Abstract
Release from arrest in G2 phase of the cell cycle causes profound changes in rat ether-à-go-go (r-eag) K+ channels heterologously expressed in Xenopus oocytes. The most evident consequence of the onset of maturation is the appearance of rectification in the r-eag current. The trigger for these changes is located downstream of the activation of mitosis-promoting factor (MPF). We demonstrate here that the rectification is due to a voltage-dependent block by intracellular Na+ ions. Manipulation of the intracellular Na+ concentration indicates that the site of Na+ block is located ∼45% into the electrical distance of the pore and is only present in oocytes undergoing maturation. Since the currents through excised patches from immature oocytes exhibited a fast rundown, we studied CHO-K1 cells permanently transfected with r-eag. These cells displayed currents with a variable degree of block by Na+ and variable permeability to Cs+. Partial synchronization of the cultures in G0/G1 or M phases of the cell cycle greatly reduced the variability. The combined data obtained from mammalian cells and oocytes strongly suggest that the permeability properties of r-eag K+ channels are modulated during cell cycle–related processes.
Keywords: cell cycle, CHO cells, electrophysiology, potassium channels, Xenopus laevis
The Xenopus oocyte maturation has proven useful for studies of development and cell cycle. Although some key processes have been identified (Cork and Robinson, 1994; Murakami and Vande Woude, 1997), the exact biochemical pathway leading to maturation remains unknown. The physiological trigger of maturation in amphibians is progesterone, which is secreted by follicular cells (Fortune et al., 1975; Schuetz and Glad, 1985). It acts on surface receptors and induces the activation of pre-MPF molecules already present in the oocyte. This is thought to happen through a process that includes the inhibition of protein kinase A and the activation of Mos protein kinase (Sagata, 1997). This initial activation is amplified by the synthesis of new cyclin B (the regulatory subunit of mitosis-promoting factor [MPF]1) and positive feedback of MPF on pre-MPF activation. Injection of exogenous MPF is able to induce oocyte maturation by itself. Hence, MPF is regarded as a key molecule in the process (Nurse, 1994).
We have recently shown that rat ether-à-go-go (r-eag) (Ludwig et al., 1994) K+ channels heterologously expressed in Xenopus oocytes undergo profound modification during maturation of the host cell (Brüggemann et al., 1997). The modification consists of a voltage-dependent block that causes rectification of the ionic currents. We also demonstrated that the modification of the channel occurs downstream from the activation of MPF, indicating that it is a cell cycle–related process and is not due to one of the collateral pathways that converge on MPF activation. In this paper, we investigate the mechanism underlying the maturation-dependent rectification.
Block of K+ channels by internal Na+ has been known, particularly in delayed rectifiers from squid axon and in Ca2+-activated K+ channels (Bezanilla and Armstrong, 1972; French and Wells, 1977; Marty, 1983; Yellen, 1984a ; Howe et al., 1992). Some cloned channels, such as Kv2.1 (Lopatin and Nichols, 1994), have been shown to be blocked by Na+ in a voltage-dependent manner. Na+ seems to act as an open channel blocker since it decreases the conductance of the channel but not its open probability. Moreover, a K+ current present in human neuroblastoma cells, which shares many properties with eag currents (Meyer and Heinemann, 1998), had previously been shown to be blocked by intracellular Na+ (Johansson et al., 1996). The intracellular Na+ concentration changes in response to many cellular events, usually related to the activation of either Na+/Ca2+ or Na+/H+ antiporters (see for example Harootunian et al., 1989; Borin and Siffert, 1991; Johnson et al., 1991). In this paper, we show that Na+ is able to block the r-eag channel only after MPF activation in oocytes, implying profound modifications in the ion-conduction pathway during cell cycle–related processes.
Materials and Methods
cRNA Preparation
All constructs used in this work are based on the pCDNA3–r-eag DNA, which was a generous gift from Prof. O. Pongs (Zentrum für Neurobiologie, Hamburg, Germany). It was used to subclone the r-eag cDNA into the high-efficiency expression vector pSGEM (M. Hollmann, Max-Planck-Institut). RNA encoding r-eag was prepared using a template with a T7 promoter following a standard protocol (Krieg and Melton, 1987), and injection into Xenopus oocytes was performed as described previously (Stühmer, 1992). Only oocytes considered to be in stages V and VI were injected. The oocytes were incubated in Barth's medium (including 0.33 mM Ca(NO3)2 and 0.41 mM CaCl2) at 18°C.
Cell Culture and Construction of Stably Transfected Cell Lines
CHO-K1 cells (CCL61; American Type Culture Collection, Rockville, MD) were maintained in 90% Ham's F-12 nutrient mixture and 10% FCS in a humidified atmosphere at 37°C. Medium was replaced twice a week, and cells were passed every 7–12 d. Transfection was performed using the Ca2+ phosphate method of Chen and Okayama (1987). Stable cell lines were selected based on their resistance to G418 encoded in the pCDNA3 vector.
Cell populations in a particular phase of the cell cycle were enriched using arresting media. To inhibit proliferation and increase the percentage of cells in G0/G1 phase, the serum concentration was reduced to 0.5% for 48 h before the measurement. To obtain an enrichment in M phase cells, 500 nM taxol (Schiff et al., 1979) was added to the medium for 18–24 h, and only round cells were used for measurements. In a few experiments, the cells were stained in vivo after 18 h in taxol with 5 μg/ml Hoechst 33342 (HO) for ∼2 min. We then recorded from cells displaying mitotic figures of their chromosomes under UV illumination.
Electrophysiology
Two-microelectrode recordings were performed 1–7 d after cRNA injection (using a Turbo TEC-10CD amplifier; npi Electronics, Tamm, Germany). The intracellular electrodes had resistances of 0.6–1 MΩ when filled with 2 M KCl. All the records presented were leak subtracted online using a P/n protocol. Recordings were performed in an external solution (normal frog Ringer [NFR]) containing (mM) 115 NaCl, 2.5 KCl, 1.8 CaCl2, 10 Hepes/NaOH, pH 7.2. Recombinant human MPF (0.75 U/μl) was purchased from Promega (Madison, WI). It was injected (∼50 nl) into oocytes during or immediately before electrophysiological measurements. For some experiments, we took advantage of spontaneous maturation, a frequent phenomenon in mammalian oocytes that also occurs in amphibian oocytes after detachment of the follicular cell layer (Zelarayan et al., 1996).
Patch-clamp (Hamill et al., 1981) experiments in macropatches from oocytes were carried out using an EPC-9 amplifier (HEKA Electronics, Lambrecht, Germany). Pipettes were prepared from aluminum-silicate glass with resistances of 0.7–1.5 MΩ when filled with the extracellular solution NFR. The bath solution contained (mM) 100 KCl, 10 EGTA, 1 1,2-bis(2-aminophenoxy)ethaneN,N,N′N′-tetraacetic acid (BAPTA), 10 Hepes/ KOH, pH 7.2. NaCl was added to the intracellular solution at the indicated concentrations without changing the KCl concentration to simplify the analysis. Equivalent increase in ionic strength and osmotic properties of the solution with KCl did not noticeably alter the current properties.
Single channel measurements were performed in the inside-out configuration of the patch clamp technique, with NFR as pipette solution and an internal solution containing (mM) 100 KCl, 10 EGTA, 1 BAPTA, 10 Hepes/KOH, pH 7.2.
All oocyte experiments were repeated at least three times using oocytes from at least two different donors. The figures show representative experiments with internal controls performed in the same oocyte or patch.
For whole-cell recordings in CHO-K1 cells, pipettes pulled from Kimax glass (2–3 MΩ) were filled with a solution containing (mM) 140 KCl or CsCl, 10 BAPTA, 10 Hepes, pH 7.2, with the corresponding hydroxide (Cs or K). The extracellular medium contained (mM) 140 NaCl, 2.8 KCl, 2 CaCl2, 2 MgCl2, 10 glucose, 10 Hepes/NaOH, pH 7.2. To measure reversal potentials, the cells were bathed in a solution containing (mM) 140 KCl, 2 CaCl2, 10 Hepes/NaOH, pH 7.2, and the pipette solution contained (mM) 140 CsCl, 10 BAPTA, 10 Hepes, pH 7.2 (CsOH). Unless otherwise stated, the holding potential for all experiments was −80 mV. Electrophysiological experiments were carried out at a room temperature of 20–22°C.
