XI) promoter significantly enhances the promoter activity in cartilage and directs expression in the notochord. An enhancer from the first intron of the α1 type II collagen gene (Col2a1) directs much stronger chondrocyte-specific expression (Horton et al., 1987; Krebsbach et al., 1996).
In this study, we have generated transgenic mice expressing recombinant CDMP-1 at different levels under the control of the α2(XI) or α1(II) collagen promoter/ enhancer sequences to define the action of CDMP-1 on endochondral bone formation and skeletal growth. These transgenic mice died before or just after birth and showed a chondrodysplasia-like phenotype with expanded primordial cartilage not only in the limb skeleton but also in the axial skeleton. The remarkable feature of the cartilage of all of the transgenic mice is the expansion of a hypertrophic zone and the reduced height of zones of proliferative chondrocytes. The transgenic mice also showed enlarged mesenchymal condensations and increased the number of prechondrogenic cells. These results suggest that CDMP-1 promotes differentiation of chondrocytes into hypertrophy and enhances commitment of mesenchymal cells into the chondrocytic lineage. Interestingly, expression of CDMP-1 in the notochord before the onset of chondrogenesis appeared to inhibit the migration of sclerotome cells to form primordial cartilage around the notochord, resulting in the absence of the vertebral bodies.
Materials and Methods
Construction of the Transgene
The α2(XI) collagen gene–based expression vectors, 742lacZ and 742lacZInt, have been described previously (Tsumaki et al., 1996). 742lacZ contains the Col11a2 promoter (−742 to +380), an SV-40 RNA splice site, the β-galactosidase reporter gene, and the SV-40 polyadenylation signal. 742lacZInt also contains 2.3 kb of the first intron sequence downstream of the SV-40 polyadenylation signal as an enhancer. The type II collagen gene–based expression vector was generated by linking Col2a1 promoter (−956 to +77), the rabbit β-globin splice site, the β-galactosidase reporter gene, the SV-40 polyadenylation signal, and the fragment of Col2a1 first intron (+2038 to +2678). The fragment of the first intron contains tissue-specific enhancer elements (Zhou et al., 1995; Krebsbach et al., 1996).
A 1.6-kb DNA fragment covering the entire coding region of the human CDMP-1 cDNA was generated by PCR using a forward primer tagged with NotI site (AAA TAT GCG GCC GCT CTA GAG TCA TTC AGC GGC TGG CCA GAG GAT) and a reverse primer with NotI site (TGT AGA TGC TGC GGC CAC AGC TTC CTG). After digestion with NotI, the PCR fragment was cloned into the NotI site of 742lacZ-, 742lacZInt-, and Col2a1-based expression vectors by replacing the β-galactosidase gene to create three CDMP1 expression vectors, 742- CDMP1, 742-CDMP1-Int, and Col2a1-CDMP1, respectively.
Generation of Transgenic Mice
The plasmids 742-CDMP1 and 742-CDMP1-Int were digested with NdeI and HindIII to release the inserts from their vector sequences. The plasmid Col2a1-CDMP1 was digested with BssHII to release the vector sequence. Transgenic mice were produced by microinjecting each of the inserts into the pronuclei of fertilized eggs from F1 hybrid mice (C57BL/6 × C3H) as described previously (Hogan et al., 1994). Transgenic embryos were identified by PCR assays of genomic DNA extracted from the placenta or skin. The DNA was subjected to transgene-specific PCR with primers derived from the human CDMP-1 cDNA (TGA GGA CAT GGT CGT CCA GTC GTC TGG) and from the SV-40 poly(A) signal region (TCA CTG CAT TCT AGT TGT GGT TTG TCC) to amplify an 192-bp product.
Staining of Skeleton
Cartilage and bones of embryos and newborn mice were stained as described (Peters, 1977). After skin and internal organs were removed, samples were fixed in 96% ethanol for 2 d followed by staining with alcian blue solution (80 ml ethanol 96%, 20 ml acetic acid, 15 mg alcian blue) for 2 d. The samples were dehydrated in 100% ethanol for 5 d and immersed in 1% KOH for 2 d. The samples were stained with 0.001% alizarin red S solution in 1% KOH for 2 d followed by dehydration in graded solutions of glycerin and stored in 100% glycerin.
Histology
Embryos were dissected with a stereomicroscope, fixed in 4% paraformaldehyde, processed, and embedded in paraffin. Serial sections were prepared and stained with hematoxylin and eosin, safranin O-fast green-iron hematoxylin. To assess the proliferative activity, silver stain for nucleolar organizer regions (AgNOR) was performed as previously described (Crocker and Nar, 1987). Then the numbers of AgNOR dots in 50–100 cells were counted. Cryostat sections of dissected tissues embedded in Tissue-Tek OCT compound were stained with hematoxylin and eosin.
Hybridization Probes
Probes included human CDMP1 cDNA (an ApaI fragment, residue 470– 1155) (Chang et al., 1994) and mouse α2(XI) collagen cDNA (pRAC2-28) (Tsumaki and Kimura, 1995). Mouse Ihh (Hh-14.1) and Shh (Hh-16.1) cDNA probes were provided by A. McMahon (Echelard et al., 1993). Mouse Pax1 cDNA (a HincII-SacI fragment) was obtained from H. Koseki and R. Balling (Deutsch et al., 1988). Mouse PTHrP cDNA (a AvrII/ SmaI fragment) and PTH/PTHrP receptor cDNA (a Sau3A/PvuII fragment) were obtained from A. Broadus (Mangin et al., 1990). Mouse α1(X) collagen cDNA (pRK26) was provided by K.S.E. Cheah (Kong et al., 1993). Mouse type IIA procollagen cDNA (exon 2) was from L.J. Sandell (Metsaranta et al., 1991).
In Situ Hybridization
Digoxigenin-11-UTP–labeled single-strand RNA probes were prepared using a DIG RNA labeling kit (Boehringer Mannheim) according to the manufacturer's instructions. cDNAs described above were used to generate antisense and sense probes. Hybridization was performed as described previously (Hirota et al., 1992). In brief, after deparaffinization, the sections were treated with 10 μg/ml of proteinase K for 15 min at room temperature, and subjected to 0.2 N HCl to inactivate endogenous alkaline phosphatase. Hybridization was performed at 50°C in 50% formamide, and washes were carried out at a stringency of 2× SSC containing 50% formamide at 55°C. Then, the slides were subjected to 10 μg/ml of RNase A in TNE (10 mM Tris-HCl, pH 8.0, 500 mM NaCl, 1 mM EDTA) at 37°C for 30 min for digestion of nonhybridized transcripts, and washed. A Genius Detection System (Boehringer Mannheim) according to the manufacturer's instructions was used to detect signals.
Northern Hybridization
Total RNA was extracted from the limb buds of 14.5-d postcoitus (d.p.c.) transgenic and normal embryos using the RNeasy Mini Kit (Qiagen), fractionated by electrophoresis through a formaldehyde agarose gel, and transferred into Nytran membrane (Schleicher & Schuell). cDNAs were labeled with [32P]dCTP using the Prime-it II kit (Stratagene). The membranes were hybridized with 32P-labeled CDMP-1 cDNA at 42°C in 50% formamide, washed first at room temperature in 1× SSC and 0.1% SDS and then at 60°C in 0.1× SSC and 0.1% SDS, and exposed to autoradiography film. The filters were rehybridized with 32P-labeled probes for mouse α2(XI) collagen, α1(X) collagen, Ihh, PTHrP, and PTH/PTHrP receptor.
Immunohistochemical Staining
Cryostat sections were fixed with acetone. Then they were treated by the primary polyclonal antibody to CDMP-1 raised in rabbits (Chang et al., 1994), washed with PBS, and incubated with the secondary antibody (FITC-labeled antibodies; Chemicon).
