Abstract
The LAMC1 gene coding for the laminin γ1 subunit was targeted by homologous recombination in mouse embryonic stem cells. Mice heterozygous for the mutation had a normal phenotype and were fertile, whereas homozygous mutant embryos did not survive beyond day 5.5 post coitum. These embryos lacked basement membranes and although the blastocysts had expanded, primitive endoderm cells remained in the inner cell mass, and the parietal yolk sac did not develop. Cultured embryonic stem cells appeared normal after targeting both LAMC1 genes, but the embryoid bodies derived from them also lacked basement membranes, having disorganized extracellular deposits of the basement membrane proteins collagen IV and perlecan, and the cells failed to differentiate into stable myotubes. Secretion of the linking protein nidogen and a truncated laminin α1 subunit did occur, but these were not deposited in the extracellular matrix. These results show that the laminin γ1 subunit is necessary for laminin assembly and that laminin is in turn essential for the organization of other basement membrane components in vivo and in vitro. Surprisingly, basement membranes are not necessary for the formation of the first epithelium to develop during embryogenesis, but first become required for extra embryonic endoderm differentiation.
Keywords: extracellular matrix, epithelium, embryogenesis, endoderm, laminin
Although basement membrane molecules have been shown to affect the differentiation and survival of cells (Streuli, 1996), the mechanisms regulating the assembly of basement membranes in vivo and the fundamental roles of basement membranes during embryogenesis are poorly defined. The best studied basement membrane proteins are the laminins which constitute the major noncollagenous basement membrane component (Timpl, 1996). Antibody inhibition of laminin binding to its cellular receptors or to other basement membrane components has been shown to perturb both basement membrane deposition and also epithelial morphogenesis in organ culture (Klein et al., 1988; Sorokin et al., 1990; Ekblom et al., 1994; Kadoya et al., 1995). However, it remains to be established if basement membranes are an absolute requirement for epithelial cell differentiation and at what stages of development these fundamental extracellular matrix structures become essential.
All characterized laminin variants are heterotrimeric molecules formed by the covalent bonding of one polypeptide from the α, β, and γ laminin subunit families, each of which comprises multiple members encoded by individual genes (Maurer and Engel, 1996). Thus, many variant laminin trimers may potentially be formed, depending on differential subunit gene expression which occurs in a time- and cell-specific manner (Paulsson, 1996). Definitive evidence for the distinct roles of some laminin variants in vivo has been provided by the phenotypes of mutations in laminin subunit genes. For example, natural mutations in any of the genes coding for subunits of laminin type-5 (kalinin) can result in junctional epidermolysis bullosa (Burgeson, 1996). Similarly, mutations of the α2 subunit (merosin) can result in autosomal forms of muscular dystrophy (Helbling-Leclerc et al., 1995), and targeted disruption of the laminin β2 chain (s-laminin) has been shown to result in disruption of neuromuscular junction development and of kidney function (Noakes et al., 1995). The characteristic postnatal phenotypes of all of these mutations reflect the restricted expression and specific functions of the minor laminin subunits concerned.
Laminin γ1 is one of the earliest expressed laminin subunits which, together with the α1 and β1 subunits of laminin type-1, is expressed in the preimplantation embryo (Shim et al., 1996) before the appearance of the first basement membrane of the trophectodermal epithelium (Dziadek and Timpl, 1985; Thorsteinsdottir, 1992). Furthermore, γ1 is the most ubiquitously expressed laminin subunit, being present in 10 of the 11 known laminin isoforms (Burgeson et al., 1994; Miner et al., 1997). Indeed, the only isoform (type-5) shown to lack the γ1 subunit has instead the other known member of this subunit family, γ2 (Kallunki et al., 1992). However, the γ2 subunit has a restricted distribution being associated with epithelial anchoring filaments rather than being a common component of basement membranes (Burgeson, 1996). This is most probably either because it has no nidogen binding domain (Mayer et al., 1993) or because it lacks the three self-interacting NH2-terminal globular domains necessary to link it to the other basement membrane components (Champliaud et al., 1996; Cheng et al., 1997). We therefore decided to use homologous recombination to target the mouse LAMC1 gene because the resulting lack of the laminin γ1 subunit would alter the formation of all known integral basement membrane laminin isoforms. This would therefore be expected to affect the structure and function of most if not all basement membranes. Analysis of the phenotype of this knockout thus defines the role of laminin in basement membrane formation in vivo, and in this in turn demonstrates the initial function of basement membranes in tissue development during embryogenesis.
Materials and Methods
Production of Targeting Constructs
A Lambda FIX®II genomic library (Stratagene) of the 129SVJ mouse line was screened using a PCR product comprising 372 bp of the 5′ untranslated region and the first 128 bases of exon one of the LAMC1 gene (Ogawa et al., 1988). Six different clones representing this area were isolated and mapped. Targeting construct 1 was formed by cloning the 6-kb HindIII fragment containing the first exon together with 2-kb upstream sequence and 3.5 kb of intron 1 into pUC 19. The IRES β-Geo cassette (Friedrich and Soriano, 1991; Mountford et al., 1994), which had a NotI linker added to its 3′ end was inserted into the unique NotI site in the first exon (see Fig. 1). The use of the cap-independent translation initiation sequence removed the need to place a Neo resistance cassette in frame to obtain expression by the LAMC1 promoter.
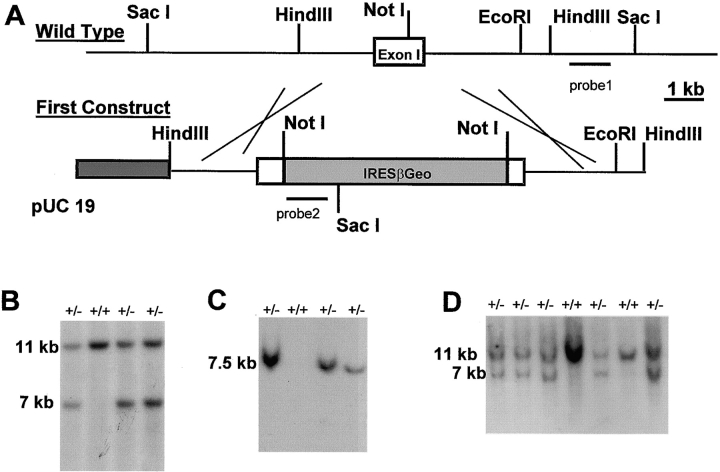
Figure 1.
Homologous recombination in the LAMC1 gene of ES cells and germline transmission in mice. (A) Restriction map including the first exon of the LAMC1 gene and the targeting construct used to disrupt the gene. (B) SacI-digested DNA from ES clones analyzed with probe 1, showing appearance of a 7-kb band of equal intensity to the 11-kb wild-type band in clones having undergone homologous recombination. (C) A single integration event was confirmed using the internal probe 2, which produced a single 7.5-kb band when hybridized against SacI-digested DNA. (D) SacI-digested tail DNA of the offspring from LAMC1 +/− mice matings hybridized with probe 1 to show the absence of animals homozygous for the mutation.
Construct 2 was an EcoRI/SacI fragment of LAMC1 cloned into KSII Bluescript (Stratagene). The sequence was interrupted at the NotI site by the insertion of the phosphoglycerate kinase (pgk) promoter (Soriano et al., 1991) 5′ to a hygromycin resistance cassette with poly A tail (Hygro). This divided the LAMC1 fragment into two arms with 6-kb homology in the 5′ arm and 2.5-kb homology in the 3′ arm (see Fig. 2).
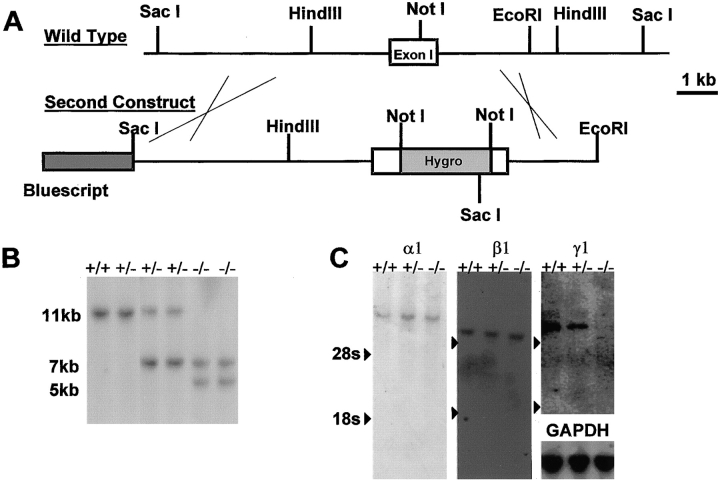
Figure 2.