Acquisition and data analysis was achieved using the Pulse-PulseFit software package (HEKA Electronics). For single-channel measurements, the analysis was performed using TAC software (Bruxton Corp., Seattle, WA). The sampling rate was set in order to obtain five times oversampling at any particular filter setting.
Results
Rectification of r-eag Current in Mature Oocytes Is Compatible with Block by an Intracellular Factor
Fig. 1 A shows a comparison of the current–voltage (I-V) relationships of an oocyte before and after maturation by progesterone (see Brüggemann et al., 1997). Before MPF activation by progesterone, the I-V relationship is fairly linear at depolarized potentials (open circles). The situation is quite different after MPF activation (filled circles); the two curves overlap for small depolarizations, but diverge at larger depolarizations (starting at about +10 mV in this particular oocyte). This phenomenon is termed “rectification” (Hille, 1992) since the membrane conductance decreases with increasing potentials.
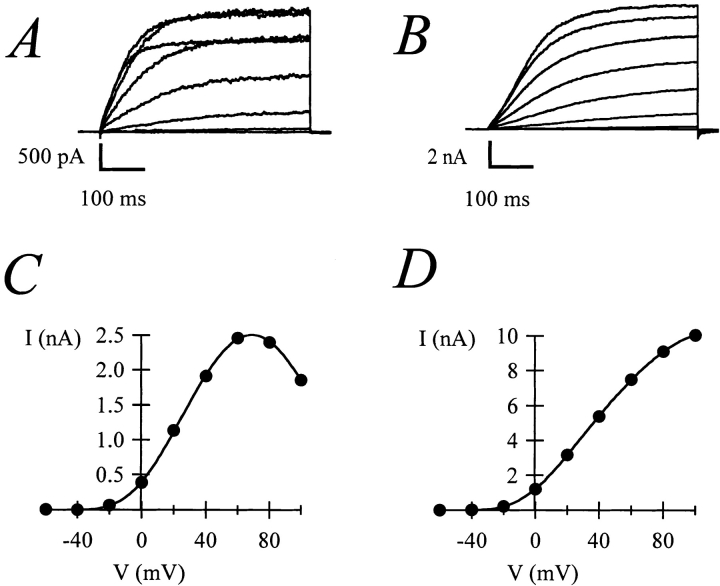
Figure 1.
(A) Current–voltage relationships obtained from the same oocyte before (open circles) and after (filled circles) treatment with 20 μg/ ml progesterone in NFR. The current amplitudes were determined by depolarizing pulses from a holding potential of −100 mV to voltages ranging between −60 and +80 mV in 20-mV steps. The amplitude was measured as mean steady-state current at the final 10% of a 500-ms pulse. (B) Current elicited by a 20-s depolarization to +80 mV from an oocyte expressing rectifying currents. (C) Normalized current obtained during different frequency and/or voltage stimulations (open circles, 2 Hz, +60 mV; filled triangles, 2 Hz, 0 mV; open squares, 4 Hz, +60 mV).
Rectification can be caused by at least two different processes (Hille, 1992). In some channels, it is caused by a block by mobile factors, such as Mg2+ or polyamines. In others, the rectification is directly caused by voltage-dependent conformational changes of the channel. A member of the eag family, human eag–related gene [HERG], belongs to the second group (Schönherr and Heinemann, 1996; Smith et al., 1996; Spector et al., 1996). HERG channels undergo inactivation with such fast kinetics that the activation of the channel is not detectable. Currents can only be observed upon repolarization while they deactivate. This prompted us to test whether a related mechanism could be responsible for the rectification of r-eag currents after MPF activation. Although no inactivation was detected in r-eag currents from immature oocytes, this property could have been conferred to the channels by MPF activation.
We attempted to evoke inactivation by applying long-lasting depolarizing pulses. Fig. 1 B shows that there is no decrease in current amplitude during long depolarizations (up to 20 s) at voltages at which the current shows substantial rectification (+80 mV), indicating that the reduction on current amplitude reaches its equilibrium before activation is completed. Cumulative inactivation during repetitive stimulation (Baukrowitz and Yellen, 1995) was also tested. Fig. 1 C shows that the current amplitude did not decrease during trains of stimuli at different frequencies. (2 and 4 Hz in this case; the strong voltage-dependence of activation [Terlau et al., 1996] precludes the use of higher frequencies.) Moreover, at a given frequency there was no detectable difference between depolarizations to potentials where there is rectification (+60 mV) or to potentials where the rectification is minimal, if at all present (0 mV).
Our observations indicate that the inactivation process, if it exists, would have very fast kinetics, and thus we favor the hypothesis of a blocking agent acting from the inside (see below).
Block by Internal Na+ Ions Can Account for the Rectification
An alternate mechanism to account for rectification is block by diffusible factors. This possibility is supported by the fact that cell-attached patches from mature oocytes exhibit rectification properties similar to the ones observed with two-electrode voltage clamp in whole oocytes. Furthermore, current amplitude increases and rectification disappears upon the excision of the patch (data not shown), as would be expected of a block by a diffusible, intracellular factor.
In a previous report (Brüggemann et al., 1997), we had tested and rejected two potential candidates that could cause rectification: Ca2+, a potent blocker from the cytoplasmic side (Stansfeld et al., 1996), and spermidine. Intracellular Ca2+ did not show a voltage-dependent block, and spermidine failed to block the channel even at high concentrations.
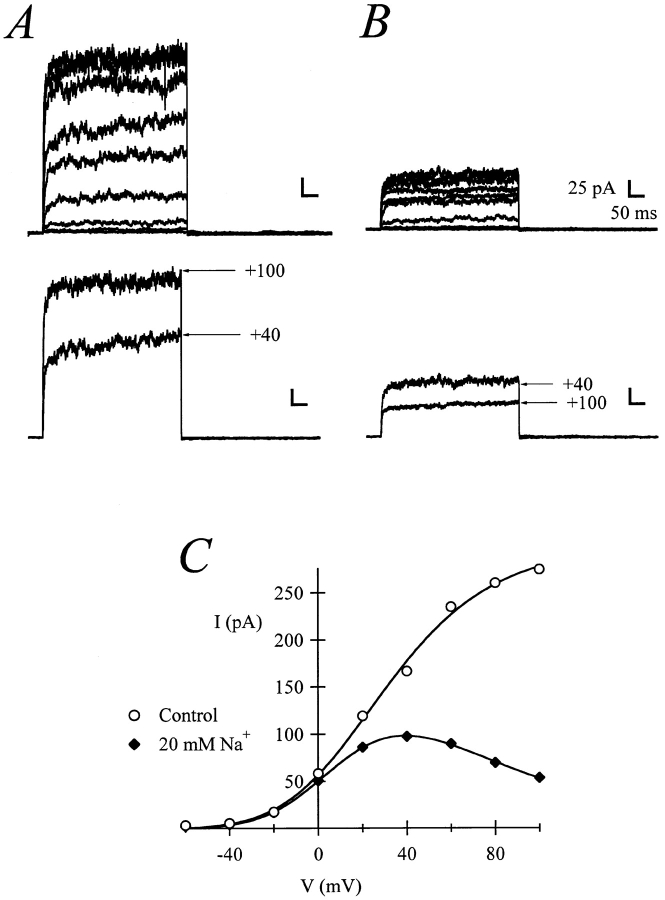
High extracellular K+ or Cs+ concentrations reversibly remove rectification (not shown). This observation is compatible with either relief of C-type inactivation (López-Barneo et al., 1993; Baukrowitz and Yellen, 1995) or block by intracellular Na+ (Yellen, 1984b ). We have already shown (Fig. 1) that inactivation is unlikely to be responsible for the rectification. Therefore, we tested intracellular Na+ as a voltage-dependent blocker. Inside-out patches taken from mature oocytes expressing r-eag recover their rectification properties in the presence of millimolar concentrations of Na+. Fig. 2, A and B, shows raw current traces, and Fig. 2 C shows the corresponding I-V relationship of r-eag currents exposed to either 0 or 20 mM NaCl from the intracellular side. This current–voltage relationship closely resembles those recorded from mature whole oocytes (see Fig. 1 A). Na+ ions are known to induce rectification in various K+ channels (Bezanilla and Armstrong, 1972; French and Wells, 1977; Marty, 1983; Yellen, 1984a ; Howe et al., 1992; Lopatin and Nichols, 1994). Mg2+, commonly a blocker of K+ channels, also causes block of r-eag currents, but this effect is only slightly voltage dependent (data not shown). Since the phenomenon described here is similar to block by Na+, we decided to further characterize the effects of intracellular Na+.