Results
Creation of Transgenic Mice Expressing CDMP-1 in Cartilage
Since the expression levels of recombinant CDMP-1 may affect the phenotype of transgenic mice, we prepared three different expression vectors derived from the regulatory regions of cartilage-specific genes for α1(II) and α2(XI) collagen chains to obtain different expression levels of recombinant CDMP-1 in transgenic mice. The first construct was CDMP-1 cDNA linked to the 742-bp α2(XI) collagen promoter and intron (742-CDMP1-Int), the second was the CDMP-1 cDNA linked to the α2(XI) collagen promoter without intron (742-CDMP1), and the third was the CDMP-1 cDNA linked to α1(II) collagen promoter with intron (Col2a1-CDMP1) (Fig. 1 A).
Figure 1.
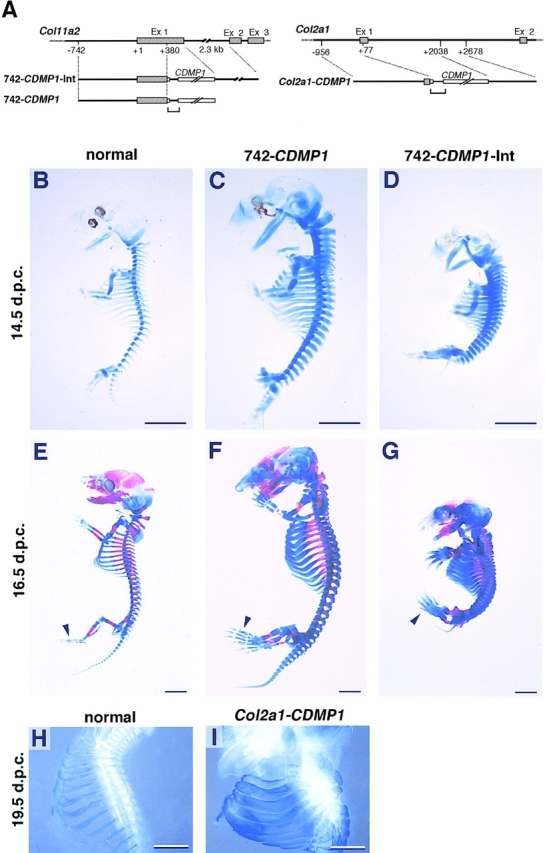
Transgene constructs and skeletal abnormalities of CDMP-1 transgenic mice. (A) Diagrams of the DNA constructs used to generate Col11a2-CDMP1 (left) and Col2a1-CDMP1 (right) transgenic mice. The gene structures of Col11a2 and Col2a1 are shown at the top of each panel. Boxes indicate the coding regions and solid lines denote noncoding sequences. The transgenes are shown below the genomic map. Brackets indicate intron cassettes. (B–I) Skeletons of CDMP-1 transgenic mice. Entire skeletons of normal (B and E), 742- CDMP1 transgenic (C and F), and 742- CDMP1-Int transgenic (D and G) embryos were stained with alcian blue (B–D) and alcian blue plus alizarin red (E–G). The rib cages of a normal mouse (H) and a Col2a1-CDMP1 transgenic mouse (I) were stained with alcian blue and shown in dark field. (B–D) Embryos at 14.5 d.p.c.; (E–G) 16.5 d.p.c.; (H and I), 19.5 d.p.c. 742- CDMP1 transgenic mice were large, whereas 742-CDMP1-Int and Col2a1-CDMP1 transgenic mice were small. The skeletal components were thick in all transgenic mice. In 742-CDMP1-Int and Col2a1 transgenic mice, the ribs were so thick that intercostal spaces were almost eliminated. Ossification had just started at metatarsals in normal and transgenic mice (E–G, arrowheads). Scale bars, 2 mm.
The linearized constructs were microinjected into fertilized eggs and generation zero (G0) embryos were collected at 11.5–19.5 d.p.c. (Table I). For the 742-CDMP1 construct, 27 embryos were collected of which 8 were transgenic (29%). For the 742-CDMP1-Int construct, 24 of the 86 embryos were transgenic (28%). Only one transgenic mouse was obtained out of 32 collected embryos for the Col2a1-CDMP1 construct (0.3%). In total, 27 out of the 33 transgenic embryos displayed detectable phenotypes with abnormalities restricted to the skeletal tissues. The remaining transgenic mice did not have a phenotypic abnormality and did not have detectable levels of recombinant CDMP-1 mRNA or protein. Two transgenic mice, one with 742-CDMP1 and the other with Col2a1-CDMP1, were born but died shortly after birth. Both showed a chondrodysplasia-like phenotype.
Table I.
Production Frequency of Transgenic Mice
| Transgene construct | Stage of sacrifice | Number of pups obtained | Number of transgenic mice | Number of transgenic mice with phenotype* | ||||
|---|---|---|---|---|---|---|---|---|
| d.p.c. | ||||||||
| 742-CDMP1 | 14.5 | 12 | 3 | 2 | ||||
| 16.5 | 13 | 4 | 3 | |||||
| 19.5 | 2 | 1 | 1‡ | |||||
| 742-CDMP1-Int | 11.5 | 9 | 3 | 3 | ||||
| 12.5 | 12 | 4 | 3 | |||||
| 14.5 | 19 | 5 | 4 | |||||
| 16.5 | 9 | 3 | 2 | |||||
| Col2a1-CDMP1 | 14.5 | 17 | 0 | 0 | ||||
| 15.5 | 1 | 0 | 0 | |||||
| 16.5 | 4 | 0 | 0 | |||||
| 18.5 | 6 | 0 | 0 | |||||
| 19.5 | 4 | 1 | 1‡ |
Abnormalities were predominantly restricted to the skeleton.
Mice died shortly after birth.
Gross Skeletal Analysis of CDMP-1 Transgenic Mice
At 14.5 d.p.c., two 742-CDMP1 transgenic G0 embryos were identified due to their large size, i.e., average size of crown-rump length was 10% larger than that of normal littermates. Anatomical and histological examination of the transgenic mice revealed no obvious abnormalities in tissues other than skeleton (data not shown). Alcian blue staining of cartilage showed that the entire cartilaginous skeleton was enlarged and thickened when compared with normal mice (Fig. 1, B and C). In contrast, nine 742- CDMP1-Int transgenic G0 embryos were identified with dwarfism, i.e., average size of crown-rump length was 20% smaller than that of normal littermates, and kyphotic posture at 14.5 d.p.c. However, the paws were disproportionally large. The cartilaginous skeleton of 742-CDMP1-Int transgenic mice was much thicker than that of normal mice and was similar to 742-CDMP1 transgenic mice (Fig. 1, B and D). The transgenic mouse with Col2a1-CDMP1 showed dwarfism and had profound abnormalities in the skeletal components at 19.5 d.p.c. Dark-field pictures of the skeleton stained with alcian blue showed that the Col2a1-CDMP1 transgenic mouse had much thicker skeletal components than normal mouse (Fig. 1, H and I).
Most of the phenotypic characteristics in skeletal thickness were maintained in all transgenic mice at 16.5 d.p.c. (Fig. 1, E–G). In 742-CDMP1-Int transgenic mice, in addition to kyphotic posture, skeletal components were so thick that the intercostal space was almost eliminated in the rib cage. At this stage, ossification is active and was visualized with alizarin red S staining in addition to alcian blue cartilage staining. As can be seen in the metatarsal, ossification in transgenic mice started at normal developmental stage (Fig. 1, E–G, arrowheads). However, endochondral ossification in the cranial region was significantly delayed (Fig. 1, E–G).
In the transgenic limbs, the distal components, such as carpals, metacarpals, and phalanges, were more affected and enlarged than the proximal components (Fig. 2, A–C) at 16.5 d.p.c., i.e., average size of the third phalange thickness was 70% larger in 742-CDMP1 transgenic mice and 150% larger in 742-CDMP1-Int transgenic mice than that of normal littermates. All abnormal 742-CDMP1- Int transgenic mice had fused joints, whereas 742-CDMP1 transgenic mice did not. The humerus, radius, and ulna of the 742-CDMP1-Int transgenic mice were united by bone. In the Col2a1-CDMP1 transgenic mouse at 19.5 d.p.c., joints were completely fused by the bone (Fig. 2, D and E). The ribs of 742-CDMP1 and 742-CDMP1-Int transgenic mice at 16.5 d.p.c. were thicker than normal, i.e., diameter of the 742-CDMP1 and 742-CDMP1-Int transgenic ribs was two to three times thicker than that of normal littermates (Fig. 2, F–H). The Col2a1-CDMP1 transgenic mouse at 19.5 d.p.c. also had thick ribs, i.e., diameter of the Col2a1-CDMP1 transgenic ribs was at least four times thicker than that of normal littermates (Fig. 2, I and J). The degree of the thickness of the skeleton and the enlargement of the paws in 742-CDMP1-Int transgenic mice was consistently more severe than those of 742-CDMP1 transgenic mice. Although stages were different, the degree of deformity in Col2a1-CDMP1 transgenic mouse appeared to be more severe than that of 742-CDMP1-Int transgenic mice.
