Abstract
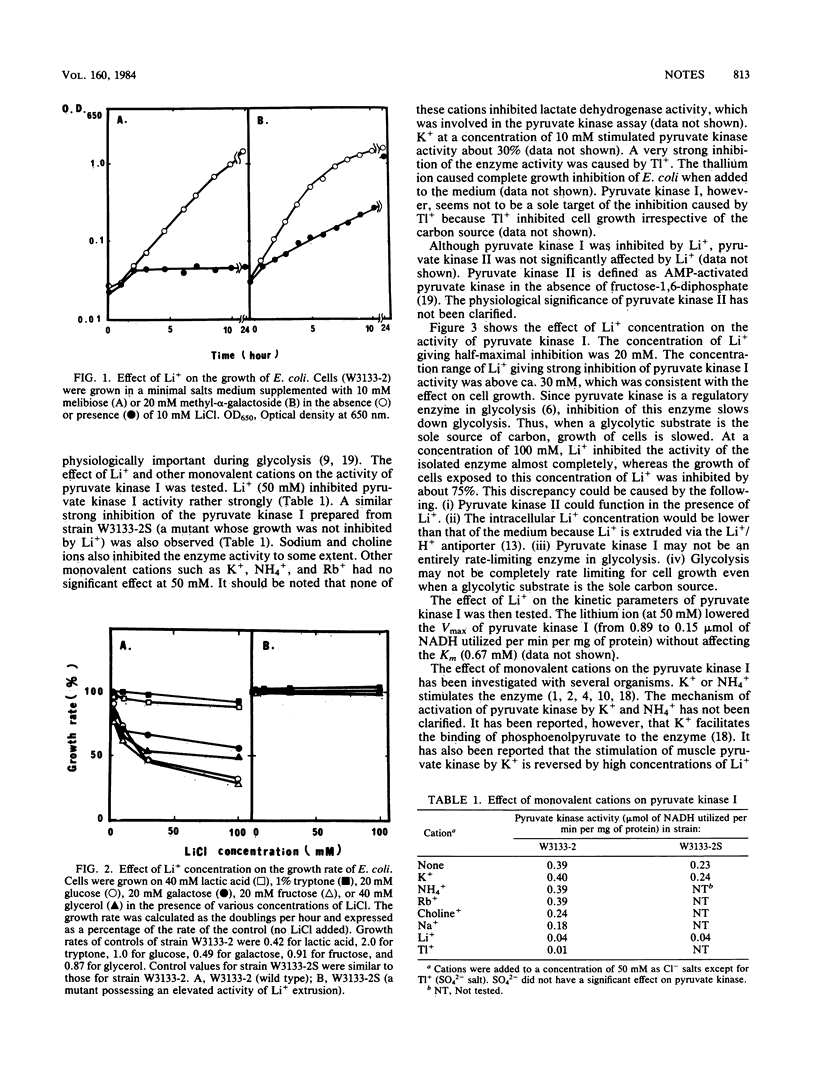
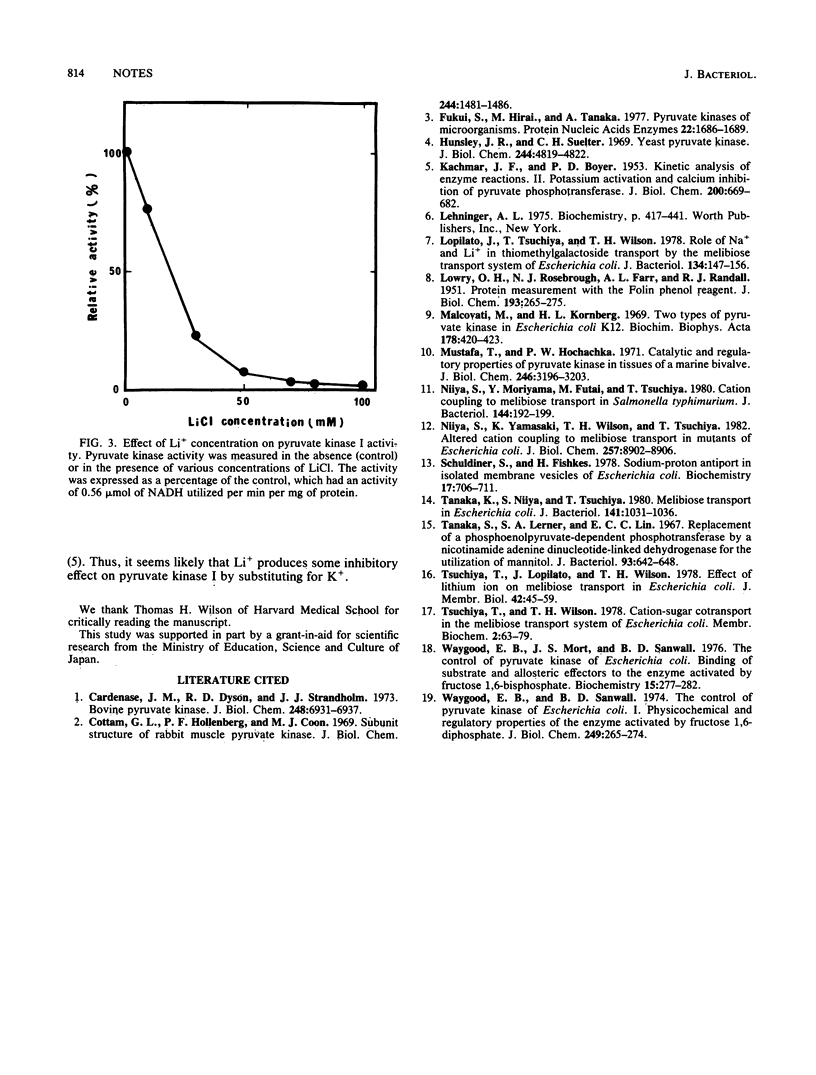
Li+ inhibited growth of Escherichia coli when glucose, galactose, fructose, or glycerol was added as the sole source of carbon. Growth inhibition was not observed when lactate or a mixture of amino acids was used as the carbon source. A mutant possessing elevated activity of Li+ extrusion was not inhibited by Li+. These results suggested that intracellular Li+ inhibited the glycolytic pathway, most likely triose metabolism, without affecting gluconeogenesis. We also found that pyruvate kinase I was inhibited by Li+.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cardenas J. M., Dyson R. D., Strandholm J. J. Bovine pyruvate kinases. I. Purification and characterization of the skeletal muscle isozyme. J Biol Chem. 1973 Oct 25;248(20):6931–6937. [PubMed] [Google Scholar]
- Cottam G. L., Hollenberg P. F., Coon M. J. Subunit structure of rabbit muscle pyruvate kinase. J Biol Chem. 1969 Mar 25;244(6):1481–1486. [PubMed] [Google Scholar]
- Fukui S., Hirai M., Tanaka A. [Pyruvate kinases of microorganisms (author's transl)]. Tanpakushitsu Kakusan Koso. 1977;22(12):1686–1689. [PubMed] [Google Scholar]
- Hunsley J. R., Suelter C. H. Yeast pyruvate kinase. II. Kinetic properties. J Biol Chem. 1969 Sep 25;244(18):4819–4822. [PubMed] [Google Scholar]
- KACHMAR J. F., BOYER P. D. Kinetic analysis of enzyme reactions. II. The potassium activation and calcium inhibition of pyruvic phosphoferase. J Biol Chem. 1953 Feb;200(2):669–682. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lopilato J., Tsuchiya T., Wilson T. H. Role of Na+ and Li+ in thiomethylgalactoside transport by the melibiose transport system of Escherichia coli. J Bacteriol. 1978 Apr;134(1):147–156. doi: 10.1128/jb.134.1.147-156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcovati M., Kornberg H. L. Two types of pyruvate kinase in Escherichia coli K12. Biochim Biophys Acta. 1969 Apr 22;178(2):420–423. doi: 10.1016/0005-2744(69)90417-3. [DOI] [PubMed] [Google Scholar]