Figure 2.
Raw current traces from the same patch in the absence (A) or in the presence of 20 mM NaCl in the internal solution (B). C shows the current–voltage relationships under both conditions. The voltage protocol consisted of a 200-ms depolarization from a holding potential of −80 mV. The amplitude was determined as mean current at the end of the test pulse. The scale bars correspond to 25 pA and 50 ms.
The degree of rectification depends on the Na+ concentration (Fig. 3 A), and the concentration required for the 50% inhibition (IC50) is strongly voltage dependent (Fig. 3 B), ranging from 10 mM at +100 mV to more than 20 mM at +20 mV. This data can be described according to Woodhull (1973) by the following equation:
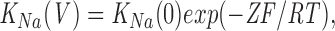 |
1 |
Figure 3.
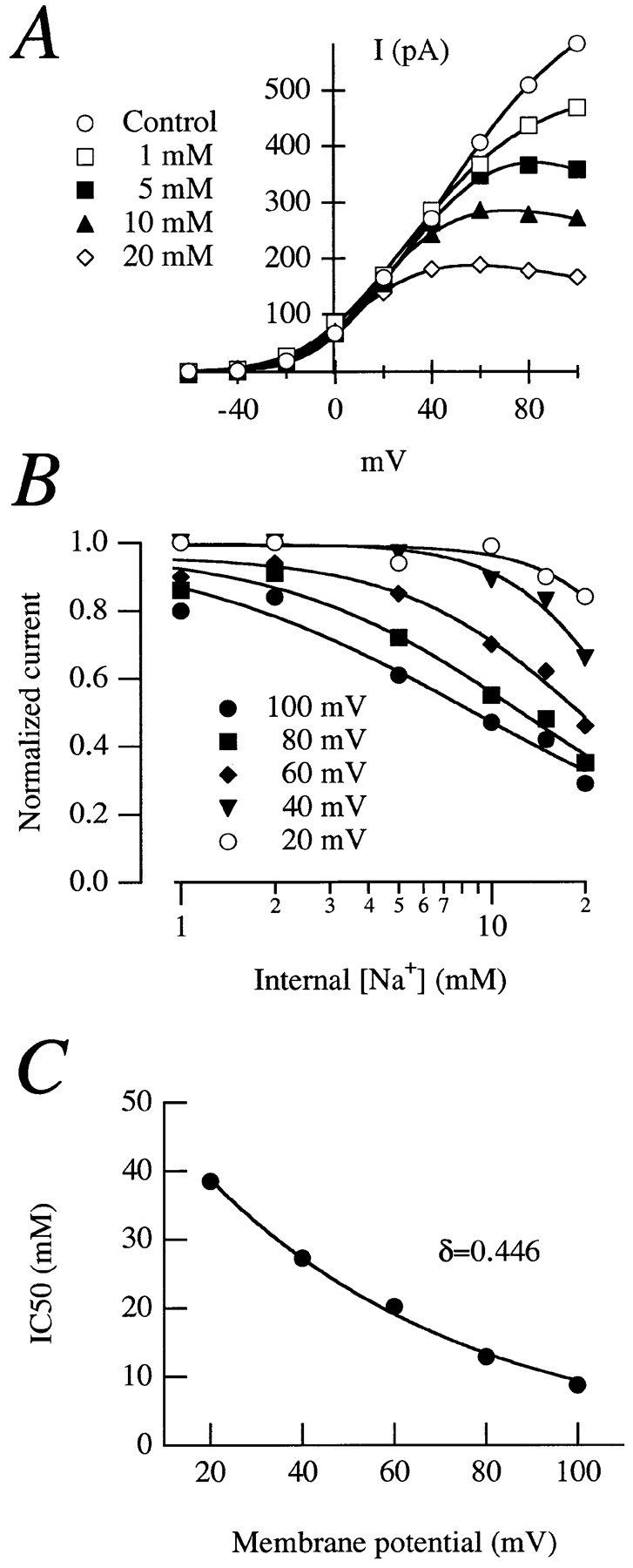
(A) Normalized current–voltage relationships in an inside-out patch from an oocyte expressing rectifying currents in the presence of different internal Na+ concentrations. The stimulation protocol was as the one in Fig. 2. (B) Dose–response plots for effects of Na+. The solid lines are fits to the Hill equation. (C) IC50 plotted versus voltage. The exponential fit to Eq. 1 gave a value for δ of 0.446.
where K Na(V) represents the IC50 for Na+ at a given voltage, K Na(0) is the IC50 at 0 mV, Z is the effective valence of the ion, and F, R, and T have their usual meaning. The effective valence of the blocking ion is Z = zδ, where z is the true valence and δ stands for the fraction of the electrical distance that the blocking ion has to enter to reach its binding site. A fit of the experimental IC50 to Eq. 1 (Fig. 3 C) gave a value of 0.446 for δ, indicating that the Na+ ion has to enter ∼45% of the electrical distance within the channel to reach the blocking site.
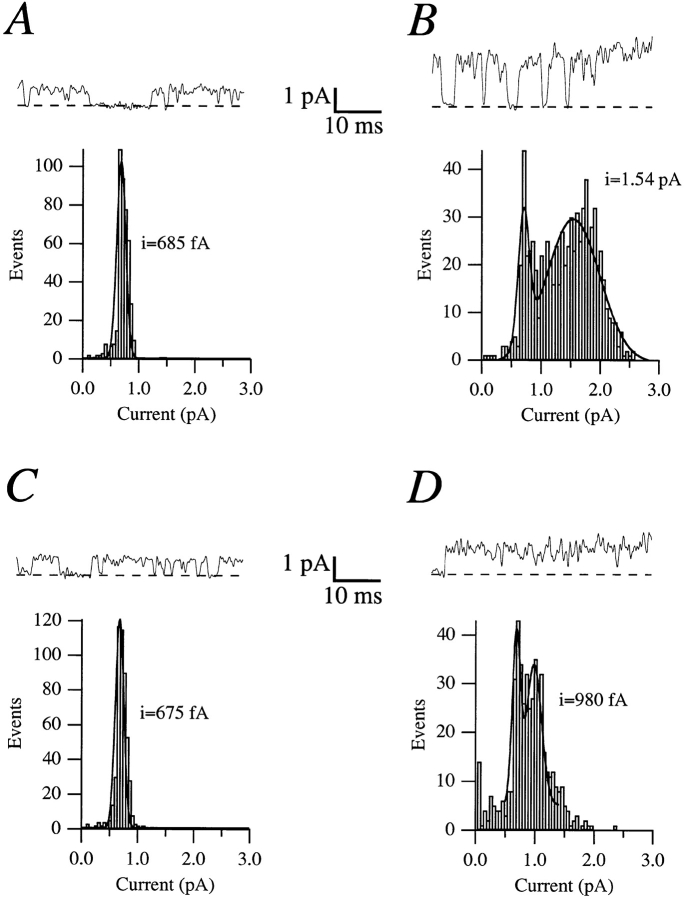
Single-channel records from inside-out patches show that Na+ induces an apparent decrease of the single-channel conductance from 1.5 to 0.9 pA at +80 mV, while at 0 mV the conductance is unaffected (Fig. 4). This behavior is typical for an open channel blocker, which possibly interferes with the K+ flow by binding to a site within the conducting pathway itself.