Analysis of ES cells lines generated by the homologous replacement of the second LAMC1 allele. (A) Restriction map of the second targeting construct used to disrupt the remaining LAMC1 allele by insertion of a hygromycin resistance cassette. (B) Southern blot analysis with probe 1 of SacI-digested DNA from ES clones. Loss of both LAMC1 alleles results in the loss of the 11-kb wild-type band and the appearance of a 5-kb band in addition to the 7-kb band generated by the first targeting event. (C) Northern blot analysis of total RNA from ES cells +/+, +/−, and −/− for the LAMC1. The cDNA probes used were for the mRNAs of LAMA1(α1), LAMB1(β1), and LAMC1(γ1). The GADPH probe was used as a loading control. Arrowheads, positions of 28S and 18S rRNA bands on the three blots. The LAMC1 message was reduced in the +/− ES cells and absent −/− cells, and there was no change observed in the levels of mRNAs coding for the other laminin subunits.
LAMC1 Gene Targeting in Embryonic Stem Cells
R1 mouse embryonic stem (ES)1 cells were grown in standard ES conditions with DME supplemented with 15% (vol/vol) fetal bovine serum (FBS), 0.1 mM β mercaptoethanol, and 1,000 U/ml of LIF (ESGRO; GIBCO BRL). 5 × 106 cells were transfected by electroporation with 25 μg of linearized construct 1 and colonies selected for resistance to G418 at 380 μg/ml in the culture medium. Surviving clones were picked, expanded, and then DNA extracted for Southern blotting. DNA from the cells digested with SacI was probed with an external 3′ probe and an internal 5′ probe (see Fig. 1). In cases of correct integration, the wild-type 11-kb fragment was reduced to 7 kb when probed with the external probe and a 7.5-kb band was seen with the internal probe (see Fig. 1).
Attempts using increased G418 concentrations up to 1.5 mg/ml failed to produce ES cells in which both the LAMC1 alleles had been targeted (Mortensen et al., 1992). Therefore, the second targeting construct was used for disruption of the second LAMC1 allele in ES cells previously targeted with construct 1 (see Fig. 2 A). After correct targeting, Southern blot hybridization of SacI genomic DNA digests with probe 1 resulted in the wild-type band being lost, whereas a 5.5-kb band appeared (see Fig. 2 B). Clones so targeted were checked for the absence of expression of the LAMC1 gene by Northern hybridization of mRNA with a probe of LAMC1 cDNA.
Production of Mice Lacking the LAMC1 Gene
Two independent ES cells lines were used to generate germ line chimeras. Blastocysts were isolated from C57Bl/6 mice 3.5 d post coitum (pc) (plug date = 0.5 d pc) and were injected with five to seven +/− ES cells. Blastocysts were then transferred into the uteri of pseudopregnant CD1 foster mothers. Chimeric male progeny were mated to C57Bl/6 females and offspring were tested for germline transmission by Southern blots of DNA extracted from tail biopsies. Heterozygous animals were mated together to obtain homozygous embryos.
Immuno- and Fragmented DNA Staining
The rabbit polyclonal primary antibodies used were: anti–laminin α1 raised against recombinant domain IVa (Schulze et al., 1996); anti–laminin γ1 raised against recombinant domain III LE3-5 (Mayer et al., 1993); anti-EHS laminin which recognizes all three subunits of laminin (Kücherer-Ehret et al., 1990); anti-nidogen raised against recombinant nidogen (Fox et al., 1991); and anti-perlecan raised against recombinant domain III3 (Schulze et al., 1995). Rabbit polyclonal antibodies against von Willebrand factor, the 200-kD neurofilament subunit, and skeletal myosin were obtained from Sigma.
Embryos were washed in phosphate buffered saline (PBS) before embedding and freezing in Tissue-Tek (Sakura Finetek Europe). Cryostat sections were fixed with 0.5% (wt/vol) paraformaldehyde in PBS for 10 min, washed with PBS, and then blocked with 5% (vol/vol) goat serum in PBS/0.1% (vol/vol) Tween 20. The primary antibodies (see below) and goat–anti rabbit Cy3 conjugate secondary antibodies (Jackson Immunodiagnostics) were used in the same solution before washing the sections in PBS and mounting in fluorescent mounting medium (Dako).
For visualization of fragmented nuclear DNA in situ, serial cryosections were fixed for 20 min in 4% (wt/vol) paraformaldehyde in PBS before staining by a modification of the terminal dUTP–biotin nick end labeling (TUNEL) method (Gavrieli et al., 1992). A TACS apoptosis detection kit™ (Trevigen) was used according to the manufacturer's instructions, fragmented DNA being end labeled with biotinylated nucleotides using the Klenow fragment, followed by detection with streptavidin-horseradish peroxidase conjugates. The sections were then counterstained with eosin.
Embryo Culture
Embryos were isolated from heterozygous matings by flushing the uterus with M2 medium and the blastocysts were cultured in M16 medium at 37°C in 5% CO2 until they had fully expanded or hatched. Where present, the zona pellucida was removed from the expanded blastocysts by a short incubation in acid tyrode solution and the blastocysts washed in PBS before fixation in 1% (wt/vol) paraformaldehyde for 10 min at room temperature. The blastocysts were permeabilized in PBS/0.02% (vol/vol) Triton X-100 containing 2% (wt/vol) bovine serum albumin for 30 min before incubation with antibodies and subsequent fluorescence microscopy.
Embryoid Bodies
Undifferentiated ES cells were trypsinized, triturated, and then resuspended in DME 10% FBS at a dilution of 1,000 cells/μl. The cells were then placed in hanging drops of 20 μl on the lower surface of the lids of plastic Petri dishes containing PBS (Wobus et al., 1991). After 24 h of culture as hanging drops, the cell aggregates were plated into plastic Petri dishes and the embryoid bodies were fixed with 4% (wt/vol) paraformaldehyde after varying culture periods before sectioning and immunostaining as described above. Frozen sections were also stained for lacZ expression as previously described (Beddington and Lawson, 1990).
To monitor cell phenotypes of differentiating ES cells after formation in hanging drops, the embryoid bodies were allowed to attach to tissue culture plastic and cultured in the above medium for 21 d. Preliminary experiments showed that under these conditions small numbers of myotubes differentiated (Kuang et al., 1998). The cultures were then fixed in 4% (wt/vol) paraformaldehyde and immunostained as above.
Northern Blots
Wild-type ES cells, and those heterozygous and homozygous for mutations in the LAMC1 alleles, were preplated in tissue culture dishes for 10 min to deplete them of the embryonic fibroblast feeder cells. The nonadherent ES cells were isolated and cultured on a gelatin-coated plate in ES media with LIF for two or three days until almost confluent. The cells were lysed and RNA extracted with guanidinium isothiocyanate (Chomczynski and Sacchi, 1987). 10 μg of total RNA was separated on a denaturing formaldehyde gel of 1% agarose and transferred by vacuum blotting to a nylon membrane (Hybond N; Amersham). After UV cross-linking of the RNA to the membrane, it was prehybridized with a 50% formamide containing buffer and hybridized against cDNA probes for laminin α1, β1, γ1, γ2, and glyceraldehyde-3-phosphate dehydrogenase (GADPH) mRNAs. After high stringency washing, the blots were exposed to autoradiographic film. The probe for the laminin γ1 mRNA was a BamHI-EcoRI fragment between bases 2,959 and 4,163 in the protein coding area (Sasaki and Yamada, 1987), whereas γ2 was a BamHI fragment spanning nucleotides 1,509–2,120 of the protein coding region (Sugiyama et al., 1995). The probe for the α1 chain was a PCR-generated fragment using GCGCATCAGAACACTCAACG (sense) and CAAGGGTGGTCATCATAAGG (antisense) primers amplifying between bases 708 and 1,203 of the protein coding region (Sasaki et al., 1988). The probe for the β1 chain was a PCR product using primers GATAACTGTCAGCACAACACC (sense) and GTGAAGTAGTAACCGGACTCC (antisense) giving a probe between bases 1,231 and 1,794 of the protein coding region (Sasaki et al., 1987).
Metabolic Labeling and Immunoprecipitation
Embryoid bodies were produced from 5 × 103 ES cells incubated in hanging drops for 2 d (Wobus et al., 1991). About 10 embryoid bodies were then cultured in 500 μl DME without methionine, supplemented with 1% FBS and 200 μCi/ml [35S]methionine (specific activity >1,000 Ci/mmol; Amersham). Labeling was carried out overnight. The medium was then collected, centrifuged, and then supernatants were stored at −70°C. Cells were washed with complete DME supplemented FBS and lysed in 50 mM Tris HCl, pH 7.5, containing 1% (vol/vol) Triton X-100, 10 mM EDTA, 0.10 M NaCl, and protease inhibitors. After trituration, insoluble material was removed by centrifugation and the supernatants were stored at −70°C.