Figure 2.

Examination of the skeleton of the forelimbs and ribs of CDMP-1 transgenic mice. (A–C) Skeletal components of the forelimb at 16.5 d.p.c. stained with alcian blue and alizarin red. In the 742-CDMP1 (B) and 742- CDMP1-Int (C) transgenic mice, the distal components (brackets), such as the carpals, metacarpals, and phalanges, were larger than those of normal mice (A), whereas the proximal component such as the scapula of the transgenic mice was not so enlarged. The extent of deformity of 742-CDMP1-Int transgenic mice was more severe than that of 742-CDMP1 transgenic mice. (D and E) Skeletal components of the forelimb at 19.5 d.p.c. stained with alcian blue shown in the dark field. In the Col2a1-CDMP1 transgenic mouse (E), the skeleton of the paws was much enlarged compared with the normal mouse (D). Note the difference in the scale between D and E. 742-CDMP1- Int and Col2a1-CDMP1 transgenic mice had fused joints, whereas 742- CDMP1 transgenic mice did not. The humerus, radius, and ulna of 742- CDMP1-Int and Col2a1-CDMP1 transgenic mice were united by bone. (F–H) Ribs at 16.5 d.p.c. stained with alcian blue and alizarin red. The ribs of 742-CDMP1 (G) and 742-CDMP1-Int (H) transgenic mice were thicker than those of normal mice (F) at 16.5 d.p.c. (I and J) Ribs at 19.5 d.p.c. stained with alcian blue shown in the dark field. The Col2a1-CDMP1 transgenic mouse (I) also had much thicker ribs than normal mice (J). The degree of the thickness of the skeleton and the enlargement of the paw in 742- CDMP1-Int transgenic mice were consistently more severe than those of 742-CDMP1 transgenic mice. Although stages were different, the degree of deformity in the Col2a1-CDMP1 transgenic mouse appeared to be more severe than that of 742-CDMP1-Int transgenic mice. s, scapula; h, humerus; r, radius; u, ulna. Brackets in A–E indicate skeletal components of paws including carpals, metacarpals, and phalanges. Scale bars, 1 mm.
Gene Expression in CDMP-1 Transgenic Mice
Northern analysis demonstrated expression of CDMP-1 in the limb buds in transgenic mice (Fig. 3 A, top). Recombinant CDMP1 mRNA was ∼3 kb in size and slightly larger than endogenous Gdf5/CDMP1 mRNA due to the length differences in both the 5′ and 3′ untranslated regions. Expression levels of the transgene in the 742-CDMP1-Int transgenic mice were about twofold higher than those in the 742-CDMP1 transgenic mice (Fig. 3 A, lanes 2 and 3). A low level of endogenous Gdf5/CDMP1 mRNA was found in normal mice (Fig. 3 A, lane 1, arrow). The expression levels of mRNAs for Indian hedgehog (Ihh) and type X collagen α1 chain (Col10a1) genes, markers for prehypertrophic and hypertrophic chondrocytes, respectively, were elevated in 742-CDMP1 and 742-CDMP1-Int transgenic mice.
Figure 3.

Gene expression of CDMP-1 transgenic mice. (A) Northern blot analysis using total RNAs extracted from limb buds of normal (lane 1), 742- CDMP1 (lane 2), and 742-CDMP1-Int (lane 3) transgenic mice at 14.5 d.p.c. For each lane, 20 μg RNA was loaded, transferred to the nylon membrane, and hybridized with corresponding probes indicated at the left of the panels. The bottom panel shows the ethidium bromide–stained gel before transfer. Transgenic CDMP-1 mRNA was expressed strongly compared with endogenous Gdf5/CDMP1 mRNA (arrowhead). The expression levels of Ihh and Col10a1 mRNAs, markers for chondrocyte differentiation, were elevated in the transgenic mice. (B) Distribution of CDMP1 mRNA in the forelimb of normal (left), 742-CDMP1 transgenic (middle), and 742-CDMP1-Int transgenic (right) mice at 14.5 d.p.c. (Top) Staining with hematoxylin and eosin. (Bottom) In situ hybridization of semiserial sections using CDMP1 antisense probe. In the transgenic mice, CDMP-1 mRNA was distributed in proliferating chondrocytes, but not in hypertrophic chondrocytes. Note that the height of the hypertrophic zone was increased in transgenic mice. (C) CDMP1 and Col11a2 expression in the rib of normal (left) and 742- CDMP1-Int transgenic (right) mice at 16.5 d.p.c. (Top) Staining with hematoxylin and eosin. (Middle) In situ hybridization of CDMP1 antisense probe of semiserial sections. (Bottom) Col11a2 antisense probe. In the transgenic mice, CDMP-1 mRNA was distributed in proliferating chondrocytes, but not in hypertrophic chondrocytes. The localization of CDMP-1 mRNA was identical to that of Col11a2 mRNA. (D) Immunohistochemical analysis of the primordial cartilage of the tarsals of normal (left) and 742- CDMP1-Int transgenic (right) mice at 14.5 d.p.c. (Top) Staining with hematoxylin and eosin. (Bottom) Immunostaining of anti–CDMP-1 polyclonal antibody showed transgenic cartilage contained a significant amount of CDMP-1. r, resting chondrocytes; p, proliferating chondrocytes; h, hypertrophic chondrocytes. Scale bars, B, 500 μm; C, 200 μm; D, 50 μm.
In situ hybridization analysis indicated that recombinant CDMP-1 mRNA was localized in resting and proliferative chondrocytes of primordial cartilage in the forelimbs of 14.5 d.p.c. and ribs of 16.5 d.p.c. transgenic mice (Fig. 3, B and C). The CDMP-1 expression levels in the 742- CDMP1-Int transgenic mice were higher than those in the 742-CDMP1 transgenic mice, similar to what was observed by Northern analysis. Expression patterns of the transgene were almost identical to those of the α2(XI) collagen gene in the rib (Fig. 3 C) and limb (Fig. 4 B, second and third rows). Immunohistochemical analysis using an anti–CDMP-1 polyclonal antibody showed that cartilage in the transgenic mice expressed a higher level of CDMP-1 compared with normal mice, suggesting that the transgene mRNA was translated (Fig. 3 D). In situ hybridization showed that CDMP1 mRNA was expressed in the cartilage in a 19.5 d.p.c. Col2a1-CDMP1 transgenic mouse (data not shown). No expression of endogenous Gdf5/ CDMP1 mRNA was detected in normal mice at this stage (data not shown).
Figure 4.

Altered endochondral bone formation in Col11a2-CDMP1 transgenic mice. (A) Histological sections of the humerus of normal (left) and 742- CDMP1-Int transgenic mice (right) at 14.5 d.p.c. (Top) Staining with Safranin O-fast green-iron hematoxylin of entire humerus. The transgenic mice (right) had the increased height of a zone of hypertrophic chondrocytes (h) compared to normal mice (left). In the transgenic mice, the primordial cartilage was wide and elbow joints were fused. (Bottom) Magnifications of the boxed regions of the distal part of the humerus in the top panels. The height of zones of proliferating chondrocytes was reduced in the transgenic mice. Note that arrays of chondrocytes at the bottom (asterisk) were different from those of chondrocytes located above in the transgenic mice. They were chondrocytes of the proximal part of the ulna. Hematoxylin and eosin staining. (B) Spatial expression of marker genes for endochondral bone formation in the forelimb of normal (left) and 742- CDMP1-Int transgenic (right) mice at 16.5 d.p.c. (Top row) Staining with Safranin O-fast green-iron hematoxylin. The skeletal components consist of proliferating and hypertrophic zones of chondrocytes and bone. In the transgenic mice, the carpals and distal part of the radius and ulna were completely fused and underwent endochondral bone formation as a single component. Semiserial sections were hybridized with cRNA probes of corresponding genes indicated on the left. Areas expressing Ihh (fourth row) and type X collagen (fifth row) mRNAs were remarkably enlarged in transgenic mice. r, resting chondrocytes; p, proliferating chondrocytes; h, hypertrophic chondrocytes. Scale bars, 100 μm.