Figure 4.
Sample traces and amplitude histograms obtained from the same patch without (A and B) or with (C and D) 5 mM NaCl added to the intracellular side, at either 0 (A and C) or +80 mV (B and D). The single channel currents are obtained from the fit to a Gaussian distribution. The smaller amplitude in the histograms at +80 mV corresponds to the amplitude of the flickering of the channel, and remains constant in the absence (B) or in the presence (D) of Na+.
The Sensitivity to Na+ Ions Is Conferred by MPF Activation
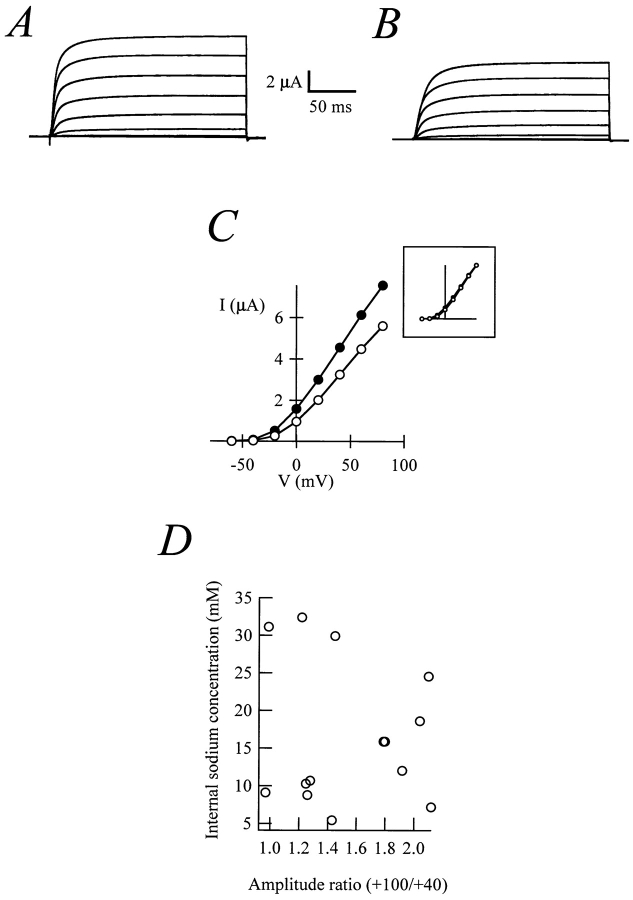
Na+ concentration is expected to rise inside the oocyte during maturation, due to the activity of the Na+/H+ antiporters (Rezai et al., 1994). The rectification of the r-eag current could simply be a consequence of this rise in Na+ concentration. To test this hypothesis, we examined the effect of a rise in Na+ concentration in immature oocytes. The Na+ increase was achieved by injection of ∼50 nl of 2 M Na+ into an oocyte (final concentration > 20 mM) while recording. Fig. 5, A–C, shows that the current amplitude is decreased after Na+ increase. Once the amplitude differences were normalized, the shape of the I-V relationship remained unchanged (Fig. 5 C, inset). Similar results were obtained increasing the Na+ concentration by incubation of the oocytes in ouabain (0.5 or 1.3 mM for 180 min; data not shown).
Figure 5.
Currents recorded from a whole oocyte before (A) and after (B) the injection of 50 nl 2 M NaCl. (C) Current–voltage plots for steady-state currents from A and B. (Inset) The normalized currents. (D) Lack of correlation between the internal Na+ concentration (calculated from the reversal potential of Na+ currents) and the degree of rectification of r-eag currents. The correlation coefficient is −0.1.
To determine the possible correlation between the intracellular Na+ concentration in a particular oocyte and the degree of rectification of r-eag currents, we coinjected cRNAs coding for Na+ channel type II from rat brain (Noda et al., 1986) and r-eag. The reversal potential for the Na+ current was then used to estimate the internal Na+ concentration in the oocyte. The recordings were performed with two-electrode voltage clamp in standard extracellular solution (NFR) containing 1 mM MgCl2 and stimulating from a holding potential of −120 mV. Both conditions were designed to slow down the activation of r-eag current (Terlau et al., 1996) and therefore minimize the interference of outward K+ currents with Na+ currents. Subsequently, the outward current was measured from a holding potential of −80 mV in the absence of extracellular Mg2+. In these experiments (Fig. 5 D), the intracellular Na+ concentration was between 5 and 35 mM. We did not observe correlation with the degree of rectification assessed as ratio between the steady-state current at +100 mV and at +40 mV. This ratio is ∼2 when the current does not show rectification (see Fig. 1 A). Taken together, these results demonstrate that an increase in Na+ concentration by itself is not sufficient to induce rectification of r-eag currents.
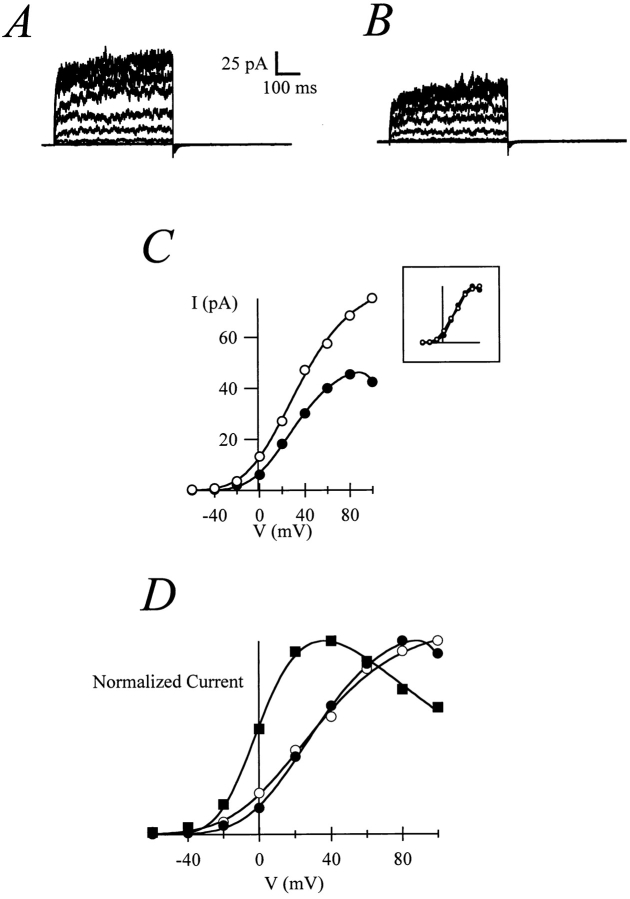
Inside-out patches from immature oocytes show a fast and strong run-down of the current. This run-down does not depend on diffusible factors since the current is not recovered after reinserting the membrane patch into the oocyte (“patch-cramming”; Kramer, 1990). This has precluded a detailed characterization of the behavior of the current in these oocytes. However, in a limited number of patches (n = 3), we were able to measure currents with only slight rectification when exposed to 20 mM internal Na+ (Fig. 6, A–C). In contrast, patches obtained from the same cell, this time after injection of MPF (Fig. 6 D, filled squares), showed a strong rectification that closely resembled the one described earlier in this paper (see Fig. 1 A). We conclude that MPF renders the channels sensitive to internal Na+.