Immunoprecipitations were carried out in the extraction buffer using Pansorbin (Calbiochem-Novabiochem) to precipitate the antibody–antigen complexes. Proteins bound to the Pansorbin were removed using boiling SDS gel electrophoresis sample buffer. Proteins were fractionated by SDS-PAGE on 5% gels under reducing conditions or on 3 and 5% gels under nonreducing conditions before fluorography.
For immunoblot analysis, embryoid bodies were cultured as above but using complete DME supplemented with 10% FBS. Cell extracts and media were concentrated by immunoprecipitation with antilaminin antiserum before SDS-PAGE under reducing conditions. After electroblotting onto nitrocellulose, the membrane was incubated with the primary antibodies which were detected using goat anti–rabbit antibodies conjugated with horseradish peroxidase (Dako). The enzymatic activity was visualized using 4-chloro-1-naphthol.
Results
Targeted Disruption of LAMC1 Genes in ES Cells
A diagram of the initial targeting of the LAMC1 gene is shown in Fig. 1 A. Of the 50 G418-resistant ES cell clones expanded and analyzed on Southern blots by hybridization with probe 1, 17 had undergone recombination at the LAMC1 gene, as shown by the appearance of a band of the expected size of 7 kb and of equal intensity to the remaining wild-type 1-kb band (Fig. 1 B). A single insertion was demonstrated by the internal probe 2 which hybridized to the expected 7.5-kb band (Fig. 1 C). Although expression of the neomycin resistance–lacZ fusion cassette was dependent on the laminin promoter (Ogawa et al., 1988) present in the targeting vector, the high frequency of homologous recombination (>30% of all G418-resistant clones) suggests that the regulatory elements needed for full expression of the LAMC1 gene in ES cells were lacking in the construct. This agrees with the recent demonstration of a strong enhancer element in the first intron of LAMC1, which was absent from our targeting construct (Chang et al., 1996). As expected, the frequency of the second targeting event with the hygromycin resistance cassette (Fig. 2 A) was much lower than that of the first: out of 200 clones, 12 had undergone the second homologous recombination shown by replacement of the wild-type LAMC1 band by a 5-kb fragment (Fig. 2 B).
Northern blotting showed the absence of laminin γ1 subunit mRNA in −/− ES cells, and the amount of LAMC1 mRNA was reduced in +/− cells relative to the wild type (Fig. 2 C). However, levels of the mRNAs coding for laminin α1 and β1 subunits were the same in undifferentiated +/+, +/−, and −/− cells (Fig. 2 C). The expression of LAMC2 remained below the level of detection in all cases (data not shown).
Consequences of LAMC1 Disruption In Vivo
LAMC1 +/− animals were phenotypically normal and have been intercrossed for at least seven generations and have also been bred into a C57Bl/6 background. More than 200 heterozygous matings produced no progeny homozygous for the mutation that were either born or found in utero after day 8.5 pc, indicating early embryonic lethality (Fig. 1 D). Day 3.5 pc preimplantation blastocysts from these matings were immunostained with polyclonal antibodies specific for the laminin γ1 subunit (Fig. 3 A) and also with antibodies against laminin-1 which recognize the α1 and β1 subunits as well as γ1 (Kücherer-Ehret et al., 1990). The majority of blastocysts showed the reported pattern of immunostaining for laminin-1 (Thorsteinsdottir, 1992): the trophectodermal basement membrane was stained together with apparently intracellular staining of cells throughout the blastocyst (Fig. 3 D). However, about one-quarter of the expanded and hatched embryos showed no immunostaining for the laminin γ1 subunit (Fig. 3, B and C) and displayed only intracellular staining using the laminin-1 antibodies (Fig. 3 D).
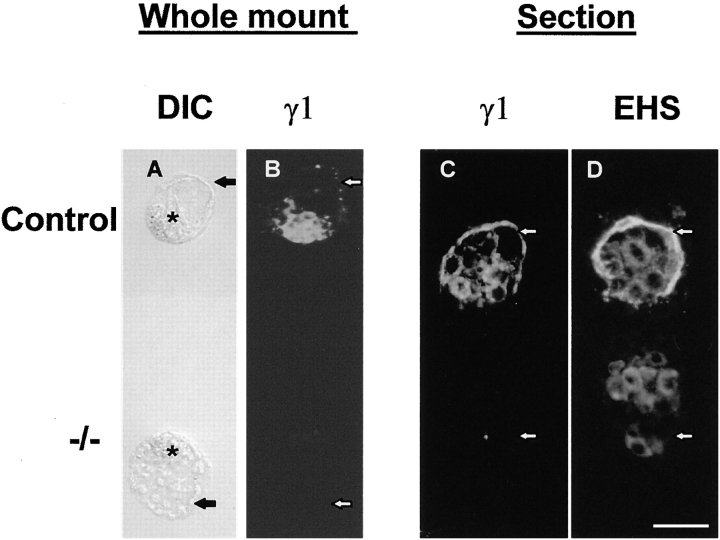
Figure 3.
Appearance of 3.5 d pc preimplantation blastocysts from heterozygote matings. (A and B) Whole mount micrographs of the same pair of blastocysts. (C and D) Frozen 7-μm serial sections. A shows the appearance of the blastocysts using Normarski optics. B and C are stained with γ1 subunit antibodies and D is stained with antibodies against laminin-1. Arrows, location of the trophectoderm; asterisks, inner cell mass. Embryos lacking laminin γ1 immunoreactivity can expand to form blastocysts of normal appearance but lack the trophectodermal basement membrane. Immunoreactivity of the other laminin subunits is intracellular, the cells showing cytoplasmic staining (D). Bars, 50 μm.
The embryos found in decidua at day 4.5 pc appeared histologically normal (Fig. 4, A and F). However, eight out of the 40 embryos sectioned and stained did not display laminin γ1 subunit immunoreactivity, although as expected there was immunoreactivity in the stroma and epithelial lining of maternal decidua (Fig. 4 J), which also stained with the other antibodies used (Fig. 4, G–I). Embryos negative for γ1 immunoreactivity demonstrated the absence of any extracellular laminin when stained with laminin-1 antibodies, although there was an accumulation of cells with intense laminin immunoreactivity in the inner cell mass (Fig. 4 G, inset). Very limited patchy deposits of nidogen and collagen type IV were seen under the trophectodermal epithelium but there was no continuous sheet characteristic of the trophectodermal basement membrane (Fig. 4, H and I).
Figure 4.
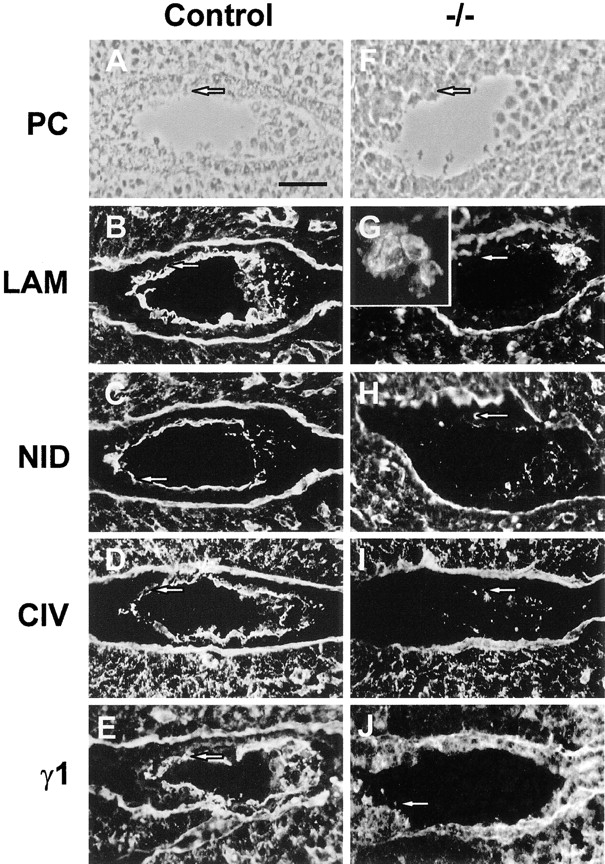
Immunofluorescence staining for basement membrane components in frozen sections of 4.5 d pc embryos in utero. (A–E) Wild-type or heterozygous embryos; (F–J) −/− Embryos, as defined by lack of laminin γ1 immunoreactivity (J). (A and F) Phase–contrast photomicrographs. Immunostaining was performed with antibodies directed against: laminin-1 (B and G); nidogen (C and H); collagen type IV (D and I); laminin γ1 subunit (E and J). In wild-type or heterozygous embryos, all antibodies show staining under the trophectoderm (arrows) and within the inner cell mass. Occasionally strongly staining parietal endoderm cells can be seen migrating over the trophectoderm (B). In the −/− embryos, discrete aggregates of nidogen and collagen IV immunoreactivity can be seen (H and I) whereas strong laminin 1 staining is mainly confined to the cells of the inner cell mass (G) and is apparently intracellular (G, inset). No basement membrane-like immunoreactivity can be seen associated with the trophectoderm in these embryos (arrows), although the maternal basement membrane underlying the uterine epithelium is clearly visible in all cases. Bar, 50 μm.