Endochondral Bone Formation of CDMP-1 Transgenic Mice
The most striking change in the cartilage of 742-CDMP1- Int transgenic mice was an enlargement of the hypertrophic zone (Fig. 4, A and B, top row). In the transgenic mice, the carpals and the distal part of the radius and ulna were completely fused and underwent endochondral bone formation as a single component (Fig. 4 B, top row). 742- CDMP1 transgenic mice also showed an enlarged hypertrophic zone compared with normal mice, but to a lesser extent than the 742-CDMP1-Int transgenic mice (Fig. 3 B). For further identification of chondrocyte types at specific stages of differentiation, we performed in situ hybridization using specific marker genes as probes (Fig. 4 B). Type XI collagen and type X collagen probes were used as markers for proliferating and hypertrophic zones, respectively. Indian hedgehog (Ihh) was used as a marker for the prehypertrophic zone located between these two zones. The areas expressing Ihh and type X collagen mRNAs were remarkably enlarged in 742-CDMP1-Int transgenic mice by 12- and 6-fold, respectively, indicating both prehypertrophic and hypertrophic zones were expanded compared with normal cartilage. Northern analysis of total RNA from 14.5 d.p.c. limb buds also showed significant elevation of the expression levels of Ihh and type X collagen mRNAs in both 742-CDMP1 and 742-CDMP1-Int transgenic mice (Fig. 3 A).
Mesenchymal Condensation and Chondroprogenitor Cells of CDMP-1 Transgenic Mice
During skeletal development, recombinant CDMP-1 expression was found in transgenic mice at 12.5 d.p.c., when mesenchymal cells aggregated to form condensation (data not shown). At this stage, mesenchymal condensations in the forelimb bud of the transgenic mice were already considerably expanded as compared with the wild type (Fig. 5, A and B) and were composed of increased numbers of mesenchymal cells. It has been demonstrated that the number of AgNORs (nucleolar organizer region–associated proteins) is indicative of proliferative cellular activity correlating well with proliferating nuclear cell antigen (PCNA) expression (Crocker and Nar, 1987; Leek et al., 1991; Heinisch and Wozel, 1995; Nagano et al., 1995). Therefore, we analyzed AgNORs by silver staining to examine proliferative cell populations in mesenchymal condensations as markers of cell proliferative activity. In normal mice, cells in the peripheral region of the condensation were characterized by a flattened shape and contained an increasing number of AgNORs staining per cell (Fig. 5 C, arrowheads) whereas cells in the central region showed a round shape (Fig. 5 C). The number of peripheral cells with these characteristics in the condensation of the transgenic mice was much higher than that of the normal mice (Fig. 5 D). Thus, the expanded mesenchymal condensation in the transgenic mice consisted of more proliferative cell populations than the normal mice. At 14.5 d.p.c., similar expansion of the proliferative cell population was also observed in the perichondrium surrounding epiphyseal cartilage of 742-CDMP1-Int transgenic mice. The perichondrium of the transgenic mice was thicker and composed of more multiple cell layers than in the normal mice (Fig. 5, E and F).
Figure 5.

Mesenchymal condensation and perichondrium of CDMP-1 transgenic mice. (A, C, and E) Normal mice. (B, D, and F) 742-CDMP1-Int transgenic mice. (A and B) Axial sections of mesenchymal condensation in the forelimb bud at 12.5 d.p.c. Mesenchymal condensation was already expanded in transgenic mice. Hematoxylin and eosin staining. (C and D) AgNORs in mesenchyme of boxed portions in top panels. In normal mice (C), peripheral cells (arrowheads) located between the central part of mesenchymal condensation (c) and surrounding mesenchymal cells (m) contained large number of AgNORs, indicative of proliferative activities. In transgenic mice (D), the number of peripheral cells was increased and formed multiple layers. (E and F) Perichondrium (arrows) of epiphyseal cartilage (e) of the distal ulna at 14.5 d.p.c. Compared with normal mice, transgenic mice showed thick perichondrium consisting of multiple cell layers. Scale bars, 50 μm.
The increased number of chondroprogenitor cells in 742-CDMP1-Int transgenic mice was also confirmed by in situ hybridization with a probe for collagen type IIA mRNA (Fig. 6). Two forms of type II procollagen are generated by alternative splicing of the exon 2 sequence (Ryan and Sandell, 1990) and the longer form (type IIA) containing the exon 2 sequence expressed predominantly by chondroprogenitor cells but not proliferative chondrocytes. Therefore, we analyzed the expression of type IIA mRNA as a marker for cells committed for a chondrocytic lineage (Sandell et al., 1994). At 13.5 d.p.c., cartilage anlages of phalanges, carpals, radius, and ulna were recognizable in the forelimb (Fig. 6 A). In CDMP-1 transgenic mice, these components were expanded and fused, forming a single anlage (Fig. 6 B). The expression of type IIA mRNA was more profound in cells surrounding the transgenic cartilage than the wild type, suggesting the increased number of chondroprogenitor cells in CDMP-1 transgenic cartilage (Fig. 6, C–F).
Figure 6.
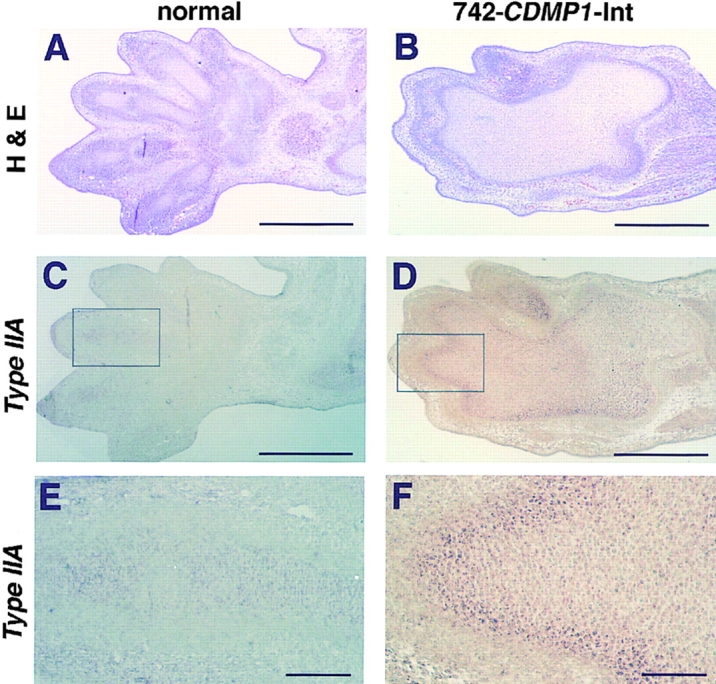
Expression of type IIA collagen mRNA in the forelimb at 13.5 d.p.c. (A and B) Hematoxylin and eosin staining. In the transgenic mice, the phalanges, carpals, and distal part of the radius and ulna were completely fused and formed single thick cartilage anlage. (C–F) In situ hybridization with the antisense type IIA collagen mRNA probe of semiserial sections (C and D) and magnification of the boxed regions (E and F). Type IIA mRNA-positive cells surrounded the cartilage anlage in transgenic mice, whereas only faint signals were detected in normal mice. Scale bars, A–D, 500 μm; E and F, 100 μm.
