Abstract
A cholera toxin mutant (CTX–K63) unable to raise cAMP levels was used to study in Vero cells the retrograde transport of the toxin A subunit (CTX-A–K63), which possesses a COOH-terminal KDEL retrieval signal. Microinjected GTP-γ-S inhibits the internalization as well as Golgi–ER transport of CTX-A–K63. The appearance of CTX-A–K63 in the Golgi induces a marked dispersion of Erd2p and p53 but not of the Golgi marker giantin. Erd2p is translocated under these conditions most likely to the intermediate compartment as indicated by an increased colocalization of Erd2p with mSEC13, a member of the mammalian coat protein II complex. IgGs as well as Fab fragments directed against Erd2p, β-COP, or p23, a new member of the p24 protein family, inhibit or block retrograde transport of CTX-A–K63 from the Golgi without affecting its internalization or its transport to the Golgi. Anti-Erd2p antibodies do not affect the binding of CTX-A to Erd2p, but inhibit the CTX-K63–induced translocation of Erd2p and p53.
Keywords: cholera toxin, COPI, KDEL-receptor, p23, p53
Resident soluble and transmembrane proteins of the ER undergo permanent retention and retrieval processes to secure their steady-state distribution to this organelle (for review see Pelham, 1994; Teasdale and Jackson, 1996). This requires that ER proteins that have escaped from the ER are transported back by retrograde transport mechanisms. Many type I and type II transmembrane proteins of the ER possess specific retrieval signals, a COOH-terminal KKXX motif for type I (Jackson et al., 1993), an NH2-terminal XXRR motif for type II (Schutze et al., 1994) transmembrane proteins. Another example is the retrograde transport of the yeast protein Sec12p, which requires its interaction with Rer1p (Sato et al., 1995, 1996). Soluble ER proteins use the COOH-terminal tetrapeptide KDEL, HDEL, or similar as a retrieval signal (Munro and Pelham, 1979). Escaped type I transmembrane proteins are believed to be sorted in the Golgi into coat protein (COP)I1-coated vesicles after an interaction of the KKXX motif with coatomer (Cosson and Letourneur, 1994; Letourneur et al., 1994). However, what really regulates the recruitment of COPI coatomers to the Golgi membranes is still a matter of debate (for a review see Gaynor et al., 1998). Coatomers seem to bind not only to the COOH- or NH2-terminal motifs of escaped ER–transmembrane proteins, but also to the cytoplasmic tails of members of a family of transmembrane proteins (p24 proteins) identified in yeast (Schimmöller et al., 1995) and mammalian (Stamnes et al., 1995; Dominguez et al., 1998) cells. Most, but not all of these proteins share a COOH-terminal KKXX or similar motif and several of them contain in positions 7 and 8 upstream from the COOH-terminal amino acid two phenylalanins followed in positions 9 and 10 by two basic amino acids (Stamnes et al., 1995). Members of the p24 family can bind to COPI (Stamnes et al., 1995; Sohn et al., 1996) as well as to COPII proteins (Belden and Barlowe, 1996; Elrod-Erickson and Kaiser, 1996; Dominguez et al., 1998). It has been shown in particular for p24 and the newly described p23 protein (Blum et al., 1996; Sohn et al., 1996), that the di-phenylalanin motif is absolutely required for coatomer binding (Fiedler et al., 1996; Sohn et al., 1996). Whereas most members of the p24 protein family under steady-state conditions exist in the ER, mammalian p23 and p24 show a Golgi-like distribution (Sohn et al., 1996; Dominguez et al., 1998).