Of the 80 decidua examined at 5.5 d pc from heterozygous matings, 28 contained laminin γ1–negative accumulations of cells, none of which conformed to any recognizable embryonic structures (Fig. 5, B and C), whereas out of the 40 decidua examined from heterozygous/wild-type matings, only five contained no recognizable embryo. Detection of fragmented nuclear DNA by TUNEL staining of serial sections revealed very few stained cells in any embryos before 5.5 d pc (data not shown). However, at 5.5 d pc we detected low numbers of stained nuclei in the γ1-positive embryos (Fig. 5 D), and the laminin γ1–negative embryos displayed either increased numbers of TUNEL-positive cells (Fig. 5 E) or very intense labeling, indicative of extensive DNA fragmentation (Fig. 5 F).
Figure 5.
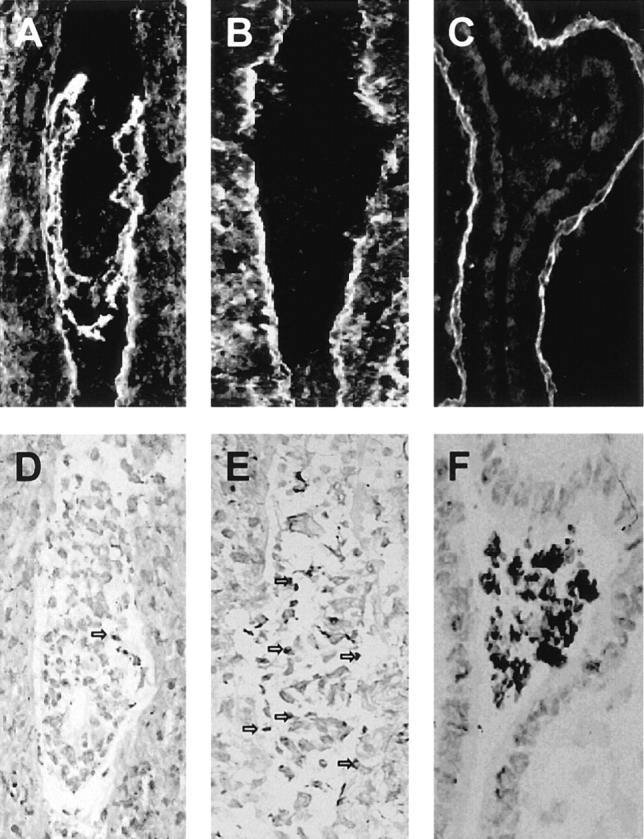
Appearance of 5.5 d pc embryos in utero. Frozen sections of embryos were immunostained with anti–laminin γ1 antibodies to show: (A) a control embryo staining for γ1 (either +/+ or +/−); (B and C) −/− embryos lacking γ1 staining. The γ1-negative embryonic cells had lost recognizable structure, large aggregates of cells being present (E and F). TUNEL staining revealed low numbers of cells with fragmented DNA in serial sections from γ1-positive embryos (D), whereas γ1-negative embryos displayed either increased (E) or intense (F) staining.
Analysis of LAMC1 Disruption In Vitro
After 48 h of suspension culture, the differentiating +/− and −/− ES cells at the periphery of developing embryoid bodies began to display intense lacZ staining (Fig. 6), indicating the expression of the LAMC1 gene. This reflects the differentiation of these cells into primitive endoderm-like cells with high levels of laminin expression (Doetschman et al., 1985). The deposition of a continuous basement membrane-like sheet of laminin, nidogen, perlecan, and collagen type IV immunoreactivity was observed towards the periphery of +/− embryoid bodies after 7 d of culture (Fig. 7, A, C, E, and G). Outside this basement membrane, there was a sheet some one to three cells thick displaying laminin immunoreactivity (Fig. 7 A). No differences in these were observed between +/+ and +/− embryoid bodies (data not shown). In contrast, in addition to the expected lack of γ1 immunoreactivity in the −/− embryoid bodies (data not shown), no deposition of basement membranes was detected when they were stained with any of the above antibodies: although there were patchy extracellular deposits of perlecan and collagen type IV, these molecules were not deposited in a continuous basement membrane-like sheet (Fig. 7, F and H). Although there was no obvious extracellular laminin staining, polyclonal antibodies to laminin-1 showed immunoreactivity to be accumulated mainly in the cells at the surface of the −/− embryoid bodies (Fig. 7 B). Staining with antibodies specific to α1 and β1 showed the same patterns of distribution as that detected with the polyclonal antibodies to laminin 1 (data not shown). Little if any intracellular or extracellular nidogen immunoreactivity was detectable in the −/− embryoid bodies (Fig. 7 D) although it was present as expected in the basement membranes of +/+ and +/− embryoid bodies (Fig. 7 C).
Figure 6.
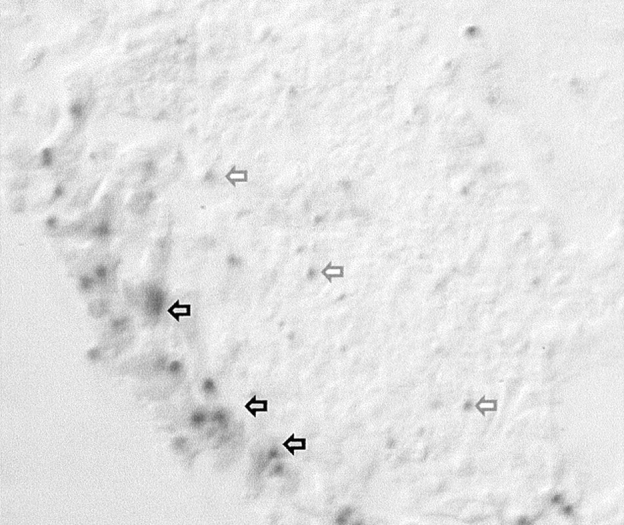
LacZ staining of +/− embryoid bodies after 48 h of culture. The lacZ reaction product is localized to cells in which the LAMC1 promoter is active are found mainly around the periphery of the embryoid body, although a few scattered weakly positive cells were also seen in the center of the embryoid body (arrows).
Figure 7.
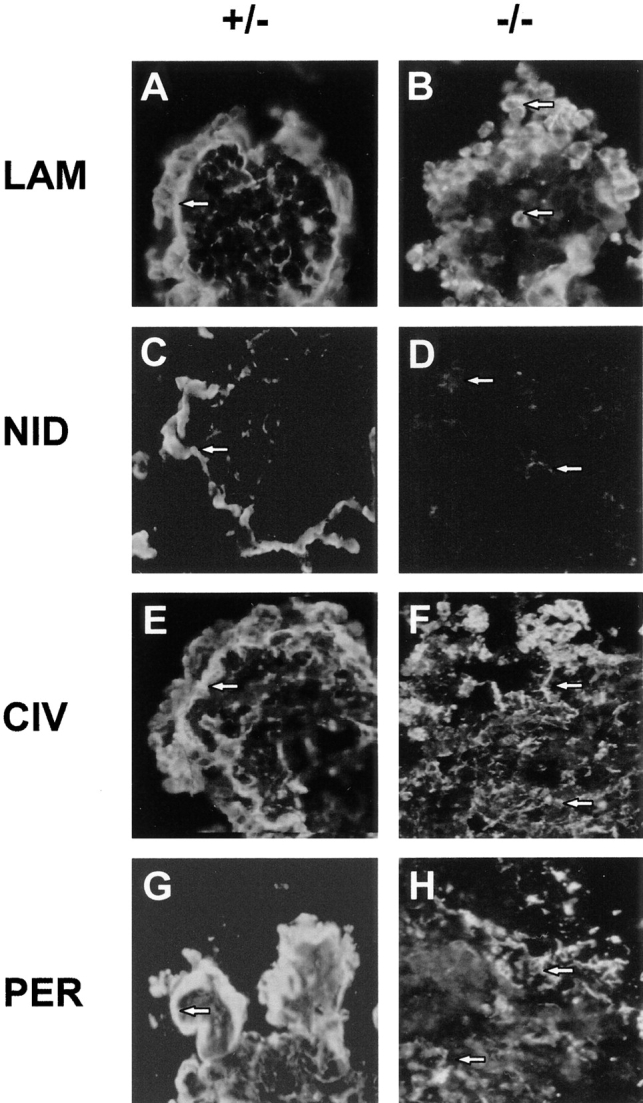
Immunofluorescence staining for basement membrane components in embryoid bodies after 7 d of culture. The primary polyclonal antibodies used were: anti–laminin-1 (A and B); anti-nidogen (C and D); anti-collagen type IV (E and F); and anti-perlecan (G and H). Genotypes of the ES cells are indicated. Arrows, location of immunofluorescence in the peripheral basement membrane of (+/−) embryoid bodies (A, C, E, and G). Arrows, intracellular laminin staining in (−/−) embryoid bodies (B) and disorganized extracellular staining of nidogen, collagen type IV, and perlecan in (−/−) embryoid bodies (D, F, and H, respectively). Note that there is very little nidogen immunoreactivity in the (−/−) embryoid body (D).