Abnormalities in the Vertebral Bodies of CDMP-1 Transgenic Mice
There is a striking difference in the formation of vertebral bodies between transgenic mice with 742-CDMP1 and 742-CDMP1-Int. In 742-CDMP1 transgenic mice, vertebral bodies were expanded (Fig. 7, B, E, and H). On the other hand, vertebral bodies were absent in 742-CDMP1- Int transgenic mice as seen in skeletal staining of the spinal column at 14.5 and 16.5 d.p.c. (Fig. 7, C, F, and I). The Col2a1-CDMP1 transgenic mouse also resembled the 742- CDMP1-Int transgenic mice (data not shown). We performed histological analysis and in situ hybridization at earlier stages to access the role of CDMP-1 in vertebral body malformation. CDMP1 mRNA was expressed specifically in the notochord of the 742-CDMP1-Int transgenic mice at 11.5 d.p.c. when chondrogenesis had not yet occurred, whereas CDMP1 mRNA was not expressed in the normal mice, agreeing with previous findings (Storm et al., 1994) (Fig. 8, C and D). Sonic hedgehog (Shh) mRNA was expressed in the notochord in both normal mice and 742-CDMP1-Int transgenic mice (Fig. 8, E and F). Since the vertebral bodies develop from Pax1-expressing sclerotome cells (Deutsch et al., 1988), we examined expression of Pax1 during development. Development of the axial structure progresses in a cranio-caudal direction. Therefore, axial sections at the caudal level represent an earlier stage of development than those at the cranial level of the embryo. At the caudal level at 11.5 d.p.c., ventral parts of sclerotome consisted of Pax1-positive cells in both normal and transgenic mice (Fig. 8, G and H). At the cranial level at 11.5 d.p.c., Pax1-positive cells were migrating from the somite to the notochord in normal mice, whereas in 742-CDMP1-Int transgenic mice, Pax1-positive cells remained near the somite (Fig. 8, I and J). At 12.5 d.p.c., Pax1-expressing cells were localized around the notochord and appeared to be forming the primordial cartilage of vertebral bodies in normal mice (Fig. 8 K). In contrast, there were no signals of Pax1 expression near the notochord of 742-CDMP1-Int transgenic mice (Fig. 8 L).
Figure 7.
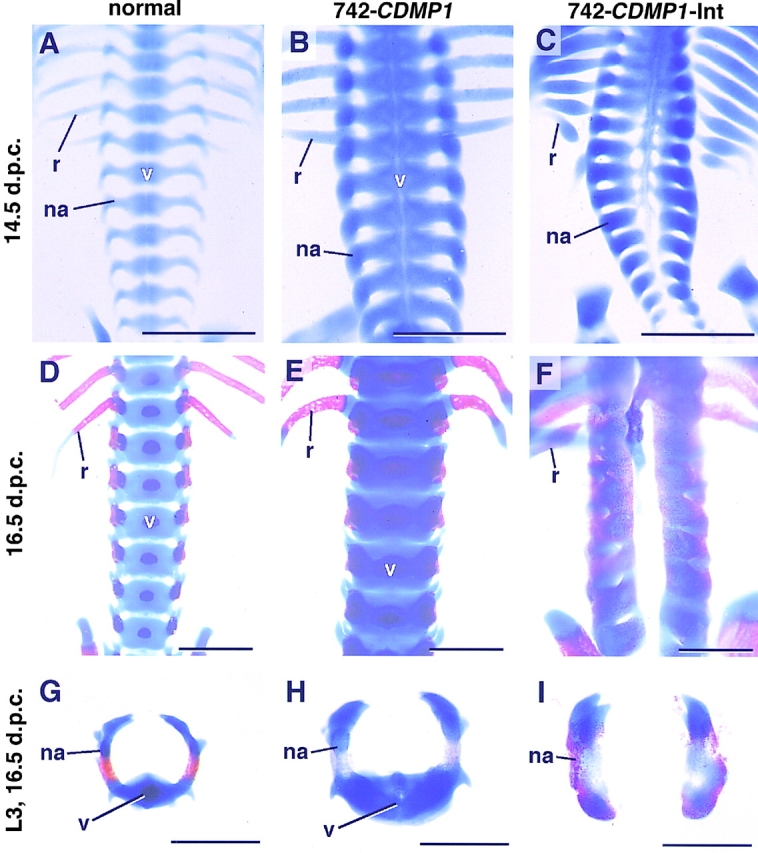
Spinal columns stained with alcian blue and alizarin red. Normal (A, D, and G), 742-CDMP1 transgenic (B, E, and H), and 742-CDMP1-Int transgenic (C, F, and I) mice. (A–F) The spinal columns with the ribs were viewed from the ventral side at 14.5 d.p.c. (A–C) and at 16.5 d.p.c. (D–F). 742-CDMP1 transgenic mice had thick skeletal components including the vertebral bodies (v), neural arches (na), and ribs (r) (B and E). In 742-CDMP1- Int transgenic mice, the vertebral bodies were absent (C and F). (G–I) Axial view of the third lumber vertebrae of mice at 16.5 d.p.c. The vertebral body was enlarged in 742-CDMP1 transgenic mice (H) and absent in 742- CDMP1-Int transgenic mice (I). v, vertebral bodies; na, neural arch; r, rib. Scale bars, 1 μm.
Figure 8.
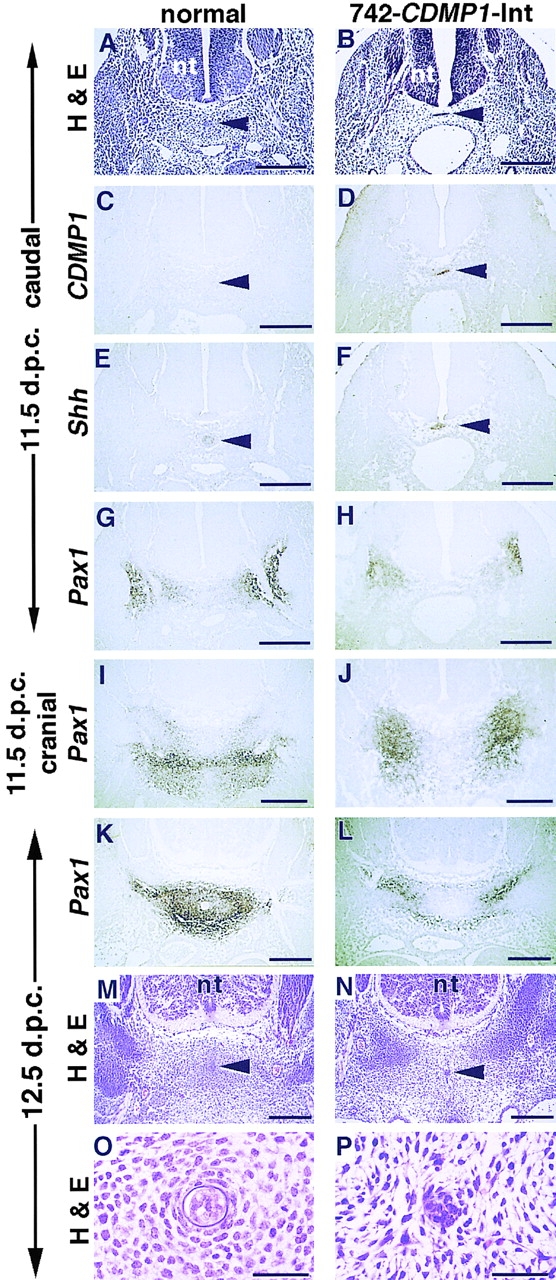
Histology and expression analysis of spine of 742- CDMP1-Int transgenic mice. (A–H) Axial semiserial sections at caudal level (in tail region) of the spine of normal (A, C, E, and G) and 742-CDMP1-Int transgenic (B, D, F, and H) embryos at 11.5 d.p.c. Staining with hematoxylin and eosin (A and B). In situ hybridization of CDMP1 (C and D), Shh (E and F), and Pax1 (G and H) antisense probes. In transgenic mice, CDMP-1 mRNA was localized in the notochord. (I and J) In situ hybridization of sections at the cranial level (thoracic region) of the spine of normal (I) and 742-CDMP1-Int transgenic (J) embryos at 11.5 d.p.c. using Pax1 antisense probe. (K–N) Axial semiserial sections of spine in the thoracic region in normal (K and M) and 742- CDMP1-Int transgenic (L and N) embryos at 12.5 d.p.c. In situ hybridization of Pax1 (K and L) and staining with hematoxylin and eosin (M and N). In normal mice, Pax1-expressing sclerotome cells migrated toward the notochord to form aggregation as the developmental stage progressed (G, I, and K). In transgenic mice, those cells remained laterally (H, J, and L). (O and P) Magnifications of the region around the notochord in M and N, respectively. In 742-CDMP1-Int transgenic mice, mesenchymal cells around the notochord were disorganized, and the sheath of the notochord was not clear. Arrowhead, notochord; nt, neural tube. Scale bars, A–N, 200 μm; O and P, 50 μm.