It has been proposed that binding of KDEL proteins in the Golgi occurs because of the lower pH in this compartment as compared with the ER, where a more alkaline pH would favor dissociation of the KDEL protein from its receptor (Wilson et al., 1993). Additional factors in the Golgi may be needed for binding since the pH within the Golgi has been estimated to be ∼6.45 (Kim et al., 1996). At this pH value, the binding of KDEL peptides, which shows a maximum at pH 5.0, is rather weak (Wilson et al., 1993). In the steady-state, the highest concentration of Erd2p exists in the cis-Golgi and the cis-Golgi network (Griffiths et al., 1994). However, it has been shown recently, that KDEL proteins can be moved by retrograde transport from the plasma membrane to the ER (Miesenböck and Rothman, 1995; Majoul et al., 1996). This could hardly occur unless some Erd2p were also accessible already in the TGN or the trans-Golgi. Overexpression of a lysozyme–KDEL fusion protein as well as overexpression of Erd2p enhances retrograde transport of Erd2p together with Golgi marker proteins leading to a new steady-state distribution of the receptor and of Golgi marker proteins (Lewis and Pelham, 1992), which resembles the effects of brefeldin A (BFA). However, it is not clear yet in which way the occupied Erd2p is transported from the Golgi to the ER. As resident ER membrane proteins bearing a COOH-terminal KKXX signal are thought to be retrieved via COPI-coated vesicles (Letourneur et al., 1994), it seems possible that KDEL proteins undergo retrograde transport via the same type of vesicles. This would be in line with experiments in yeast (Lewis and Pelham, 1996) where it was shown that the mutation of the target SNAP receptor (t-SNARE) Ufe1p inhibited the recycling of the overexpressed HDEL receptor as well as of Emp47p, a protein with a dibasic retrieval signal (Schröder et al., 1995). In these experiments, the recycling of the overexpressed HDEL receptor served as the transport parameter. On the other hand, it has been reported recently (Scheel et al., 1997), that anti–β-COP antibodies inhibited retrograde translocation of Golgi markers to the ER induced by BFA, but not the relocation of Erd2p from the Golgi to the intermediate compartment and that, therefore, retrograde transport of Erd2p could not involve a mechanism sensitive to an inhibitory anti–β-COP antibody. However, it is unlikely that the redistribution of proteins from the Golgi to the ER induced by BFA reflects the physiological conditions of retrograde transport. Moreover, it is necessary to study sorting and transport of the occupied rather than that of the free Erd2p to gain insights into the mechanisms of retrieval of soluble protein cargo. Therefore, we have used the retrograde transport of cholera toxin in Vero cells to examine for proteins involved in the retrograde transport of the occupied Erd2p. After binding of the CTX-B subunit to the ganglioside GM1 (Sattler et al., 1978) and endocytosis CTX becomes separated into its B subunits and its A subunit (CTX-A) in a late endosomal or a trans-Golgi compartment (Bastiaens et al., 1996). CTX-A binds to Erd2p via its COOH-terminal KDEL motif followed by retrograde transport to the IC and ER (Lencer et al., 1995; Majoul et al., 1996). For our studies we have used a CTX with a mutated ADP ribose binding site in CTX-A, thus avoiding any stimulation of adenylate cyclase. Immunofluorescence studies show that upon arrival of CTX-A in the Golgi the Golgi-like distribution of Erd2p undergoes a transient dispersion with a concomitant translocation into a mSEC13-positive compartment, most likely reflecting the IC.
Microinjected GTP-γ-S inhibited internalization of CTX and its transport to the Golgi as well as the transport of CTX-A from the Golgi to the ER. In contrast, microinjected IgGs or Fab fragments directed against the cytoplasmic tails of Erd2p, of β-COP (anti-EAGE), or of p23 strongly inhibited transport of CTX-A from the Golgi to the ER, but not its internalization or transport to the Golgi. Interestingly, antibodies directed against the cytoplasmic tail of Erd2 inhibited or abolished the CTX-K63– induced translocation of Erd2p as well as of p53. These observations indicate that the increased occupancy of Erd2p induces not only an increased sorting of occupied Erd2p into COPI vesicles but also an enhanced formation or budding of these vesicles.
Materials and Methods
Wild-Type and Mutant CTX
Wild-type CTX and CTX-A were obtained from Calbiochem-Novabiochem (Bad Soden, Germany). CTX with a mutated A subunit was generated as previously described (Fontana et al., 1995). We have used a mutation in which serine63 of the mature CTX-A had been replaced by a lysine (CTX–K63). The mutated protein is completely unable to ADP ribosylate polyarginine when tested according to Lai et al. (1981) and does not induce a rise of cAMP (results not shown here).
Antibodies
Antibodies were raised in rabbits against CTX-A and against the following peptides, which all contained an additional NH2-terminal cysteine: COOH-LYITKVLKGKKLSLPA (COOH-terminal peptide of Erd2p), COOH-KKEAGELKPEEEITVGPVQK (residues 494–513 of β-COP) (Duden et al., 1997), COOH-RRFFKAKKLIE (COOH terminus of p23; Sohn et al., 1996), and COOH-YLKRFFEVRRVV (COOH terminus of p24; Stamnes et al., 1995). The peptides were coupled to keyhole limpet hemocyanin via the NH2-terminal cysteines using the bifunctional reagent sulfo-SMCC (Pierce Chemical Co., Rockford, IL). The antibodies against CTX-A reacted also with the mutated CTX-A–K63. IgGs from the antisera were purified via protein A–Sepharose chromatography. The resulting IgGs were purified by affinity chromatography on the immobilized peptides or immobilized CTX-A (anti–CTX-A antibodies). The purity of the antibodies was checked by immunoblotting with extracts from Vero cells. All microinjested IgGs were equilibrated with “internal medium” (120 mM potassium glutamate, 5 mM NaCl, 5 mM MgCl2) by gel filtration before microinjection. An mAb against p53 and antibodies against giantin came from H.P. Hauri (Biocenter, University of Basel, Basel, Switzerland) and an affinity-purified antibody against recombinant mSEC13 from B.L. Tang (Institute of Molecular and Cellular Biology, Singapore, Singapore).