To determine if the differentiation of ES cells was affected by the absence of the γ1 subunit, embryoid bodies were allowed to attach to tissue culture plastic and cultured for up to 3 wk. At this time, the LAMC1 +/− cells displayed basement membrane-like sheets of laminin immunoreactivity (Fig. 8 A), small numbers of isolated von Willebrand–positive cells were seen (Fig. 8 C) and neurofilament-positive cell bodies and neurites were identified (Fig. 8 E). Furthermore, the cultures contained low numbers of myotubes that stained with antibodies to skeletal myosin (Fig. 8 G). As expected from Fig. 7, the LAMC1 −/− cells did not deposit basement membranes although individual permeabilized cells displayed intense laminin immunoreactivity (Fig. 8 B). Although no differences from controls were seen in the von Willebrand– and neurofilament-positive cells (Fig. 8, D and F, respectively), no normal myotubes were observed, myosin-positive cells being present in large aggregates which extended thin processes (Fig. 8 H).
Figure 8.
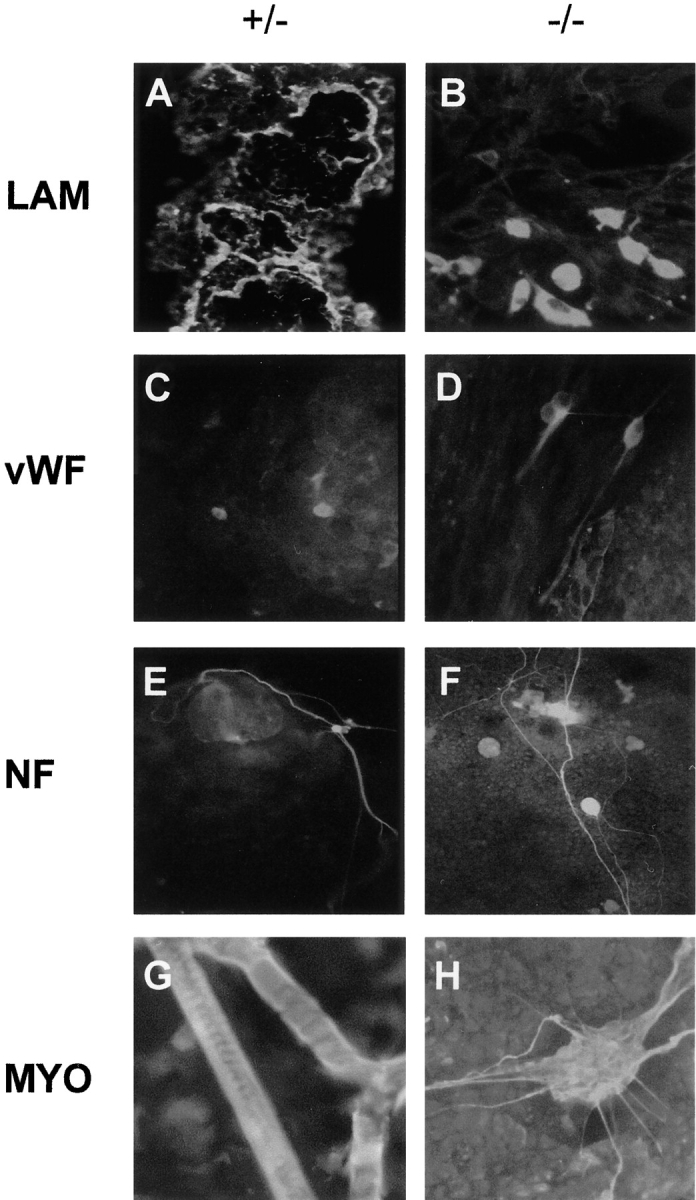
Immunofluorescence staining for laminin and cell-specific markers in +/− and −/− ES cells after 21 d of culture under differentiating conditions. The primary polyclonal antibodies used were: anti–laminin-1 (A and B); anti–von Willebrand factor (C and D); anti-neurofilament 200 subunit (E and F); and anti-skeletal myosin (G and H). Note the presence of myosin-positive accumulations of cells with processes rather than typical myotubes in the −/− ES cell cultures (H).
After overnight [35S]methionine metabolic labeling, a band of ∼800 kD on nonreducing SDS-PAGE was immunoprecipitated with laminin-1 antibodies from both culture medium and extracts of the +/+ and +/− embryoid bodies (Fig. 9 A, lanes 1–4). This corresponds to the expected size of laminin type 1. In contrast, neither the cell extracts nor the culture medium from the −/− embryoid bodies contained detectable laminin of 800 kD, although lower molecular weight bands were immunoprecipitated (Fig. 9 A, lanes 5 and 6).
Figure 9.
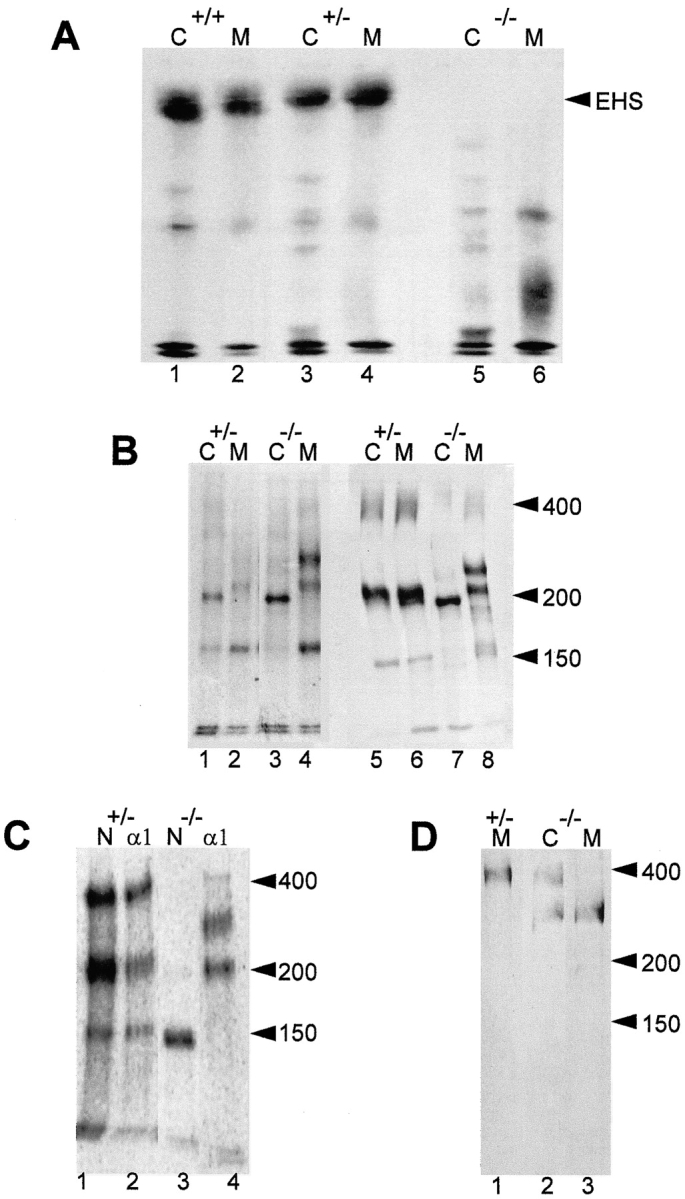
Embryoid bodies lacking the laminin γ1 subunit fail to produce intact laminin but do secrete a truncated α1 subunit and free nidogen. (A) Immunoprecipitation with anti–laminin-1 antibodies and 3% SDS-PAGE under nonreducing conditions of 35S-labeled laminin from cell extracts (1, 3, and 5) and media (2, 4, and 6) of embryoid bodies +/+ (1 and 2), +/− (3 and 4) and −/− (5 and 6) for the γ1 subunit. (B) 5% SDS-PAGE under nonreducing (1–4) and reducing conditions (5–8) of immunoprecipitated 35S-labeled laminin from cell extracts (1, 3, 5, and 7) and media (2, 4, 6, and 8) obtained from +/− (1, 2, 5, and 6) and −/− (3, 4, 7, and 8) embryoid bodies. (C) Immunoprecipitation and electrophoresis under reducing conditions of 35S-labeled protein from culture media of +/− (1 and 2) and −/− (3 and 4) embryoid bodies with antibodies specific for the laminin α1 subunit (2 and 4) and nidogen (1 and 3). (D) Anti–laminin α1 subunit immunoblots of proteins from +/− (1) and −/− (2 and 3) embryoid bodies after electrophoresis under reducing conditions. (1 and 3) Proteins present in the media; (2) proteins present in cell extracts.