Histological sections of 12.5 d.p.c. embryos showed that mesenchymal cells were condensing around the notochord of normal mice (Fig. 8, M and O). In contrast, mesenchymal cells around the notochord of 742-CDMP1-Int transgenic mice were disorganized (Fig. 8, N and P). In addition, the sheath of the notochord was not clearly seen in the transgenic mice (Fig. 8 P). These results suggest that CDMP-1 expression in the notochord blocks migration of Pax1-expressing cells from the sclerotome to the notochord and thus blocks the vertebral body formation.
Discussion
We have studied the phenotypic changes in transgenic mice by targeted expression of CDMP-1 to cartilage to gain insights into the mechanism of action of BMPs in skeletal development. The transgenic mice developed abnormal phenotypes characteristic of chondrodysplasia and died before or just after birth. Many of the cartilage elements in the transgenic mice were thick and enlarged containing reduced height of the proliferating zone and expanded prehypertrophic and hypertrophic zones. The number of cells within the mesenchymal condensations and the number of prechondrogenic cells in the transgenic mice were increased. These results suggest that CDMP-1 enhances the commitment of mesenchymal cells into the chondrogenic lineage and their differentiation toward hypertrophy. Ectopic expression of CDMP-1 in the notochord before onset of chondrogenesis inhibited mesenchymal cell condensation around the notochord, which led to failure of vertebral body formation. Although there are many reports which suggest the important role for BMPs in chondrogenesis and osteogenesis using primary cells and progenitor cell lines, our results convincingly show that CDMP-1 affects skeletal growth and chondrocyte maturation in vivo.
Skeletal Abnormalities of CDMP-1 Transgenic Mice
We used three different promoter/enhancer sequences to induce various levels of expression of recombinant CDMP-1 in the developing skeleton. In situ hybridization and immunostaining revealed that the transgene was transcribed and produced CDMP-1 protein in cartilage. The chondrodysplasia-like phenotypes of the transgenic mice were consistent among individual founder mice for each construct, confirming that the abnormal phenotypes were caused by the expression of recombinant CDMP-1. The three different expression vectors generated transgenic mice with distinct phenotypes, though all showed characteristics for chondrodysplasia. The differences were found in body size, joint formation, and the extent of deformity of the skeleton and appear to be caused by different expression levels of recombinant CDMP-1. The Col2a1-CDMP1 construct produced a very severe phenotype with a low production frequency (0.3%) of transgenic mice. It is likely that most Col2a1-CDMP1 transgenic embryos were lethal at very early stages of development because the expression level of CDMP-1 directed by the type II collagen promoter/enhancer was very high. Only one Col2a1-CDMP1 transgenic mouse survived until birth, probably because the expression level was incidentally low due to the site of integration. A major difference between the 742-CDMP1 and 742-CDMP1-Int transgenic mice was in body size. All 742-CDMP1 mice were larger than the normal mice, whereas 742-CDMP1-Int mice showed dwarfism. The difference is likely due to the absence of vertebral bodies and fusion of joints in 742-CDMP1-Int transgenic mice.
Primordial Cartilage
A phenotype common to all of the transgenic mice was the expansion of the primordial cartilage. The expansion was more prominent in distal skeletal components, such as the paws, than in proximal components of the limb. The degree of the expansion seemed to be correlated with the promoter activities used for transgene constructions. Our finding of greatly expanded paws in transgenic mice is inconsistent with the phenotype of GDF5/CDMP1 null mutations in mice and humans which cause shortening of appendicular skeleton, with prominent reduction in size of paws or hand and foot skeletal components (Storm et al., 1994; Thomas et al., 1996). These results strongly suggest that GDF5/CDMP-1 controls the size of skeletal components in the distal part of the limb.
In normal development, GDF5/CDMP-1 is not expressed in axial skeleton, such as rib and spine, which accounts for the unique chondrodysplasia phenotype of the mutations in mice and humans with little abnormalities in the axial skeleton. However, expansion of the ribs in CDMP-1 transgenic mice indicates that CDMP-1 functions effectively in the axial skeleton. This activity is probably mediated by BMP receptors which are expressed in axial skeleton as well as in the limb skeleton (Dewulf et al., 1995). These results suggest that CDMP-1 can effectively transmit cellular signaling in tissues where other BMPs normally function in skeletal formation. Members of the BMP family apparently share common functions. However, unique expression patterns and affinity to the specific receptors play an important role in regulating the patterning and formation of different skeletal components. Our results clearly support the notion that the spatial regulation of BMP expression is important for controlling the size, number, and shape of individual skeletal components (Kingsley, 1994).
Cellular signaling by BMPs is mediated by two types of serine/threonine kinase receptors, type I (BMPR-I) and type II (BMPR-II). There are two type I receptors, type IA (BMPR-IA) and IB (BMPR-IB). It has been reported that CDMP-1/GDF5 binds to BMPR-IB and BMPR-II, but not to BMPR-IA (Nishitoh et al., 1996). Recently, Zou et al. (1997) showed that overexpression of constitutively activated BMPR-IB elicited extensive mesenchymal condensation and expansion of the primordial cartilage in chick limb buds by means of retrovirus vectors. However, expression of constitutively activated BMPR-IA delayed chondrocyte differentiation (Zou et al., 1997). Stimulation of mesenchymal condensation and subsequent expansion of the primordial cartilage in CDMP-1 transgenic mice suggest that recombinant CDMP-1 may bind to BMPR-IB and induces intracellular signaling for the expansion of cartilage. Promotion of chondrocyte differentiation also suggests that recombinant CDMP-1 may not bind effectively to BMPR-IA even if it was overexpressed in transgenic mice. Thus, our findings are inconsistent with those previous reports.
During the skeletal development of CDMP-1 transgenic mice, cell expansion was already apparent in mesenchymal condensations. At this stage, expansion was primarily caused by the increased number of cells organizing condensation. At 13.5 d.p.c. when cartilage anlages are recognizable, transgenic cartilage was surrounded by type IIA mRNA-positive cells, whereas the signal for type IIA mRNA in normal mice was much weaker. Because type IIA mRNA is a good marker for chondroprogenitor cells (Sandell et al., 1994), cells surrounding transgenic cartilage are likely representing mesenchymal cells committed to a chondrocytic lineage. Taken together with the enlargement of mesenchymal condensation, the increased numbers of chondroprogenitor cells in transgenic mice suggest that CDMP-1 enhanced recruitment of mesenchymal cells to the chondrocytic lineage, contributing to the formation of thicker transgenic cartilage.
Promotion of Chondrocytic Differentiation
A striking feature in the cartilage common to all of the transgenic mice was the increased size of prehypertrophic and hypertrophic zones accompanied with the decreased size of the proliferating chondrocyte zone. Endochondral bone formation is initiated when chondrocytes in the center of primordial cartilage proliferate to expand cell population and differentiate into hypertrophic chondrocytes. Hypertrophic chondrocytes eventually degenerate and die and are replaced by osteoblasts to form bone. This process radiates outward with the formation of the growth plates at both ends of primordial cartilage. These temporal events are represented spatially by zones for proliferative and hypertrophic chondrocytes (Fig. 4 A, second row, and Fig. 9) (Horton, 1993). The reduction in the height of the proliferating zone in 724-CDMP1-Int transgenic mice is likely due to accelerated cell differentiation into hypertrophic chondrocytes (Fig. 9). It is conceivable that enhanced differentiation of these cells into hypertrophic chondrocytes could result in the increased height of zone of hypertrophy in transgenic mice. In situ hybridization with the Ihh and type X collagen genes, markers for prehypertrophic and hypertrophic chondrocytes, showed the enlargement of the prehypertrophic and hypertrophic zones. These results suggest that CDMP-1 expression promotes differentiation of chondrocytes to hypertrophy. Recently, it has been reported that BMP signaling mediated by BMPR-II is necessary for the maintenance of the differentiation phenotype (Enomoto-Iwamoto et al., 1998). In addition, BMP responsive elements were found in the promoter region of the type X collagen gene (Volk et al., 1998). In transgenic mice, CDMP-1 could bind to the type II receptor and activate a BMP signaling to promote differentiation of chondrocytes.