Antibodies or BSA were labeled as recommended by the manufacturer with the disuccinimides of Cy2, Cy3, or Cy5 to a molar dye/protein ratio of 1:1 to 2:1. Cy dye–labeled primary antibodies allowed double- and triple-immunofluorescence studies without the need for secondary antibodies. Staining for p24 and mSEC13 was performed using the unlabeled primary antibodies and fluorescein- or TRITC-labeled goat anti–rabbit secondary antibodies.
Fab fragments were prepared by digestion of the corresponding IgGs with agarose-immobilized papain (Sigma-Aldrich Chemie GmbH, Deisenhofen, Germany) followed by blocking of free SH groups with iodoacetamide and removal of agarose–papain by centrifugation. Complete digestion of IgGs was controlled by SDS-PAGE (Fig. 1). Iodoacetamide was removed and the Fab fragments equilibrated with “internal medium” by gel filtration. Dye-labeled Fab fragments were prepared in the same way from dye-labeled IgGs. Fab fragments were used within 12 h after preparation.
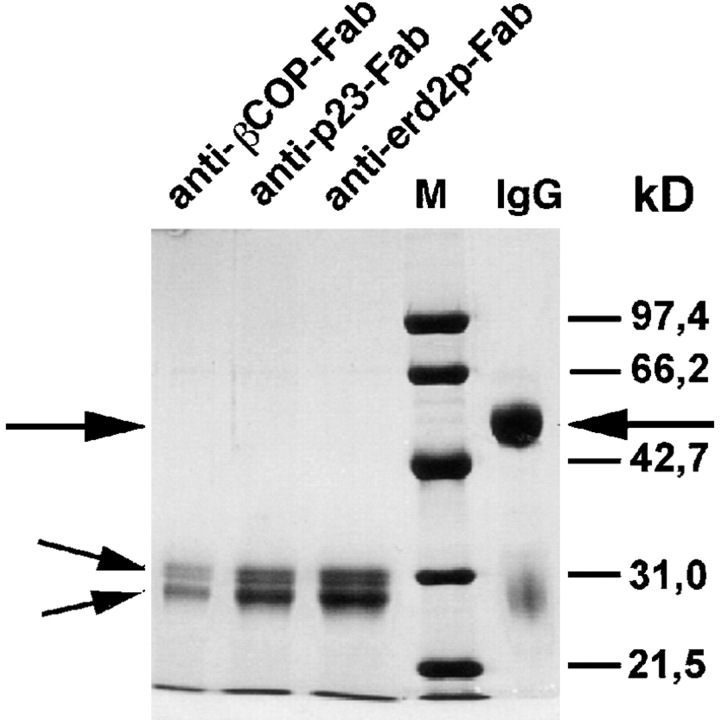
Figure 1.
SDS-PAGE (reducing conditions) of Fab fragments prepared from IgGs directed against the EAGE peptide of β-COP, and the cytoplasmic tails of p23 or Erd2p. Note the complete disappearance of uncleaved IgG (large arrow, IgG heavy chains) and the appearance of a low molecular mass double band (small arrows) representing Fab and Fc fragments respectively. M, molecular mass markers.
Bacculovirus Expression System
Bacculo-Gold™ DNA was obtained from PharMingen (Hamburg, Germany).
Methods
All transport studies were performed with Vero cells, which had been grown on coverslips to ∼70% confluency. Binding of WT–CTX (0.5 μg/ml) or the CTX–K63 (0.5 μg/ml) was performed at 0°C. Following removal of the unbound toxin the uptake was initiated and the intracellular transport followed at 37°C and 5% CO2 as described previously (Majoul et al., 1996). Unless otherwise mentioned, antibodies or GTP-γ-S were either injected 20 min before addition of CTX, or 15–20 min after start of CTX uptake, when some of the CTX-A had already reached the Golgi (Majoul et al., 1996).
As none of the microinjected IgGs affected the plasma membrane– Golgi transport of CTX-A–K63, Fab fragments were microinjected immediately before addition of CTX–K63 to the cells. For microinjection coverslips were transferred to DME, 10% FCS, 10 mM Hepes, pH 7.4, in a 3.5-cm Petri dish with a central hole (1-cm-diam) that had been closed from the bottom side by a glued glass coverslip. Microinjection was then performed over a period of ≤5 min using a semi-automatic micromanipulation and injection unit and Eppendorf femtotips (Eppendorf-Netheler-Hinz GmbH, Hamburg, Germany). After microinjection, the cells were incubated for the indicated times at 37°C and 5% CO2. Cells injected with GTP-γ-S were identified by coinjecting Cy2-labeled BSA. Cells microinjected with antibodies or Fab fragments directed against β-COP, p23, or Erd2p were identified by coinjection of the same antibodies or Fab fragments labeled with Cy2. The ratio of unlabeled IgGs or Fab fragments to Cy-labeled IgGs or Fab fragments was ∼3:1. At the appropriate time points, cells were fixed with 3.5% PFA, permeabilized with 0.1% (wt/vol) saponine, and then immunostained as previously described (Majoul et al., 1996).