To better characterize these bands, electrophoresis was performed on higher percentage SDS-polyacrylamide gels under nonreducing and reducing conditions. The pattern of reduced protein bands immunoprecipitated from the media of the −/− embryoid bodies differed from that of the −/− embryoid body cell extracts (Fig. 9 B, lanes 7 and 8), whereas the patterns were the same from the +/− embryoid body cell extracts and media (Fig. 9 B, lanes 5 and 6). These differences are consistent with the observation that most of the laminin immunoreactivity of +/+ and +/− embryoid bodies was seen in a basement membrane and hence extracellular (Fig. 6 A), whereas that seen in the −/− embryoid bodies appeared to be intracellular (Fig. 6 B). Although a nidogen band of 150 kD was present in the medium of these cells (Fig. 9 B, lanes 4 and 8; Fig. 9 C, lane 3), there was little or no nidogen detectable in the −/− embryoid body extracts under both nonreducing (Fig. 9 B, lane 3) and reducing conditions (Fig. 9 B, lane 7). Thus, the lack of nidogen immunoreactivity in −/− embryoid bodies (Fig. 9 D) is not due to lack of nidogen synthesis, but rather results from nidogen being lost from the embryoid bodies into the medium (Fig. 9 B, lanes 4 and 8).
The nonreduced extracts of +/− and −/− embryoid bodies were similar in that they contained a prominent band of ∼200 kD (Fig. 9 B, lanes 1 and 3). However, a novel strong band of ∼300 kD under both non–reducing and reducing conditions was immunoprecipitated from −/− embryoid body medium (Fig. 9 B, lanes 4 and 8), indicating that this secreted protein was not disulfide-bonded to other laminin subunits. To identify the novel band, immunoprecipitation and immunoblotting experiments were performed with laminin α1 subunit-specific antibodies directed against the IVa domain. In addition to the 400-kD α1 chain, an equally strong band corresponding to the 300-kD protein was also found in −/− cell extracts (Fig. 9 D, lane 2), and this band alone was detected in the −/− medium (Fig. 9 D, lane 3) although it was absent from the +/− medium (Fig. 9 D, lane 1). Immunoprecipitation of this band from the −/− cell medium with antibodies to the laminin α1 subunit also coprecipitated a band of 200 kD but no nidogen was detected (Fig. 9 C, lane 4). Conversely, immunoprecipitation of nidogen from −/− cell medium with antibodies against nidogen failed to precipitate any laminin subunits (Fig. 9 C, lane 3). Thus although the −/− ES cells secrete a modified laminin α1 subunit and nidogen, they are not associated as normal (Fig. 9 C, lanes 1 and 2), consistent with the absence of the laminin γ1 subunit in the −/− ES cells.
Discussion
We have used homologous recombination to target one or both of the LAMC1 alleles coding for the laminin γ1 subunit in mouse embryonic stem cells. By so doing, we have disrupted the formation of all described laminin isoforms with the exception of laminin 5. Although the null mutation resulted in the absence of basement membranes and hence was an early embryonic lethal, surprisingly, preimplantation development appeared to be normal in that a pumping trophectodermal epithelium allowed expansion of the blastocysts. Basement membranes were first found to be necessary for differentiation of primitive endodermal cells, in their absence Reichert's membrane failing to form. In vitro analysis of the null mutation showed that the laminin γ1 subunit was necessary for the differentiation of stable myotubes and for assembly of other covalently bonded laminin subunits. In turn, the lack of intact laminin deposition was necessary for the assimilation of other components into a continuous basement membrane in vitro, consistent with the disruption of formation of the first basement membrane to be formed during development in vivo.
The LAMC1-null Mutation Causes Early Embryonic Lethality
Although mice heterozygous for the LAMC1 gene are healthy and fertile, the analysis of different gestational ages showed that LAMC1-null mutant embryos did not survive later than day 5.5 pc. Immunostaining of pre- and postimplantation embryos up to 4.5 d pc showed laminin γ1–negative embryos to have an apparently normal morphology although they lacked basement membranes as defined by staining for other basement membrane components. Disruptions of cell–extracellular matrix interactions in the developing lung, kidney, and salivary gland have been shown to inhibit epithelial morphogenesis (Ekblom et al., 1994; Kadoya et al., 1997; Klein et al., 1988). However, the fact that the null mutant embryos described here could develop a functional pumping trophectodermal epithelium means that a basement membrane is not an absolute requirement for the differentiation of epithelia. Although this result points to the primary importance of cell–cell interactions in epithelial development (Watson et al., 1990), it does not rule out a role for basement membranes in the maintenance of specific epithelia or the differentiation of other epithelial cell properties. Indeed, although a decidual reaction occurred in the uterine wall adjacent to −/− embryos, it may be that in the absence of the trophectodermal basement membrane that the trophoblast was unable to successfully implant into the uterus.
Although both Reichert's membrane and underlying parietal endoderm cells were absent in the LAMC1-null embryos, cells staining strongly for intracellular laminin α1 or β1 subunits were seen in the inner cell mass, characteristic of primitive endodermal cells (Doetschman et al., 1985; Dziadek and Timpl, 1985). Thus, although these cells had evidently started to differentiate along the extra–embryonic endodermal pathway, the absence of a trophectodermal basement membrane had prevented further development involving the migration of parietal endodermal cells and/or the differentiation of primitive endodermal cells. The need for cellular interactions with laminin at this stage of development is consistent with the observation that embryos lacking the β1 integrin subunit or α-dystroglycan also die rapidly after day 4.5 pc (Fässler and Meyer, 1995; Stephens et al., 1995). It has previously been shown that the formation of the proamniotic cavity in the epiblast is due to death of the cells unable to interact with the extracellular matrix via a β1-containing integrin receptor (Coucouvanis and Martin, 1995). At 5.5 d pc we were unable to find anything other than disrupted laminin γ1–negative embryos, the cells of which displayed increased DNA fragmentation. The fact that the increased extent of DNA fragmentation in these embryos varied widely is consistent with a rapid onset of apoptotic cell death subsequent to disruption of embryo structure.
The early embryonic lethality of the LAMC1 knockout in vivo precludes an analysis of the roles of basement membranes in subsequent postimplantation development. However, by observing the differentiation of −/− ES cells in culture we were able to see that developing myotubes were affected. It has recently been shown that the stability of myotubes derived from LAM2A −/− ES cells is compromised (Kuang et al., 1998). Taken together with our observation of aggregates of myosin-positive LAMC1 −/− cells with processes characteristic of retraction, these results are consistent with the laminin γ1 subunit being required for the formation of α2/γ1-containing laminin isoforms that are necessary for the maintenance of myotubes.
Covalent Laminin Trimers Fail to Form in the Absence of the γ1 Subunit
In the absence of the γ1 subunit, no covalently bonded laminin subunits were produced by differentiating ES cells. Previous studies have indicated that intracellular laminin transport and secretion is limited by the assembly of the α1 subunit to form a triple coiled-coil α-helix with preassembled βγ dimers (Peters et al., 1985; Hunter et al., 1990; De Arcangelis et al., 1996; Yurchenco et al., 1997). However, despite the lack of extracellular laminin deposition in the embryoid bodies, our immunoprecipitation experiments clearly showed that some laminin subunits were secreted from the ES cells. However, the α1 chain had undergone cleavage to produce a fragment of ∼300 kD that was released into the medium. The size of this fragment is consistent with cleavage occurring at or close to the terminal globular domain of the α1 subunit. A similar but incomplete cutting of the laminin α1 subunit upon secretion from transfected cells has recently been demonstrated, and it was suggested that when unable to assemble into a coiled-coil structure, the α1 subunit adopts a conformation laying it open to cleavage (Yurchenco et al., 1997). Although the truncated α1 subunit in the −/− ES cell medium described here was noncovalently associated with a protein band of 200 kD, the fact that the α1 subunit was cleaved to a 300-kD fragment indicates that it is also unlikely to have been associated with any other laminin subunits via a coiled-coil interaction.