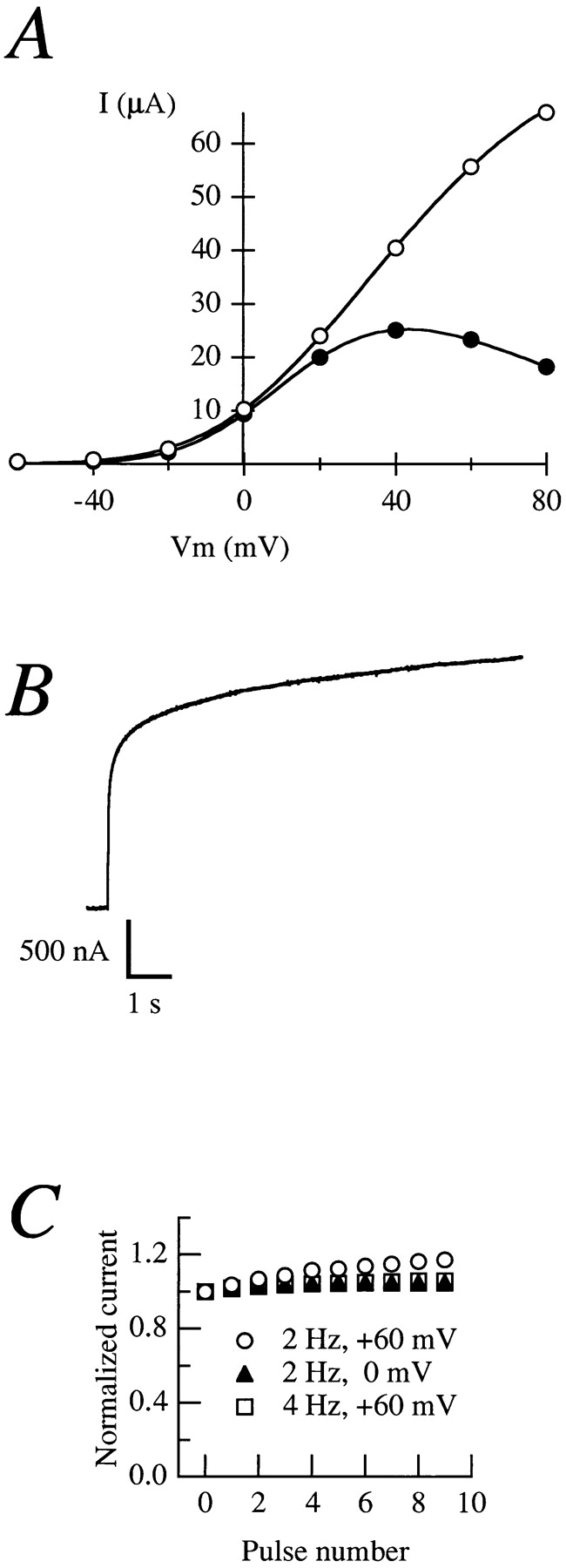
Figure 6.
Before maturation, patches expressing r-eag currents show only slight rectification with 20 mM Na+ (A, control traces; B, 20 mM Na+), as can be seen in the I-V plot (C; open circles, control; solid circles, 20 mM NaCl) and more clearly after normalization of the current amplitude (inset). After MPF injection, patches from the same oocyte show rectification comparable to mature oocytes (D; solid squares); for comparison, the normalized current amplitudes from A and B have been included (symbols as in C).
r-eag Currents Expressed in Mammalian Cells Are Blocked by Internal Na+
We checked the ability of internal Na+ ions to block eag currents in CHO-K1 cells stably expressing r-eag currents. For this purpose, we measured the currents in the whole-cell configuration, in the presence of 10 mM NaCl in the internal solution. We detected rectification in 9 out of 12 cells. In six cases, the current amplitude peaked around +80 mV when the I-V relationship was determined between −60 and +100 mV, and in the other three the peak current was obtained at a less positive potential (+60 mV). The I-V relationship resembled the one obtained in oocytes, although the rectification was usually not strong (Fig. 7).
Figure 7.
Variability in the degree of blockade by internal Na+ in CHO-K1 cells. (A and B) Raw currents traces obtained from two apparently identical cells (500-ms depolarizations to voltages between −60 and +100 mV, in 20-mV increments). (C and D) Corresponding I-V relationships. The internal solution contained 140 mM KCl and 10 mM NaCl.
This weak rectification could depend on differences between the oocyte system and mammalian cells, but most surprising were the differences within the mammalian cell line itself. A tempting hypothesis is that the differences from cell to cell are due to cells that are at different stages of the cell cycle since oocytes are arrested at G2 phase of the cell cycle and CHO-K1 cells are not. To test this hypothesis, we performed analogous experiments in cells treated with 500 nM taxol to enrich the population of cells in M phase. Only cells with spherical morphology typical of mitotic cells and/or displaying mitotic figures of chromosomes stained in vivo with HO were selected for recording. None of the cells tested (n = 8) showed rectification, supporting the hypothesis that the variability observed within cells from the same culture may be due to cell cycle–related events.
Since Na+ seems to enter deeply into the pore to block the channel, and the site appears to be only accessible under certain conditions, we addressed the possibility that changes in the conducting pathway of r-eag are occurring during cell cycle–related processes. If this was the case, some of the selectivity properties of r-eag might be altered as well. We decided to study the behavior of the current in the presence of Cs+ as the intracellular charge carrier.
r-eag Channels Are Permeable to Cs+ Ions
Drosophila eag channels are half as permeant to Cs+ as to K+ (Brüggemann et al., 1993). This is an unexpected property for a K+ channel. In contrast, r-eag channels appeared to be Cs+ impermeant when measured in whole oocytes with the two-electrode voltage clamp technique (Ludwig et al., 1994). However, we have seen some Cs+ outward current when Cs+ is the only available monovalent cation in the inside of patches obtained from immature oocytes. As previously mentioned, we have not succeeded to measure in patches from immature oocytes long enough to completely rule out any contamination by K+ in the internal solution. Therefore we decided to characterize the Cs+ permeability in transfected CHO-K1 cells.
Most cells in the whole-cell configuration with pipettes containing Cs+ as the sole charge carrier gave outward currents (Fig. 8 A). In symmetrical Cs+, outward and inward tail currents are at equilibrium around 0 mV (Fig. 8 B). When exposed to different external K+ to Cs+ ratios, the behavior of the tail currents is more complex (Fig. 8 C). Increasing Cs+ concentration results in a reduction of the tail amplitude, which is almost abolished at 35 mM Cs+. The amplitude, however, increases again with further reductions in the K+ concentration (with the concomitant increase in Cs+ concentration), indicating a close interaction between Cs+ and K+ (Fig. 8, C and D). This “anomalous mole fraction” effect is analogous to the one observed in Ca2+ channels for Ca2+ and Na+ or for Ca2+ and Ba2+, and also for the interaction between K+ and Tl+ in the inward rectifier of echinoderm eggs (Hille, 1992). The anomalous mole fraction indicates that there is interaction between both ions in the conducting pathway, i.e., that both K+ and Cs+ go through the same pore.
Figure 8.