Microscopy and Image Analysis
Standard immunofluorescence was performed with a Zeiss Axioplan microscope (Carl Zeiss, Oberkochen, Germany) equipped with a Zeiss Plan Neofluar 40×/0.75 objective and a Plan Neofluar 100×/1.30 oil objective. Cy2 and Cy3 were exited at 488 and 514 nm, respectively. Images were collected with a digital CF8/1DX camera (Kappa, Reinhausen, Germany).
Confocal laser scanning microscopy was performed with a Zeiss LSM410 microscope with a 40× 0.9 Plan Neofluar objective and a 63× 1.4 Plan Neofluar objective. Excitation was performed at 488 nm (argon laser, Cy2), 543 nm (Cy3), and 633 nm (Cy5) (both Helium/Neodym laser). The following emission filters were used: LP 515 (Schott, Mainz, Germany) or BP530 (Omega Optical, Brattleboro, VT) for Cy2, LP 570 (Schott) or 543BP12 (Omega Optical) for Cy3, and LP665 (Schott) for Cy5. Reconstruction of images was performed using the NIH-Image software and Photoshop software (version 4 for Macintosh; Adobe Systems, Inc., Mountain View, CA). Background intensity quantitation was performed using the Scil Image software (Technical University, Delft, The Netherlands).
Binding of 125I-Labeled CTX-A to Erd2p in the Presence of Anti-Erd2p Antibodies
The cDNA coding for human Erd2p was cloned into the Bacculovirus transfer vector and Sf9 cells were co-transfected with this vector together with BacculoGold DNA (PharMingen). The transfected cells were cultivated for 4–5 d in TNM FH medium at 27°C. Virus particles were isolated by centrifugation and stored at −80°C. Sf9 cells grown to 80–90% confluency were harvested and seeded at a density of 1.9 × 107 cells/plate (15 cm). After attachment of the cells they were infected with virus stock solution, cultivated for 3 d, and then harvested. The Sf9 cells overexpressing Erd2p were homogenized with a glass-glass Potter homogenizer in 0.25 M sucrose, 10 mM Tris-Cl, pH 7.4 (five times the volume of the sedimented cells). The homogenate (800 μl) was mixed with 900 μl of 2.3 M sucrose, 10 mM Tris-Cl, pH 7.4, and 100 μl 100 mM Na2EDTA and pipetted onto the bottom of a Beckman SW50 centrifuge tube (Beckman Instruments GmbH, München, Germany), followed by overlays with 1.2 M sucrose, 10 mM Tris-Cl, pH 7.4, 0.8 M sucrose, 10 mM Tris-Cl, pH 7.4. After centrifugation for 3.5 h at 90,000 g, the Golgi-enriched fraction was recovered from the 1.2 M sucrose/0.8 M sucrose interphase. Golgi-enriched fractions were mixed with an equal volume of 10 mM Hepes-KOH, pH 7.5, and spun for 1 h at 100,000 g. The pellet from 600 μl of a Golgi-enriched fraction was suspended in 200 μl of 0.1 M Na2CO3 for 10 min and spun again for 30 min at 100,000 g. The sediment was resuspended in 200 μl 10 mM Hepes-KOH, pH 7.5, and used for the binding assays.
The binding assay was performed in cacodylate buffer as described by Wilson et al. (1993). Although the maximum binding of CTX-A and of KDEL peptides to Erd2p occurred at pH 5 under our conditions (confirming the data presented first by Wilson et al. [1993]), we have observed (unpublished results) that the decrease in binding upon raising the pH is much less for 125I-KDEL proteins than for various 125I-KDEL peptides. To study the effects of anti-Erd2p antibodies on the binding of 125I–CTX-A, the permeabilized Golgi vesicles were preincubated at pH 7.2 with anti-Erd2p IgGs at room temperature for 30 min. Control vesicles were either incubated in buffer or with the same dilution of IgGs from the preimmune serum. After preincubation, the samples were brought to pH 6 with cacodylate, and the binding assays performed with different concentrations of 125I–CTX-A. Controls with 125I–CTX-A but without membranes were subtracted. Using Iodobeads CTX-A was 125I-labeled in PBS at pH 7.4 to a specific radioactivity of 2.2 μCi/μg.
Results
WT–CTX and CTX with a Mutation in the CTX-A Subunit (CTX–K63) That Abolishes Binding of ADP Ribose Are Transported in a Similar Way
After binding to the ganglioside GM1 via its B subunits, CTX undergoes cellular uptake (Janicot and Debuquois, 1987; Sofer and Futerman, 1987; Tran et al., 1987; Nambiar et al., 1993; Orlandi et al., 1993) via caveolae (Orlandi and Fishman, 1998; Wolf et al., 1998). In a late endosomal or a Golgi compartment, CTX-A and CTX-B dissociate from each other (Bastiaens et al., 1996). Only CTX-A is transported further by retrograde transport to the intermediate compartment and the ER (Lencer et al., 1995; Majoul et al., 1996). CTX induces an increase in cAMP. As increase in cAMP had been reported to stimulate transport of CTX-A to the ER (Sandvig et al., 1996), we have compared transport of WT–CTX and a mutated form of CTX–K63 that lacks any ADP-ribosylating activity and therefore does not induce an increase of cellular cAMP.