Figure 9.
Schematic drawing of the action of CDMP-1 on cartilage formation and differentiation. (Left) Skeletogenesis is initiated with commitment of mesenchymal condensation to the chondrocytic lineage and followed by a series of differentiation process of chondrocytes and replacement by bone in endochondral bone formation. These temporal events are represented spatially with zone structures named as resting (r), proliferative (p), and hypertrophic zones (h) which are arrayed from end to center of the primordial cartilage. (Right) We propose that CDMP-1 promotes the recruitment of mesenchymal cells into the chondrogenic lineage, resulting in the radial expansion of the primordial cartilage. CDMP-1 affects endochondral ossification by accelerating differentiation of chondrocytes to hypertrophy, resulting in the expansion of bones. Through these mechanisms, CDMP-1 regulates the width and length of the skeletal components.
Ossification in the transgenic mice apparently initiated at the normal developmental stages except the cranial bone. These results suggest different mechanisms of bone formation in these tissues.
Vertebral Bodies
Formation of the primordial cartilage of vertebral bodies is unique and complex because precursor cells migrate from remote places to form aggregation. Sclerotome cells located lateral to the neural tube migrate toward the notochord ventral to the neural tube to form primordial cartilage of vertebral bodies around the notochord (Christ and Wilting, 1992). This migration is believed to be guided by morphogens such as sonic hedgehog (Shh) secreted from the notochord (Fan and Tessier-Lavigne, 1994; Johnson et al., 1994). Intensive studies have shown that several BMPs are expressed in the dorsal part of the neural tube and surface ectoderm. These BMPs induce dorsal cell types in neural tube (Liem et al., 1995) and spinal column (Monsoro-Burq et al., 1996) and are believed to antagonize the ventralization signals from the notochord. In 742- CDMP1-Int transgenic mice, CDMP1 was expressed in the notochord before the onset of chondrogenesis. Expression of Shh in the notochord was found in the transgenic mice similar to that seen in normal mice, and Pax1 expression was induced in the sclerotome normally. However, Pax1-expressing sclerotome cells did not migrate toward the notochord, resulting in formation of the Pax1-negative region with a diameter of 200 μm in the transgenic mice. Because CDMP-1 binds and signals through receptor complexes used by other BMPs which mediate the dorsalizing signals (Nishitoh et al., 1996), it is tempting to assume that recombinant CDMP-1 expressed in the notochord of the transgenic mice antagonizes the ventralizing signals. Inhibition of migration of Pax1-positive cells to the notochord resulted in the failure of vertebral body formation at later stages. Abnormal appearance of the notochord suggests that CDMP-1 might affect the notochord itself as well.
Acknowledgments
We thank Andrew McMahon, Haruhiko Koseki, Arthur Broadus, Kathryn Cheah, and Linda Sandell for probes. We thank Kathy Malinda, Peter Burbelo, and Hynda Kleinman for comments.
Abbreviations used in this paper
- AgNOR
silver stain for nucleolar organizer regions
- BMP
bone morphogenetic protein
- CDMP-1
cartilage-derived morphogenetic protein-1
- d.p.c.
days postcoitus
- GDF5
growth and differentiation factor 5
Footnotes
Frank P. Luyten's present address is Division of Rheumatology, Universitaire Ziekenhuizen Leuven, U.Z. Pellenberg, Weligerveld 1, 3212 Lubbeek (Pellenberg), Belgium.
References
- Chang SC, Hoang B, Thomas JT, Vukicevic S, Luyten FP, Ryba NJ, Kozak CA, Reddi AH, Moos M., Jr Cartilage-derived morphogenetic proteins. New members of the transforming growth factor-β superfamily predominantly expressed in long bones during human embryonic development. J Biol Chem. 1994;269:28227–28234. [PubMed] [Google Scholar]
- Christ B, Wilting J. From somites to vertebral column. Anat Anz. 1992;174:23–32. doi: 10.1016/s0940-9602(11)80337-7. [DOI] [PubMed] [Google Scholar]
- Crocker J, Nar P. Nuclear organizer regions in lymphomas. J Pathol. 1987;151:111–118. doi: 10.1002/path.1711510203. [DOI] [PubMed] [Google Scholar]
- Deutsch U, Dressler GR, Gruss P. Pax 1, a member of a paired box homologous murine gene family, is expressed in segmented structures during development. Cell. 1988;53:617–625. doi: 10.1016/0092-8674(88)90577-6. [DOI] [PubMed] [Google Scholar]
- Dewulf N, Verschueren K, Lonnoy O, Morén A, Grimsby S, Vande K, Spiegle, Miyazono K, Huylebroeck D, Ten P, Dijke Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 1995;136:2652–2663. doi: 10.1210/endo.136.6.7750489. [DOI] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Enomoto-Iwamoto M, Iwamoto M, Mukudai Y, Kawakami Y, Nohno T, Higuchi Y, Takemoto S, Ohuchi H, Noji S, Kurisu K. Bone morphogenetic protein signaling is required for maintenance of differentiated phenotype, control of proliferation, and hypertrophy in chondrocytes. J Cell Biol. 1998;140:409–418. doi: 10.1083/jcb.140.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan CM, Tessier-Lavigne M. Patterning of mammalian somites by surface ectoderm and notochord: evidence for sclerotome induction by a hedgehog homolog. Cell. 1994;79:1175–1186. doi: 10.1016/0092-8674(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Heinisch G, Wozel G. Determination of epidermal proliferative activity in experimental mouse tail test by AgNOR analysis. Exp Toxicol Pathol. 1995;47:19–23. doi: 10.1016/S0940-2993(11)80276-9. [DOI] [PubMed] [Google Scholar]
- Hirota S, Ito A, Morii E, Wanaka A, Tohyama M, Kitamura Y, Nomura S. Localization of mRNA for c-kitreceptor and its ligand in the brain of adult rats: an analysis using in situ hybridization histochemistry. Brain Res Mol Brain Res. 1992;15:47–54. doi: 10.1016/0169-328x(92)90150-a. [DOI] [PubMed] [Google Scholar]
- Hogan, B., R. Beddington, F. Constantini, and E. Lacy. 1994. Manipulating the Mouse Embryo: A Laboratory Manual. 2nd edition. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Horton W, Miyashita T, Kohno K, Hassell JR, Yamada Y. Identification of a phenotype-specific enhancer in the first intron of the rat collagen II gene. Proc Natl Acad Sci USA. 1987;84:8864–8868. doi: 10.1073/pnas.84.24.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton, W.A. 1993. Morphology of connective tissue: cartilage. In Connective Tissue and Its Heritable Disorders. B.S.P.M. Royce, editor. Wiley-Liss, Inc., New York. 73–84.