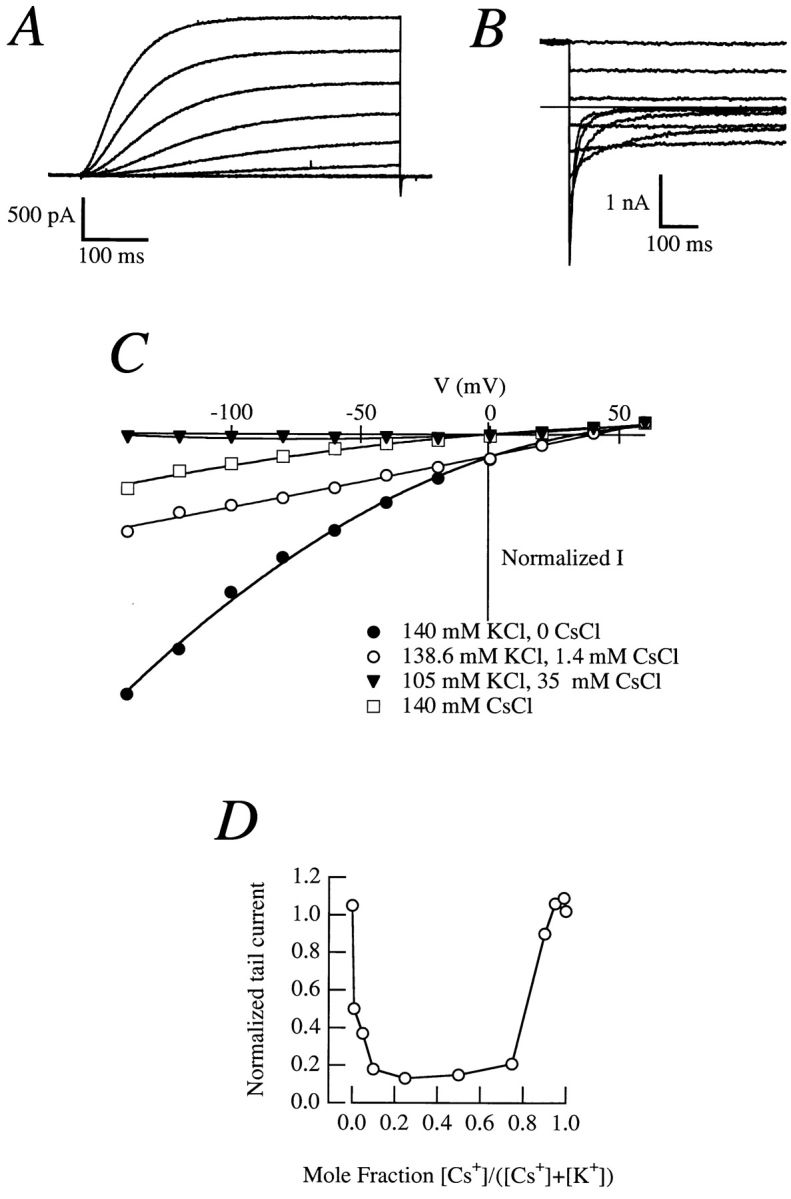
(A) Outward currents in a CHO-K1 cell expressing r-eag exposed to an internal solution containing Cs+ as the sole charge carrier; the same internal solution was used in B–D. (B) Tail currents in a cell exposed to an external solution containing (mM) 140 CsCl, 2 CaCl2, 2 MgCl2, 10 Hepes/CsOH, pH 7.2. The reversal potential is ∼0 mV. (C) Instantaneous I-V plots in cells with Cs+ internal solution and varying extracellular K+ and Cs+ concentrations. (D) Anomalous mole fraction effect obtained from tail currents in a cell perfused with different K+-Cs+ mixtures. The tail current at −100 mV (extrapolation to t = 0 of the exponential decay of the amplitude) was normalized to the outward current at +60 mV (mean amplitude at the final 10% of the pulse).
The Permeability Ratio Cs+/K+ Changes in Different Phases of the Cell Cycle
Approximately 90% of the cells tested (n > 100) showed Cs+ outward currents, but some were impermeable to Cs+, although they showed K+ inward currents, proving that r-eag channels were indeed expressed. These cell-to-cell differences could be explained in several alternative ways. We show in this paper that r-eag channels are blocked by internal Na+ once MPF is activated, and that Na+ has to enter the pore to almost one half of its electrical distance. We have also shown that most (but not all) of the transfected CHO-K1 cells show a similar behavior, and that this variability is abolished by synchronization of the cells in M phase. This indicates that the r-eag pore properties change during progression through the cell cycle.
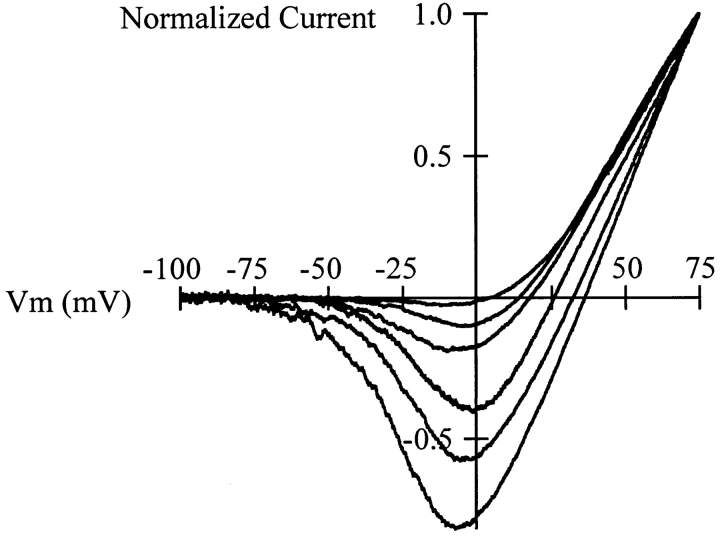
The reversal potential measured in cells with Cs+ as internal ion and K+ in the outside was also strongly variable. Fig. 9 shows some examples of the currents elicited by depolarizing ramps from −100 to +75 mV lasting 1 s. The currents have been normalized to the maximal amplitude, and evidently the reversal potential varies strongly from cell to cell, although other features of the current remain unaltered. We determined the value of the reversal potential using either voltage ramps, as in the experiments depicted in Fig. 9, or discrete depolarizations lasting 500 ms to potentials between −60 and +75 mV in 15-mV increments. Both methods gave essentially identical results. The reversal potential in these cells (Table I) ranged between −28 and +60 mV, and the coefficient of variation was 0.9. Thus, the Cs+/K+ permeability ratio varied between 0.09 and 2.76 (mean 0.38). In these experiments, we did not find any correlation between the reversal potential and cell size, current density, or series resistance.
Figure 9.
Normalized currents obtained during voltage ramps in CHO-K1 cells expressing r-eag currents. The internal solution contained Cs+ as charge carrier, and the external solution contained K+. From a holding potential of −100 mV, the cell was progressively depolarized to +75 mV during 1 s. Notice the strong variability in the reversal potential, while the shape of the current is similar in all cases.
Table I.
Reversal Potentials
| Reversal potential | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Mean | SD | CV | n | Range | |||||
| mV | mV | mV | ||||||||
| None | 24.5 | 21.2 | 0.9 | 16 | 88 | |||||
| 0.5% FCS | 16.5 | 9.8 | 0.6 | 19 | 34 | |||||
| Taxol | 32.6 | 8.3 | 0.3 | 15 | 33 | |||||
| Taxol + HO | 37.8 | 5.0 | 0.1 | 5 | 13 | |||||
Reversal potentials obtained from CHO-K1 cells permanently transfected with r-eag under biionic conditions (Cs+ in, K+ out). Control untreated cultures have the biggest standard deviation (SD) and coefficient of variation (CV). Cells selected by the presence of mitotic figures (Taxol + HO) gave values not significantly different from taxol alone, but with smaller CVs.
To test the possibility that the Cs+/K+ permeability ratio changes during progression through the cell cycle, we measured the reversal potential of the current under the same conditions in cultures enriched in G0/G1-phase cells (by withdrawal of growth factors, i.e., 0.5% FCS in the medium). These cells showed much less variation, ranging between −2 and +34 mV, with a coefficient of variation of 0.6 (Table I).
We also tested cultures enriched in M phase cells. After taxol treatment (18 h at 0.5 μM), cells having a spherical morphology had reversal potential in the range of +16 to +49 mV (Table I). Again, the coefficient of variation (0.3) was much smaller than the one of untreated cells. We think that the narrower interval is due to better recognition of M phase cells.
To further assure the identification of cells in M phase, we stained the DNA of a batch of cells in vivo using HO. Cells with clear mitotic figures had variations in their reversal potential within the limits of the voltage clamp errors (E rev = 37.8 ± 5 mV, n = 5, coefficient of variation 0.1).
To ensure that the level of expression of eag channels was sufficiently high to overcome any endogenous current contribution, we only used cells showing a robust K+ inward tail current. Neglecting cells with small tail currents systematically excludes cells that have very negative reversals caused by increased Cs+ permeability. Therefore, the measured variability in reversal potentials is a lower estimate of the true variance. We never detected Cs+ currents, and K+ currents never exceeded 0.1 nA in untransfected cells, neither in normal cultures (n = 20), nor in cultures containing 0.5% FCS (n = 3) or treated with 500 nM taxol (n = 5).