- Mustafa T., Hochachka P. W. Catalytic and regulatory properties of pyruvate kinases in tissues of a marine bivalve. J Biol Chem. 1971 May 25;246(10):3196–3203. [PubMed] [Google Scholar]
- Niiya S., Moriyama Y., Futai M., Tsuchiya T. Cation coupling to melibiose transport in Salmonella typhimurium. J Bacteriol. 1980 Oct;144(1):192–199. doi: 10.1128/jb.144.1.192-199.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niiya S., Yamasaki K., Wilson T. H., Tsuchiya T. Altered cation coupling to melibiose transport in mutants of Escherichia coli. J Biol Chem. 1982 Aug 10;257(15):8902–8906. [PubMed] [Google Scholar]
- Schuldiner S., Fishkes H. Sodium-proton antiport in isolated membrane vesicles of Escherichia coli. Biochemistry. 1978 Feb 21;17(4):706–711. doi: 10.1021/bi00597a023. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Niiya S., Tsuchiya T. Melibiose transport of Escherichia coli. J Bacteriol. 1980 Mar;141(3):1031–1036. doi: 10.1128/jb.141.3.1031-1036.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T., Lopilato J., Wilson T. H. Effect of lithium ion on melibiose transport in Escherichia coli. J Membr Biol. 1978 Jul 21;42(1):45–59. doi: 10.1007/BF01870393. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Wilson T. H. Cation-sugar cotransport in the melibiose transport system of Escherichia coli. Membr Biochem. 1978;2(1):63–79. doi: 10.3109/09687687809063858. [DOI] [PubMed] [Google Scholar]
- Waygood E. B., Mort J. S., Sanwal B. D. The control of pyruvate kinase of Escherichia coli. Binding of substrate and allosteric effectors to the enzyme activated by fructose 1,6-bisphosphate. Biochemistry. 1976 Jan 27;15(2):277–282. doi: 10.1021/bi00647a006. [DOI] [PubMed] [Google Scholar]
- Waygood E. B., Sanwal B. D. The control of pyruvate kinases of Escherichia coli. I. Physicochemical and regulatory properties of the enzyme activated by fructose 1,6-diphosphate. J Biol Chem. 1974 Jan 10;249(1):265–274. [PubMed] [Google Scholar]