Transport of both A-subunits, CTX-A–K63 and WT– CTX-A from the Golgi to ER-like structures occurred with a similar time course (Fig. 2). This indicates that under our conditions the stimulation of adenylate cyclase by CTX (Majoul et al., 1996) does not have a significant effect on the retrograde transport of the toxin. Unless otherwise mentioned, all subsequent experiments were carried out with the CTX–K63 mutant.
Figure 2.
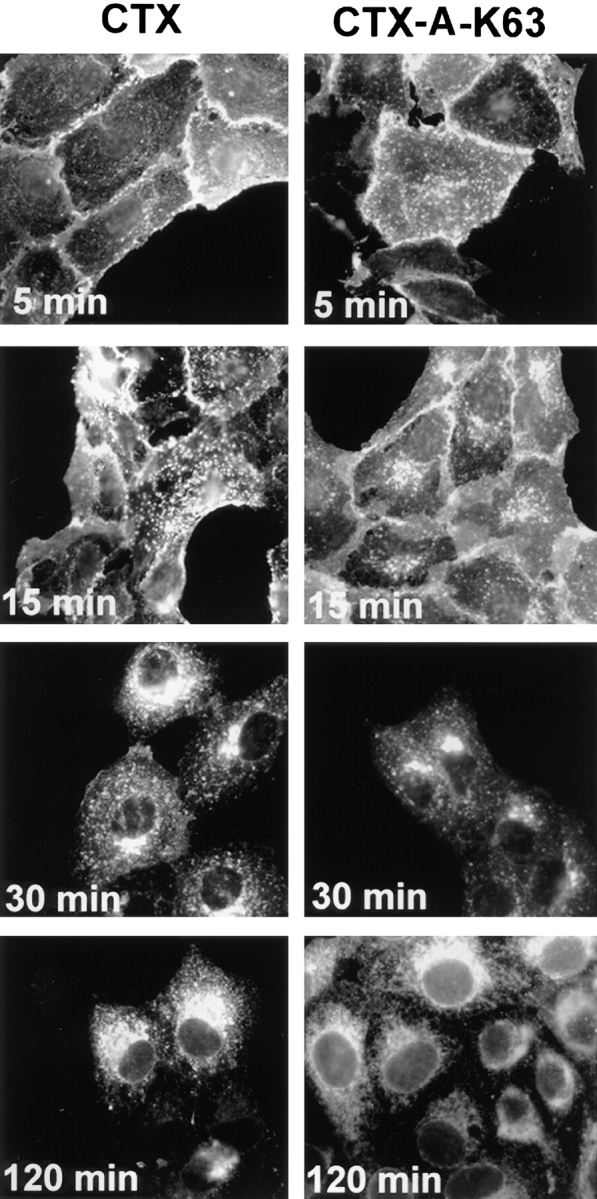
Time-dependent transport of wild-type CTX-A and mutant CTX-A (CTX-A–K63) from the plasma membrane to the ER in Vero cells after incubation with either wild-type CTX or CTX–K63, in which serine63 of the A subunit has been replaced by lysine. The initial concentration of the toxins was 0.5 μg/ml. Binding and initiation of uptake of wild-type or mutated CTX was performed as given in the Materials and Methods section (under “Methods”).
GTP-γ-S Inhibits Internalization of CTX–K63 As Well As Retrograde Transport of CTX-A–K63 from the Golgi
GTP-γ-S has been shown to inhibit intracellular vesicular transport at various steps (Melancon et al., 1987; Schwaninger et al., 1992; Tan et al., 1992; Taylor at al., 1992; Barlowe et al., 1993; Oka and Nakano, 1994; Barthel et al., 1995; Hidalgo et al., 1995). Microinjection of GTP-γ-S immediately before addition of CTX–K63 to the medium led to a retention of CTX–K63 at the plasma membrane and in endosomal structures under conditions where control cells showed an appearance of CTX-A–K63 in the Golgi (Table I).
Table I.
GTP-γ-S, But Not IgGs Directed Against Erd2p, β-COP, or p23 Inhibit the Transport of CTX–K63 from the Plasma Membrane (PM) to the Golgi
| Localization of CTX-A–K63 | ||||
|---|---|---|---|---|
| PM | Golgi | |||
| % of counted microinjected cells | ||||
| Controls | 5 | 95 | ||
| BSA | 6 | 94 | ||
| GTP-γ-S | 73 | 27 | ||
| Anti-Erd2p | 10 | 90 | ||
| Anti-β-COP | 7 | 93 | ||
| Anti-p23 | 12 | 88 | ||
The IgGs or GTP-γ-S were microinjected 10 min before initiating the uptake of CTX– K63 as in Fig. 2. The cells were fixed 40 min after start of CTX–K63 uptake and prepared for immunofluorescence using Cy3-labeled antibodies against CTX-A–K63. Cells microinjected with GTP-γ-S were identified by coinjection of dye-labeled BSA. Cells injected with the various antibodies were identified by coinjecting Cy2-labeled antibodies of the same kind that had been mixed with non-labeled antibodies at a 1:3 ratio. In each group 25–30 microinjected cells were analyzed.