Discussion
Modulation of ion channels during cell cycle has been reported (see for example Day et al., 1993; Moody, 1995; Kuga et al., 1996), although the molecular mechanisms underlying such modulation remain unclear. MPF is the ubiquitous molecule controlling the G2-to-M transition of the cell cycle, both during meiotic and mitotic divisions (Nurse, 1994). Furthermore, somatic cells and oocytes share many common effects after MPF activation, although usually at different stages of the cell cycle. r-eag channels heterologously expressed in Xenopus oocytes are strongly modulated during MPF-dependent maturation. We studied the modulation of r-eag by MPF as a tool to characterize cell cycle–related changes in conductance properties of an ion channel. This modulation might give further insight into r-eag function in native cells.
As a first conceivable source of rectification, inactivation was excluded since MPF is unable to induce inactivation in r-eag channels. The next possible cause of rectification, block by intracellular agents such as Mg2+ and Na+, was examined. This was attempted despite technical problems caused by run-down of the currents in oocytes and their fragility after MPF injection. Although Mg2+ and Ca2+ are able to block the channel, the block is not voltage dependent. Na+ acts as an open channel blocker of r-eag channels, analogous to other delayed rectifier K+ channels. During maturation, there is an activation of the Na+/H+ antiporter (Rezai et al., 1994), and consequently the intracellular Na+ concentration will increase. Rectification in r-eag could therefore be only due to a rise in intracellular Na+ concentration. However, Na+ block alone does not explain the rectification since (a) the injection of Na+ does not induce rectification in immature oocytes; (b) the rise in Na+ concentration caused by ouabain incubation does not induce rectification in immature cells, nor does it increase the rectification present in spontaneously mature cells; (c) there is no correlation between the Na+ content of an oocyte and the degree of rectification of the current; and (d) the increase in Na+ concentration happens before MPF activation, in contrast to the induction of rectification, which requires active MPF.
From the voltage dependence of the block, we found that Na+ ions cross 45% of the electrical distance within the channel. Since no block is observed in immature cells, either Na+ ions are unable to reach the blocking site, or they do not block because they can permeate the channel. Both cases would implicate a change in pore properties of the channel during maturation. This was tested using Cs+—usually a blocker of K+-channels—as the intracellular permeant ion in mammalian cells permanently transfected with r-eag. We found that Cs+ can permeate the channel when extracellular K+ is absent. Exposing the cells to mixtures containing both ions caused a dramatic reduction in current. This anomalous mole fraction effect has been explained as a competition for binding sites (Hille, 1992) and proves that the two ions share the same conduction pathway. The presence of a Cs+ outward current allows us to define the channel as permeable to Cs+. This property of r-eag K+ channels is reminiscent of the lack of selectivity of Ca2+ channels in the absence of Ca2+ and has not been described for any other K+ channel.
Strikingly, the channel is not always permeable to Cs+ since not all cells tested gave Cs+ outward currents. Neither were currents blocked in all cells by Na+, and several cells produced measurable Na+ currents (not shown). Together with the oocyte data, there seems to be a population of cells in which the r-eag channels are permeable to Cs+ and are not blocked by Na+. After progression through the cell cycle, these channels become impermeable to Cs+ and blockable by Na+. A direct comparison between the data obtained in oocytes and in CHO cells is not possible at the moment since the effects observed in oocytes correspond to the onset of the M phase, and we have only measured CHO cells in which M phase was already clearly established. At present we cannot define different permeability states at various phases of the cell cycle. We have measured permeability properties in M phase cells based on morphological features and the possibility to stain mitotic chromosomes with vital stains. All of these cells show Cs+ permeability with a ratio P Cs/P K of 0.2 (as calculated from reversal potentials) and no Na+ block.
The differences between currents measured in oocytes and in mammalian cells could be explained if some factors, such as accessory subunits, are missing and/or different in one of the cell types. This would, however, produce homogeneous currents in all cells of the same type, and it cannot explain the variability observed between cells of the same type.
Assuming that the permeability properties of the r-eag channel change because of cell cycle–related events, there are two possible mechanisms to explain such modulation. First, it is possible that the channel molecule itself is physically changed (for example, by phosphorylation), or secondly, some other factor (subunit or attached protein) linked to the channel might induce the change. In the latter case, the modifying factor has to interact tightly with the channel since the Na+ block is maintained under cell-free conditions.
Our data demonstrate that one and the same channel can carry different currents depending on the stage of the cell cycle, indicating a novel possibility for ion channel modulation. We have shown that the permeability properties of channels can themselves be modulated during cell cycle–related processes, independently of changes in expression levels and kinetic properties. A similar variability of channel properties has been recently described for cardiac Na+ channels that can permeate Ca2+ in a modulated manner (Santana et al., 1998). Here, we have detected changes in the conducting properties of r-eag channels using a biophysical approach. The precise molecular mechanisms leading to these profound changes remain to be elucidated. However, given that these changes are modulated by the cell cycle itself, they harbor the potential for being relevant to cellular biology.
Acknowledgments
We thank Dr. O. Pongs for the generous gift of the r-eag clone, Dr. M. Stocker for the Na+ channel cRNA, Dr. M. Hollmann for the pSGEM vector, Drs. S. Beckh and G. Busch for critical comments on the manuscript, and V. Díaz-Salamanca, S. Voigt, and B. Scheufler for expert technical assistance.
Abbreviations used in this paper
- BAPTA
1,2-bis(2-aminophenoxy)ethaneN,N,N′N′-tetraacetic acid
- HO
Hoechst 33342
- IC50
concentration required for 50% inhibition
- I-V
current–voltage
- MPF
mitosis-promoting factor
- NFR
normal frog Ringer
- r-eag
rat ether-à-go-go K+ channel
Footnotes
Andrea Brüggemann's present address is Hoechst Marion Roussel, DG Cardiovascular, H 821, D-65926 Frankfurt, Germany. Tel.: 49-69-305-13547. Fax: 49-69-305-16393.
Address all correspondence to Luis A. Pardo, Max-Planck-Institut für experimentelle Medizin, Hermann-Rein-Str. 3, D-37075 Göttingen, Germany. Tel.: 49-551-3899-643. Fax: 49-551-389-9644. E-mail: pardo@mail.mpiem.gwdg.de
References
- Baukrowitz T, Yellen G. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 1995;15:951–960. doi: 10.1016/0896-6273(95)90185-x. [DOI] [PubMed] [Google Scholar]
- Bezanilla F, Armstrong CM. Negative conductance caused by entry of Na+ and Cs+ ions into the K+channels of squid axons. J Gen Physiol. 1972;60:588–608. doi: 10.1085/jgp.60.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borin M, Siffert W. Stimulation by thrombin increases the cytosolic free Na+ concentration in human platelets. Studies with the novel fluorescent cytosolic Na+ indicator Na+-binding benzofuran isophthalate. J Biol Chem. 1990;265:19543–19550. [PubMed] [Google Scholar]
- Brüggemann A, Pardo LA, Stühmer W, Pongs O. Ether-à-go-go encodes a voltage-gated channel permeable to K+ and Ca 2+and modulated by cAMP. Nature. 1993;365:445–448. doi: 10.1038/365445a0. [DOI] [PubMed] [Google Scholar]
- Brüggemann A, Stühmer W, Pardo LA. Mitosis-promoting factor-mediated suppression of a cloned delayed rectifier K+ channel expressed in Xenopusoocytes. Proc Natl Acad Sci USA. 1997;94:537–542. doi: 10.1073/pnas.94.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cork RJ, Robinson KR. Second messenger signaling during hormone-induced Xenopusoocyte maturation. Zygote. 1994;2:289–299. doi: 10.1017/s0967199400002112. [DOI] [PubMed] [Google Scholar]
- Day ML, Pickering SJ, Johnson MH, Cook DI. Cell cycle control of a large-conductance K+channel in mouse early embryos. Nature. 1993;365:560–562. doi: 10.1038/365560a0. [DOI] [PubMed] [Google Scholar]
- Fortune JE, Concannon PW, Hansel W. Ovarian progesterone levels during in vitro oocyte maturation and ovulation in Xenopus laevisoocytes and eggs. Biol Reprod. 1975;13:561–567. doi: 10.1095/biolreprod13.5.561. [DOI] [PubMed] [Google Scholar]
- French RJ, Wells JB. Na+ ions as blocking agents and charge carriers in the K+channel of the squid giant axon. J Gen Physiol. 1977;70:707–724. doi: 10.1085/jgp.70.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harootunian AT, Kao JP, Eckert BK, Tsien RY. Fluorescence ratio imaging of cytosolic free Na+in individual fibroblasts and lymphocytes. J Biol Chem. 1989;264:19458–19467. [PubMed] [Google Scholar]
- Hille, B. 1992. Ionic Channels of Excitable Membranes. Sinauer Associates, Inc., Sunderland, MA. 607 pp.