Basement Membrane Components Fail to Assemble in the Absence of Laminin
Experiments in vitro have shown that collagen type IV can self-assemble into a characteristic chicken wire network (Yurchenco and O'Rear, 1993). Furthermore, there are reports of basement membrane-like structures lacking either type IV collagen (Brauer and Keller, 1989) or laminin (Hahn et al., 1980). However, the present work demonstrates in both embryos and embryoid bodies that laminin is necessary for the incorporation of collagen type IV into a continuous basement membrane. Although we cannot rule out the hitherto undocumented existence of other laminin γ subunits, it is clear that the γ1 isoform is a prerequisite for the formation of that laminin variant necessary for the assembly of the first basement membranes in the preimplantation embryo. Furthermore, the available data are consistent with that variant being laminin type-1, the α1, and β1 subunits of which have also been shown to be expressed in the preimplantation embryo (Shim et al., 1996). In the absence of the laminin, collagen IV and perlecan were seen in disorganized deposits within the embryoid bodies. However, little if any nidogen was deposited with them, but instead it was released into the culture medium. Although nidogen has been shown to be able to bind to both collagen IV and perlecan in solid-phase binding assays in vitro (Battaglia et al., 1992; Dziadek et al., 1985), the present experiments indicate that this apparently does not occur in the absence of laminin in embryoid bodies. Clearly factors other than binding interactions between its individual components regulate basement membrane deposition in vivo. In this regard, it should be noted that basement membrane organization is disrupted by targeted deletions of the β1 integrin subunit (Fässler and Meyer, 1995; Stephens et al., 1995) or α-dystroglycan (Williamson et al., 1997), pointing to the involvement of cellular receptors for laminin in basement membrane deposition.
Taken together, the present results show that other basement membrane components require laminin for their assembly into an organized basement membrane structure. Furthermore, although cell–cell contacts may be sufficient for epithelium formation during preimplantation development, these do not form a sufficient basis for postimplantation embryonic development when basement membranes are first required for endoderm differentiation.
Acknowledgments
This work was supported by a European Union SCIENCE Programme Grant (SCC-CT90-0021) and Wellcome Trust grant to D. Edgar. N. Smyth, C. Frie, and M. Paulsson were supported by a grant from the Bundesministerium fuer Bildung und Forschung in the framework of the Centre for Molecular Medicine Cologne. P. Murray was supported by a postgraduate research studentship (G610/47) of the Medical Research Council.
Abbreviations used in this paper
- ES
embryonic stem
- pc
post coitum
- TUNEL
terminal dUTP–biotin nick end labeling
Footnotes
We are greatly indebted to H. Thoenen (Max Planck Institute for Psychiatry, Munich, Germany) for providing encouragement, advice, and facilities for the initial stages of this work, to H. Thorun and B. Kunkel (both from Max Planck Institute for Psychiatry) who provided skilled technical assistance with tissue culture and microinjection, and to A. Fichard (Max Planck Institute for Psychiatry) who was involved in preliminary experiments leading to this project. We thank U. Mayer and R. Timpl (both from Max Planck Institute for Biochemistry) for generously making their antibodies available to us and also P. Soriano (Fred Hutchinson Cancer Center Research Center, Seattle, WA) and A. Nagy (Samuel Lunenfeld Research Institute, Mt. Sinai Hospital, Toronto, Canada) who provided the IRES construct and R1 ES cells, respectively.
References
- Battaglia C, Mayer U, Aumailley M, Timpl R. Basement membrane heparin sulfate proteoglycan binds to laminin by its heparan sulfate chains and to nidogen by its protein core. Eur J Biochem. 1992;208:359–366. doi: 10.1111/j.1432-1033.1992.tb17195.x. [DOI] [PubMed] [Google Scholar]
- Beddington, R.S.P., and K.A. Lawson. 1990. Clonal analysis of cell lineages. In Postimplantation Mammalian Embryos: A Practical Approach. A.J. Copp and D.L. Cockroft, editors. IRL Press at Oxford University Press, London, UK. 267–292.
- Brauer PR, Keller JM. Ultrastructure of a model basement membrane lacking type IV collagen. Anat Rec. 1989;223:376–383. doi: 10.1002/ar.1092230405. [DOI] [PubMed] [Google Scholar]
- Burgeson, R.E. 1996. Laminins in epidermal structures. In The Laminins. P. Ekblom and R. Timpl, editors. Harwood Academic Publishers, Amsterdam, The Netherlands. 217–233.
- Burgeson RE, Chiquet M, Deutzmann R, Ekblom P, Engel J, Kleinman HK, Martin GR, Meneguzzi G, Paulsson M, Sanes JR, Timpl R, Tryggvason K, Yamada Y, Yurchenco PD. A new nomenclature for the laminins. Matrix Biol. 1994;14:209–211. doi: 10.1016/0945-053x(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Champliaud M, Lunstrum GP, Rousselle P, Nishiyama T, Keene DR, Burgeson RE. Human amnion contains a novel laminin variant, laminin 7, which like laminin 6, covalently associates with laminin 5 to promote stable epithelial-stromal attachment. J Cell Biol. 1996;132:1189–1198. doi: 10.1083/jcb.132.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HS, Kim NB, Phillips SL. Positive elements in the laminin gamma 1 gene synergize to activate high level transcription during cellular differentiation. Nucleic Acids Res. 1996;24:1360–1368. doi: 10.1093/nar/24.7.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Champliaud M, Burgeson RE, Marinkovich MP, Yurchenco PD. Self-assembly of laminin isoforms. J Biol Chem. 1997;272:31525–31532. doi: 10.1074/jbc.272.50.31525. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analyt Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coucouvanis E, Martin GR. Signals for death and survival a two-step mechanism for cavitation in the vertebrate embryo. Cell. 1995;83:279–287. doi: 10.1016/0092-8674(95)90169-8. [DOI] [PubMed] [Google Scholar]
- De Arcangelis A, Neuville P, Boukamel R, Lefebvre O, Kedinger M, Simon-Assmann P. Inhibition of laminin alpha-1 chain expression leads to alteration of basement membrane assembly and cell differentiation. J Cell Biol. 1996;133:417–430. doi: 10.1083/jcb.133.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- Dziadek M, Timpl R. Expression of laminin and nidogen in basement membranes during mouse embryogenesis and in teratocarcinoma cells. Dev Biol. 1985;111:372–382. doi: 10.1016/0012-1606(85)90491-9. [DOI] [PubMed] [Google Scholar]
- Dziadek M, Paulsson M, Timpl R. Identification and interaction repertoire of large forms of the basement membrane protein nidogen. EMBO (Eur Mol Biol Organ) J. 1985;4:2513–2518. doi: 10.1002/j.1460-2075.1985.tb03964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom P, Ekblom M, Fecker L, Klein G, Zhang HY, Kadoyz Y, Chu ML, Mayer U, Timpl R. Role of mesenchymal nidogen for epithelial morphogenesis in vitro. Development (Camb) 1994;120:2003–2014. doi: 10.1242/dev.120.7.2003. [DOI] [PubMed] [Google Scholar]
- Fässler R, Meyer M. Consequences of lack of β1 integrin gene expression in mice. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- Fox JW, Mayer U, Nischt R, Aumailley M, Reinhardt D, Wiedemann H, Mann K, Timpl R, Krieg T, Engel J, Chu M-L. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO (Eur Mol Biol Organ) J. 1991;10:3137–3146. doi: 10.1002/j.1460-2075.1991.tb04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich G, Soriano P. Promoter traps in embryonic stem cells a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991;5:1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Bensasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn E, Wick G, Pencev D, Timpl R. Distribution of basement membrane proteins in normal and fibrotic human liver collagen type IV, laminin and fibronectin. Gut. 1980;21:63–69. doi: 10.1136/gut.21.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbling-Leclerc A, Zhang X, Topaloglu H, Cruaud C, Tesson F, Weissenbach J, Tome FM, Schwartz K, Fardeau M, Tryggvason K. Mutations in the laminin alpha 2-chain gene (LAMA2) cause merosin-deficient congenital muscular dystrophy. Nature Genet. 1995;11:216–218. doi: 10.1038/ng1095-216. [DOI] [PubMed] [Google Scholar]
- Hunter I, Schulthess T, Bruch M, Beck K, Engel J. Evidence for a specific mechanism of laminin assembly. Eur J Biochem. 1990;188:205–211. doi: 10.1111/j.1432-1033.1990.tb15391.x. [DOI] [PubMed] [Google Scholar]
- Kadoya Y, Kadoya K, Durbeej M, Holmvall K, Sorokin L, Ekblom P. Antibodies against domain E3 of laminin-1 and integrin α6 subunit perturb branching epithelial morphogenesis of submandibular gland, but by different modes. J Cell Biol. 1995;129:521–534. doi: 10.1083/jcb.129.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoya Y, Salmivirta K, Talts JF, Kadoya K, Mayer U, Timpl R, Ekblom P. Importance of nidogen binding to laminin-1 for branching epithelial morphogenesis of the submandibular gland. Development (Camb) 1997;124:683–691. doi: 10.1242/dev.124.3.683. [DOI] [PubMed] [Google Scholar]
- Kallunki P, Sainio K, Eddy R, Byers M, Kallunki T, Sariola H, Beck K, Hirvonen H, Shows TB, Tryggvason K. A truncated laminin chain homologous to the B2 chain: structure, spatial expression, and chromosomal assignment. J Cell Biol. 1992;119:679–693. doi: 10.1083/jcb.119.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G, Langegger M, Timpl R, Ekblom P. Role of laminin A chain in the development of epithelial cell polarity. Cell. 1988;55:331–341. doi: 10.1016/0092-8674(88)90056-6. [DOI] [PubMed] [Google Scholar]
- Kuang W, Xu H, Vachon PH, Engvall E. Disruption of the lama2 gene in embryonic stem cells: laminin alpha-2 is necessary for sustenance of mature muscle cells. Exp Cell Res. 1998;241:117–125. doi: 10.1006/excr.1998.4025. [DOI] [PubMed] [Google Scholar]
- Kücherer-Ehret A, Pottgiesser J, Kreutzberg GW, Thoenen H, Edgar D. Developmental loss of laminin from the interstitial extracellular matrix correlates with decreased laminin gene expression. Development (Camb) 1990;110:1285–1293. doi: 10.1242/dev.110.4.1285. [DOI] [PubMed] [Google Scholar]
- Maurer, P., and J. Engel. 1996. Structure of laminins and their chain assembly. In The Laminins. P. Ekblom and R. Timpl, editors. Harwood Academic Publishers, Amsterdam, The Netherlands. 27–50.