When GTP-γ-S was microinjected 15–20 min after start of CTX uptake, i.e., when most of CTX-A had appeared in the Golgi (Majoul et al., 1996), CTX-A–K63 remained in perinuclear compartments or in perinuclear tubular-vesicular structures over the following 85 min, whereas under control conditions most of the CTX-A–K63 had arrived in the ER (Table II). Thus, GTP-γ-S inhibits internalization of CTX as well as transport of the A subunit of CTX from the Golgi to the ER.
Table II.
Effects of Microinjected GTP-γ-S or IgGs Directed Against Erd2p, β-COP, or p23 on the Retrograde Transport of CTX-A–K63 from the Golgi to the ER in Vero Cells
| Localization of CTX-A–K63 | ||||
|---|---|---|---|---|
| Golgi | ER | |||
| % of counted microinjected cells | ||||
| Controls | 5 | 95 | ||
| BSA | 6 | 94 | ||
| GTP-γ-S | 90 | 10 | ||
| Controls | 5 | 95 | ||
| Preimmune IgGs | 6 | 94 | ||
| Anti-Erd2p | 58 | 42 | ||
| Controls | 2 | 98 | ||
| Preimmune IgGs | 7 | 93 | ||
| Anti–β-COP IgGs | 74 | 26 | ||
| Controls | 4 | 96 | ||
| Preimmune IgGs | 9 | 91 | ||
| Anti–p23 IgGs | 72 | 28 | ||
Uptake of CTX–K63 (0.5 μg/ml) was initiated as in Fig. 2. After 15–20 min the cells were microinjected and the transport of CTX-A–K63 followed for another 80 min. The cells were then fixed and analyzed for the distribution of CTX-A–K63 by immunofluorescence. Microinjected cells were identified by either dye-labeled BSA mixed with GTP-γ-S, or by mixing Cy2-labeled primary IgGs to the corresponding antibodies at a 1:3 ratio. The effect of microinjection itself was controlled in experiments where preimmune IgGs were injected. In each group 25–30 microinjected cells were analyzed.
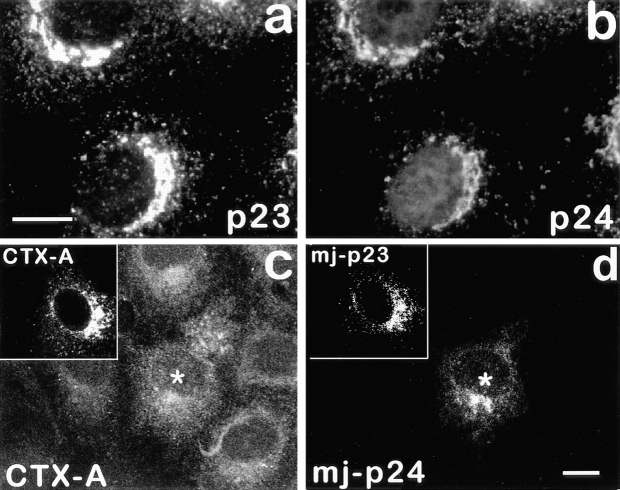
Cholera Toxin Induces a Transient Change in the Distribution of Erd2p
Arrival of CTX-A–K63 in the Golgi led to a dispersed distribution of Erd2p, which starts already 15 min after start of CTX–K63 uptake (Fig. 3). Giantin, a resident membrane protein of the Golgi (Linstedt and Hauri, 1993) remained in the more condensed perinuclear structures characteristic for Golgi cisternae under conditions where Erd2p showed a CTX-induced dispersion (Fig. 3). Apparently the increased occupancy of Erd2p resulting from the “wave” of CTX-A–K63 had led to a transient change of the steady-state distribution of Erd2p, most likely by an enhanced budding from the Golgi of vesicles containing occupied Erd2p. The dispersion of Erd2p was accompanied by a reproducible shift in the distribution of the ERGIC marker p53 (Fig. 4).