- Howe JR, Baker M, Ritchie JM. On the block of outward K+ current in rabbit Schwann cells by internal Na+ions. Proc R Soc Lond B Biol Sci. 1992;249:309–316. doi: 10.1098/rspb.1992.0120. [DOI] [PubMed] [Google Scholar]
- Johansson S, Sundgren AK, Kahl U. Potential-dependent block of human delayed rectifier K+ channels by internal Na+ . Am J Physiol. 1996;39:C1131–C1144. doi: 10.1152/ajpcell.1996.270.4.C1131. [DOI] [PubMed] [Google Scholar]
- Johnson EM, Theler JM, Capponi AM, Vallotton MB. Characterization of oscillations in cytosolic free Ca2+ concentration and measurement of cytosolic Na+concentration changes evoked by angiotensin II and vasopressin in individual rat aortic smooth muscle cells. Use of microfluorometry and digital imaging. J Biol Chem. 1991;266:12618–12626. [PubMed] [Google Scholar]
- Kramer RH. Patch cramming: monitoring intracellular messengers in intact cells with membrane patches containing detector ion channels. Neuron. 1990;2:335–341. doi: 10.1016/0896-6273(90)90046-i. [DOI] [PubMed] [Google Scholar]
- Krieg PA, Melton DA. In vitroRNA synthesis with SP6 RNA polymerase. Methods Enzymol. 1987;155:397–415. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- Kuga T, Kobayashi S, Hirakawa Y, Kanaide H, Takeshita A. Cell cycle-dependent expression of L- and T-type Ca2+currents in rat aortic smooth muscle cells in primary culture. Circ Res. 1996;79:14–19. doi: 10.1161/01.res.79.1.14. [DOI] [PubMed] [Google Scholar]
- Lopatin AN, Nichols CG. Internal Na+ and Mg2+ blockade of DRK1 (Kv2.1) K+ channels expressed in Xenopusoocytes. Inward rectification of a delayed rectifier. J Gen Physiol. 1994;103:203–216. doi: 10.1085/jgp.103.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Barneo J, Hoshi T, Heinemann SH, Aldrich RW. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker K+channels. Recept Channels. 1993;1:61–71. [PubMed] [Google Scholar]
- Ludwig J, Terlau H, Wunder F, Brüggemann A, Pardo LA, Marquardt A, Stühmer W, Pongs O. Functional expression of a rat homologue of the voltage gated ether a go-go K+ channel reveals differences in selectivity and activation kinetics between the Drosophilachannel and its mammalian counterpart. EMBO (Eur Mol Biol Organ) J. 1994;13:4451–4458. doi: 10.1002/j.1460-2075.1994.tb06767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A. Blocking of large unitary Ca2+-dependent K+ currents by internal Na+ions. Pflügers Arch. 1983;396:179–181. doi: 10.1007/BF00615524. [DOI] [PubMed] [Google Scholar]
- Meyer R, Heinemann SH. Characterization of an eag-like K+channel in human neuroblastoma cells. J Physiol (Lond) 1998;508:49–56. doi: 10.1111/j.1469-7793.1998.049br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody WJ. Critical periods of early development created by the coordinate modulation of ion channel properties. Persp Dev Neurobiol. 1995;2:309–315. [PubMed] [Google Scholar]
- Murakami MS, Vande GF, Woude Mechanisms of Xenopusoocyte maturation. Methods Enzymol. 1997;283:584–600. doi: 10.1016/s0076-6879(97)83046-7. [DOI] [PubMed] [Google Scholar]
- Noda M, Ikeda T, Suzuki H, Takeshima H, Takahashi T, Kuno M, Numa S. Expression of functional Na+channels from cloned cDNA. Nature. 1986;322:826–828. doi: 10.1038/322826a0. [DOI] [PubMed] [Google Scholar]
- Nurse P. Ordering S phase and M phase in the cell cycle. Cell. 1994;79:547–550. doi: 10.1016/0092-8674(94)90539-8. [DOI] [PubMed] [Google Scholar]
- Rezai K, Kulisz A, Wasserman WJ. Protooncogene product, c-mos kinase, is involved in upregulating Na+/H+ antiporter in Xenopusoocytes. Am J Physiol. 1994;267:C1717–C1722. doi: 10.1152/ajpcell.1994.267.6.C1717. [DOI] [PubMed] [Google Scholar]
- Sagata N. What does Mos do in oocytes and somatic cells? . Bioessays. 1997;19:13–21. doi: 10.1002/bies.950190105. [DOI] [PubMed] [Google Scholar]
- Santana LF, Gomez AM, Lederer WJ. Ca2+ flux through promiscuous cardiac Na+channels—slip-mode conductance. Science. 1998;279:1027–1033. doi: 10.1126/science.279.5353.1027. [DOI] [PubMed] [Google Scholar]
- Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Schönherr R, Heinemann SH. Molecular determinants for activation and inactivation of HERG, a human inward rectifier K+channel. J Physiol (Lond) 1996;493:635–642. doi: 10.1113/jphysiol.1996.sp021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz AW, Glad R. In vitro production of meiosis inducing substance (MIS) by isolated amphibian (Rana pipiens)follicle cells. Dev Growth Differ. 1985;27:201–211. doi: 10.1111/j.1440-169X.1985.00201.x. [DOI] [PubMed] [Google Scholar]
- Smith PL, Baukrowitz T, Yellen G. The inward rectification mechanism of the HERG cardiac K+channel. Nature. 1996;379:833–836. doi: 10.1038/379833a0. [DOI] [PubMed] [Google Scholar]
- Spector PS, Curran ME, Zou A, Keating MT, Sanguinetti MC. Fast inactivation causes rectification of the IKr channel. J Gen Physiol. 1996;107:611–619. doi: 10.1085/jgp.107.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfeld CE, Roper J, Ludwig J, Weseloh RM, Marsh SJ, Brown DA, Pongs O. Elevation of intracellular Ca2+ by muscarinic receptor activation induces a block of voltage-activated rat ether-à-go-gochannels in a stably transfected cell line. Proc Natl Acad Sci USA. 1996;93:9910–9914. doi: 10.1073/pnas.93.18.9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer W. Electrophysiological recordings from Xenopusoocytes. Methods Enzymol. 1992;207:319–339. doi: 10.1016/0076-6879(92)07021-f. [DOI] [PubMed] [Google Scholar]
- Terlau H, Ludwig J, Steffan R, Pongs O, Stühmer W, Heinemann SH. Extracellular Mg2+ regulates activation of rat eag K+channel. Pflügers Arch. 1996;432:301–312. doi: 10.1007/s004240050137. [DOI] [PubMed] [Google Scholar]
- Woodhull AM. Ionic blockage of Na+channels in nerve. J Gen Physiol. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+channels of bovine chromaffin cells. J Gen Physiol. 1984a;84:157–186. doi: 10.1085/jgp.84.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Relief of Na+ block of Ca2+-activated K+channels by external cations. J Gen Physiol. 1984b;84:187–199. doi: 10.1085/jgp.84.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelarayan L, Oterino J, Buhler MI. Spontaneous maturation in Bufo arenarumoocytes—participation of protein kinase C. Zygote. 1996;4:257–262. doi: 10.1017/s0967199400003191. [DOI] [PubMed] [Google Scholar]