- Mayer U, Nischt R, Poschl E, Mann K, Fukuda K, Gerl M, Yamada Y, Timpl R. A single EGF-like motif of laminin is responsible for high affinity nidogen binding. EMBO (Eur Mol Biol Organ) J. 1993;12:1879–1885. doi: 10.1002/j.1460-2075.1993.tb05836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Patton BL, Lentz SI, Gilbert DJ, Jenkins NA, Copeland NG, Sanes JR. The laminin alpha chains: expression, developmental transitions, and chromosomal locations of alpha 1-5, identification of heterotrimeric laminins 8-11, and cloning of a novel alpha 3 isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen RM, Conner DA, Chao S, Geisterfer-Lowrance AA, Seidman JG. Production of homozygous mutant ES cells with a single targeting construct. Mol Cell Biol. 1992;12:2391–2395. doi: 10.1128/mcb.12.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountford P, Zevnik B, Duwel A, Nichols J, Li M, Dani C, Robertson M, Chambers I, Smith A. Dicistronic targeting constructs: reporters and modifiers of mammalian gene expression. Proc Natl Acad Sci USA. 1994;91:4303–4307. doi: 10.1073/pnas.91.10.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin beta 2. Nature. 1995;374:258–262. doi: 10.1038/374258a0. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Burbelo PD, Sasaki M, Yamada Y. The laminin B2 chain promoter contains unique repeat sequences and is active in transient transfection. J Biol Chem. 1988;263:8384–8389. [PubMed] [Google Scholar]
- Paulsson, M. 1996. Biosynthesis, tissue distribution and isolation of laminins. In The Laminins. P. Ekblom and R. Timpl, editors. Harwood Academic Publishers, Amsterdam, The Netherlands. 217–233.
- Peters BP, Hartle RJ, Krzesick RF, Kroll TG, Perini FC, Balun JE, Goldstein IJ, Ruddon RW. The biosynthesis, processing and secretion of laminin by human choriocarcinoma cells. J Biol Chem. 1985;260:14732–14742. [PubMed] [Google Scholar]
- Sasaki M, Yamada Y. Structure of the laminin B2 chain shows multidomain structures homologous to the B1 chain. J Biol Chem. 1987;26211:17111–17117. [PubMed] [Google Scholar]
- Sasaki M, Kato S, Kohno K, Martin GR, Yamada Y. Sequence of cDNA encoding the laminin B1 chain reveals a multidomain protein containing cysteine-rich repeats. Proc Natl Acad Sci USA. 1987;84:935–939. doi: 10.1073/pnas.84.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Kleinman HK, Huber H, Deutzmann R, Yamada Y. Laminin, a multidomain protein. The A chain has a unique globular domain and homology with the basement membrane proteoglycan and the laminin B chains. J Biol Chem. 1988;263:16536–16544. [PubMed] [Google Scholar]
- Schulze B, Mann K, Battistuta R, Wiedemann H, Timpl R. Structural properties of recombinant domain III-3 of perlecan containing a globular domain inserted into an EGF-like motif. Eur J Biochem. 1995;231:551–556. doi: 10.1111/j.1432-1033.1995.tb20731.x. [DOI] [PubMed] [Google Scholar]
- Schulze B, Mann K, Pöschl E, Yamada Y, Timpl R. Structural and functional analysis of the globular domain IVa of the laminin α1 chain and its impact on an adjacent RGD site. Biochem J. 1996;314:847–851. doi: 10.1042/bj3140847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim C, Kwon HB, Kim K. Differential expression of laminin chain-specific mRNA transcripts during mouse preimplantation embryo development. Molec Reproduct Dev. 1996;44:44–55. doi: 10.1002/(SICI)1098-2795(199605)44:1<44::AID-MRD5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Sorokin L, Sonnenberg A, Aumailley M, Timpl R, Ekblom P. Recognition of the laminin E8 cell-binding site by an integrin possessing the α6subunit is essential for epithelial polarization in developing kidney tubules. J Cell Biol. 1990;111:1265–1273. doi: 10.1083/jcb.111.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of β1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- Streuli, C.H. 1996. Basement membrane as a differentiation and survival factor. In The Laminins. P. Ekblom and R. Timpl, editors. Harwood Academic Publishers, Amsterdam, The Netherlands. 217–233.
- Sugiyama S, Utani A, Yamada S, Kozak CA, Yamada Y. Cloning and expression of the mouse laminin γ2 (B2t) chain, a subunit of epithelial cell laminin. Eur J Biochem. 1995;228:120–128. doi: 10.1111/j.1432-1033.1995.tb20239.x. [DOI] [PubMed] [Google Scholar]
- Thorsteinsdottir S. Basement membrane and fibronectin matrix are distinct entities in the developing mouse blastocyst. Anat Rec. 1992;232:141–149. doi: 10.1002/ar.1092320116. [DOI] [PubMed] [Google Scholar]
- Timpl R. Macromolecular assembly of basement membranes. Curr Opin Cell Biol. 1996;8:618–624. doi: 10.1016/s0955-0674(96)80102-5. [DOI] [PubMed] [Google Scholar]
- Watson AJ, Damsky CH, Kidder GM. Differentiation of an epithelium factors affecting the polarized distribution of Na+, K+-ATPase in mouse trophectoderm. Dev Biol. 1990;141:104–114. doi: 10.1016/0012-1606(90)90105-r. [DOI] [PubMed] [Google Scholar]
- Williamson RA, Henry MD, Daniels KJ, Hrstka RF, Lee JC, Sunada Y, Ibraghimov-Beskrovnaya O, Campbell KP. Dystroglycan is essential for early embryonic development disruption of Reichert's membrane in Dag1-null mice. Hum Mol Genet. 1997;6:831–841. doi: 10.1093/hmg/6.6.831. [DOI] [PubMed] [Google Scholar]
- Wobus AM, Wallukat G, Hescheler J. Pluripotent mouse embryonic stem-cells are able to differentiate into cardiomyocytes expressing chronotropic responses to adrenergic and cholinergic agents and Ca2+ channel blockers. Differentiation. 1991;48:173–182. doi: 10.1111/j.1432-0436.1991.tb00255.x. [DOI] [PubMed] [Google Scholar]
- Yurchenco, P.D., and J.J. O'Rear. 1993. Supramolecular organization of basement membranes. In Molecular and Cellular Aspects of Basement Membranes. D.H. Rohrbach and R. Timpl, editors. Academic Press Inc., San Diego, CA. 19–47.
- Yurchenco PD, Quan A, Colognato H, Mathus T, Harrison D, Yamada Y, O'Rear JJ. The α chain of laminin-1 is independently secreted and drives secretion of its β and γ chain partners. Proc Natl Acad Sci USA. 1997;94:10189–10194. doi: 10.1073/pnas.94.19.10189. [DOI] [PMC free article] [PubMed] [Google Scholar]