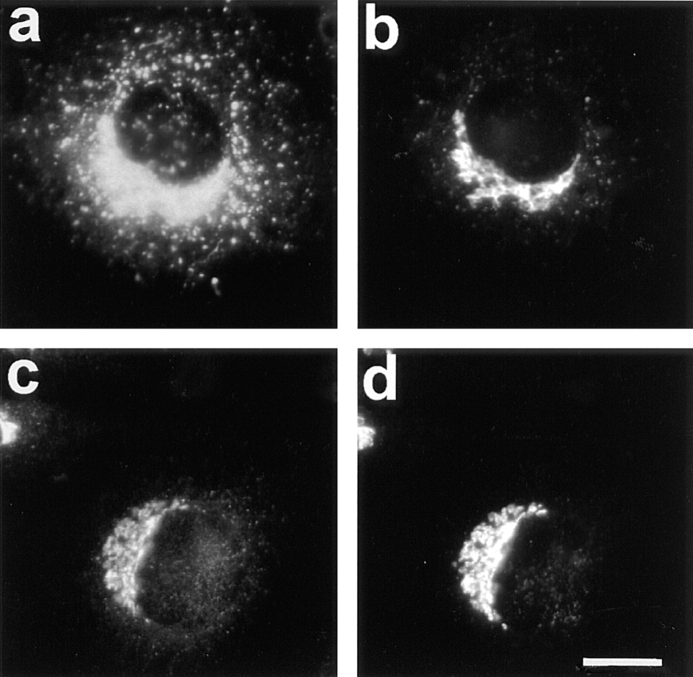
Figure 3.
Dispersion of Erd2p (a), but not of giantin (b) 40 min after start of uptake of CTX–K63 (0.5 μg/ml). (c and d) Distribution of Erd2p and giantin, respectively, in control cells incubated in the absence of CTX–K63. The experiment was performed as given in the legend to Fig. 2. Bar, 10 μm.
Figure 4.
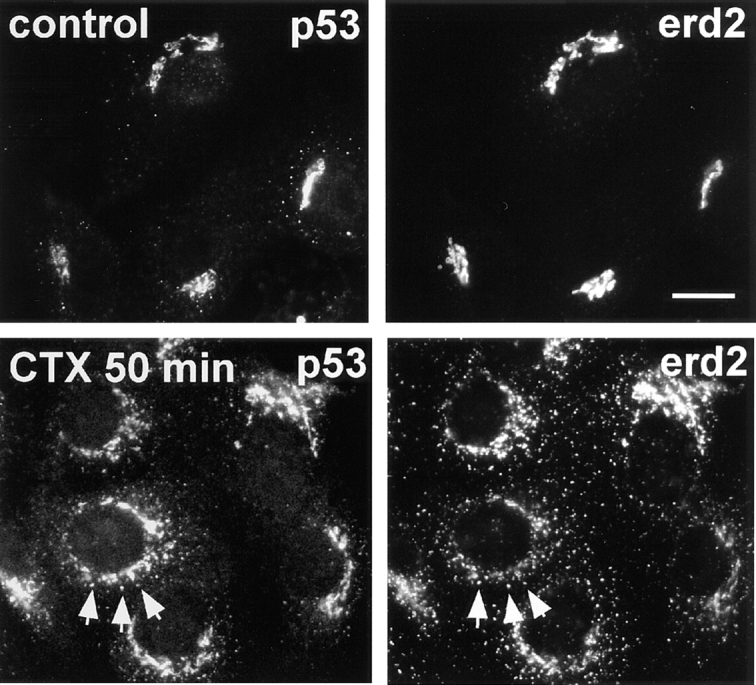
Uptake of CTX–K63 leads to a concomitant dispersion of Erd2p and p53 in Vero cells. Vero cells were incubated with 0.5 μg/ml CTX–K63 and the uptake started as in Fig. 2. At zero time (top panels) and after 50 min (bottom panels) cells were fixed and immunostained for Erd2p and p53 using Cy2-labeled anti-Erd2p (rabbit) and a mouse anti-p53 antibody. Note that Erd2p as well as p53 change from a cap-like to a more circular distribution in the presence of the KDEL cargo (CTX-A–K63). Bar, 10 μm.
While the distribution of Erd2p and mSEC13 (a member of the COPII complex), was clearly different in the absence of CTX (Fig. 5), Erd2p assumed an increased colocalization with mSEC13 between 45 and 100 min after start of CTX–K63 uptake (Fig. 6).
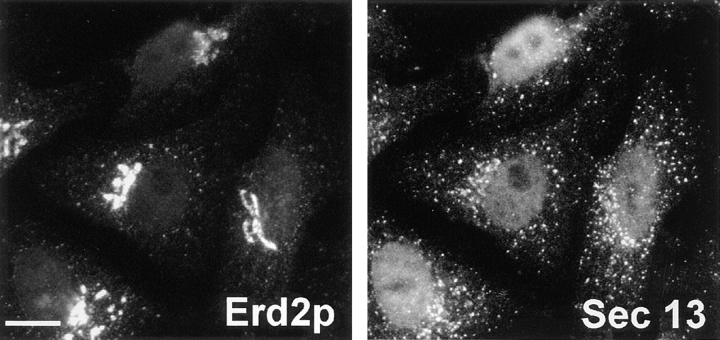
Figure 5.
Distribution of Erd2p and mSEC13 in Vero cells under steady-state conditions. Bar, 10 μm.
Figure 6.