- Hötten GC, Matsumoto T, Kimura M, Bechtold RF, Kron R, Ohara T, Tanaka H, Satoh Y, Okazaki M, Shirai T, et al. Recombinant human growth/differentiation factor 5 stimulates mesenchyme aggregation and chondrogenesis responsible for the skeletal development of limbs. Growth Factors. 1996;13:65–74. doi: 10.3109/08977199609034567. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Laufer E, Riddle RD, Tabin C. Ectopic expression of Sonic hedgehog alters dorsal-ventral patterning of somites. Cell. 1994;79:1165–1173. doi: 10.1016/0092-8674(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Kingsley DM. What do BMPs do in mammals? Clues from the mouse short-ear mutation. Trends Genet. 1994;10:16–21. doi: 10.1016/0168-9525(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Kong RY, Kwan KM, Lau ET, Thomas JT, Boot-Handford RP, Grant ME, Cheah KS. Intron-exon structure, alternative use of promoter and expression of the mouse collagen X gene, Col10a-1. . Eur J Biochem. 1993;213:99–111. doi: 10.1111/j.1432-1033.1993.tb17739.x. [DOI] [PubMed] [Google Scholar]
- Krebsbach PH, Nakata K, Bernier SM, Hatano O, Miyashita T, Rhodes CS, Yamada Y. Identification of a minimum enhancer sequence for the type II collagen gene reveals several core sequence motifs in common with the link protein gene. J Biol Chem. 1996;271:4298–4303. doi: 10.1074/jbc.271.8.4298. [DOI] [PubMed] [Google Scholar]
- Leek RD, Alison MR, Sarraf CE. Variations in the occurrence of silver-staining nucleolar organizer regions (AgNORs) in non-proliferating and proliferating tissues. J Pathol. 1991;165:43–51. doi: 10.1002/path.1711650108. [DOI] [PubMed] [Google Scholar]
- Liem KF, Jr, Tremml G, Roelink H, Jessell TM. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82:969–979. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Lyons KM, Pelton RW, Hogan BL. Patterns of expression of murine Vgr-1 and BMP-2a RNA suggest that transforming growth factor-β-like genes coordinately regulate aspects of embryonic development. Genes Dev. 1989;3:1657–1668. doi: 10.1101/gad.3.11.1657. [DOI] [PubMed] [Google Scholar]
- Macias D, Ganan Y, Sampath TK, Piedra ME, Ros MA, Hurle JM. Role of BMP-2 and OP-1 (BMP-7) in programmed cell death and skeletogenesis during chick limb development. Development. 1997;124:1109–1117. doi: 10.1242/dev.124.6.1109. [DOI] [PubMed] [Google Scholar]
- Mangin M, Ikeda K, Broadus AE. Structure of the mouse gene encoding parathyroid hormone-related peptide. Gene. 1990;95:195–202. doi: 10.1016/0378-1119(90)90362-u. [DOI] [PubMed] [Google Scholar]
- Metsaranta M, Toman D, de Crombrugghe B, Vuorio E. Mouse type II collagen gene. Complete nucleotide sequence, exon structure, and alternative splicing. J Biol Chem. 1991;266:16862–16869. [PubMed] [Google Scholar]
- Monsoro-Burq AH, Duprez D, Watanabe Y, Bontoux M, Vincent C, Brickell P, Le Douarin N. The role of bone morphogenetic proteins in vertebral development. Development. 1996;122:3607–3616. doi: 10.1242/dev.122.11.3607. [DOI] [PubMed] [Google Scholar]
- Nagano T, Yonenobu K, Miyamoto S, Tohyama M, Ono K. Distribution of the basic fibroblast growth factor and its receptor gene expression in normal and degenerated rat intervertebral discs. Spine. 1995;20:1972–1978. doi: 10.1097/00007632-199509150-00002. [DOI] [PubMed] [Google Scholar]
- Nishitoh H, Ichijo H, Kimura M, Matsumoto T, Makishima F, Yamaguchi A, Yamashita H, Enomoto S, Miyazono K. Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. J Biol Chem. 1996;271:21345–21352. doi: 10.1074/jbc.271.35.21345. [DOI] [PubMed] [Google Scholar]
- Peters, P.W.J. 1977. Double staining of fetal skeletons for cartilage and bone. In Methods in Prenatal Toxicology. H.J.M.D. Neuberg and T.E. Kwasigroch, editors. Georg Thieme Verlag, Stuttgart, Germany. 153–154.
- Polinkovsky A, Robin NH, Thomas JT, Irons M, Audrey L, Goodman FR, Reardon W, Kant SG, Brunner HG, van der Burgt I, et al. Mutations in CDMP1cause autosomal dominant brachydactyly type C. Nat Genet. 1997;17:18–19. doi: 10.1038/ng0997-18. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Sandell LJ. Differential expression of a cysteine-rich domain in the amino-terminal propeptide of type II (cartilage) procollagen by alternative splicing of mRNA. J Biol Chem. 1990;265:10334–10339. [PubMed] [Google Scholar]
- Sampath TK, Reddi AH. Dissociative extraction and reconstitution of extracellular matrix components involved in local bone differentiation. Proc Natl Acad Sci USA. 1981;78:7599–7603. doi: 10.1073/pnas.78.12.7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell LJ, Nalin AM, Reife RA. Alternative splice form of type II procollagen mRNA (IIA) is predominant in skeletal precursors and non-cartilaginous tissues during early mouse development. Dev Dyn. 1994;199:129–140. doi: 10.1002/aja.1001990206. [DOI] [PubMed] [Google Scholar]
- Storm EE, Kingsley DM. Joint patterning defects caused by single and double mutations in members of the bone morphogenetic protein (BMP) family. Development. 1996;122:3969–3979. doi: 10.1242/dev.122.12.3969. [DOI] [PubMed] [Google Scholar]
- Storm EE, Huynh TV, Copeland NG, Jenkins NA, Kingsley DM, Lee SJ. Limb alterations in brachypodism mice due to mutations in a new member of the TGF β-superfamily. Nature. 1994;368:639–643. doi: 10.1038/368639a0. [DOI] [PubMed] [Google Scholar]
- Thomas JT, Lin K, Nandedkar M, Camargo M, Cervenka J, Luyten FP. A human chondrodysplasia due to a mutation in a TGF-β superfamily member. Nat Genet. 1996;12:315–317. doi: 10.1038/ng0396-315. [DOI] [PubMed] [Google Scholar]
- Thomas JT, Kilpatrick MW, Lin K, Erlacher L, Lembessis P, Costa T, Tsipouras P, Luyten FP. Disruption of human limb morphogenesis by a dominant negative mutation in cartilage-derived morphogenetic protein-1. Nat Genet. 1997;17:58–64. doi: 10.1038/ng0997-58. [DOI] [PubMed] [Google Scholar]
- Tsumaki N, Kimura T. Differential expression of an acidic domain in the amino-terminal propeptide of mouse pro-α 2(XI) collagen by complex alternative splicing. J Biol Chem. 1995;270:2372–2378. doi: 10.1074/jbc.270.5.2372. [DOI] [PubMed] [Google Scholar]
- Tsumaki N, Kimura T, Matsui Y, Nakata K, Ochi T. Separable cis-regulatory elements that contribute to tissue- and site-specific α2(XI) collagen gene expression in the embryonic mouse cartilage. J Cell Biol. 1996;134:1573–1582. doi: 10.1083/jcb.134.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumaki N, Kimura T, Tanaka K, Kimura JH, Ochi T, Yamada Y. Modular arrangement of cartilage- and neural tissue-specific cis-elements in the mouse α2(XI) collagen promoter. J Biol Chem. 1998;273:22861–22864. doi: 10.1074/jbc.273.36.22861. [DOI] [PubMed] [Google Scholar]
- Urist MR, Mikulski A, Lietze A. Solubilized and insolubilized bone morphogenetic protein. Proc Natl Acad Sci USA. 1979;76:1828–1832. doi: 10.1073/pnas.76.4.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk SW, Luvalle P, Leask T, Leboy PS. A BMP responsive transcriptional region in the chicken type X collagen gene. J Bone Miner Res. 1998;13:1521–1529. doi: 10.1359/jbmr.1998.13.10.1521. [DOI] [PubMed] [Google Scholar]
- Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Zhou G, Garofalo S, Mukhopadhyay K, Lefebvre V, Smith CN, Eberspaecher H, de Crombrugghe B. A 182 bp fragment of the mouse pro α1(II) collagen gene is sufficient to direct chondrocyte expression in transgenic mice. J Cell Sci. 1995;108:3677–3684. doi: 10.1242/jcs.108.12.3677. [DOI] [PubMed] [Google Scholar]
- Zou H, Wieser R, Massague J, Niswander L. Distinct roles for type I bone morphogenetic protein receptors in the formation and differentiation of cartilage. Genes Dev. 1997;11:2191–2203. doi: 10.1101/gad.11.17.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]