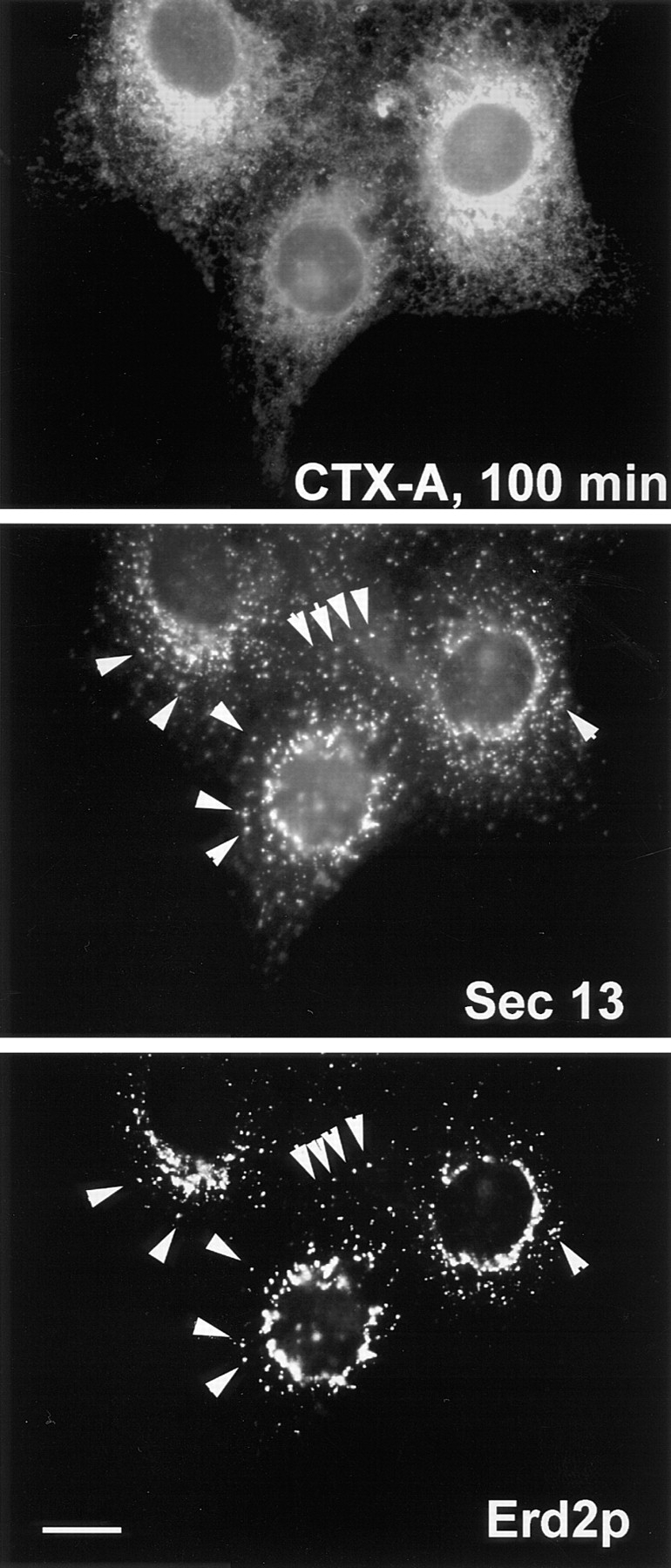
Retrograde transport of CTX-A–K63 induces an increased colocalization (arrows) of Erd2p and mSEC13. Cells were fixed and analyzed 100 min after start of CTX–K63 uptake by using triple immunofluorescence. Note that the distribution of mSEC13 shows only minor changes in the presence of CTX–K63 (compare with Fig. 5), but that Erd2p exhibits a shift from a Golgi-like distribution towards punctated structures costaining for mSEC13. Bar, 10 μm.
IgGs or Fab Fragments Directed Against the COOH Terminus of Erd2p Inhibit Transport of CTX-A–K63 from the Golgi to the ER and the CTX-K63–induced Translocation of Erd2p and p53
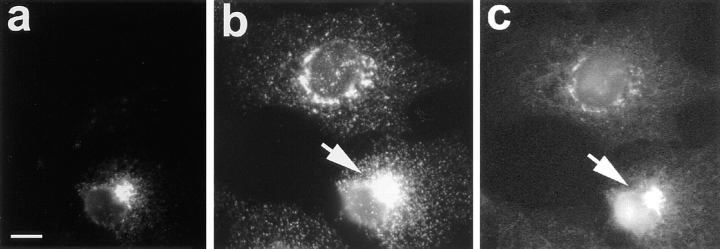
To analyze for a possible contribution of the cytoplasmically oriented COOH terminus of Erd2p to the sorting and the retrograde transport of the occupied Erd2p we analyzed the effect of antibodies raised against the COOH terminus of Erd2p on the retrograde transport of CTX-A–K63. Anti-Erd2p IgGs injected before start of CTX– K63 uptake, did not significantly affect the retrograde transport from the plasma membrane to the Golgi (Table I). However, when the IgGs were injected 10–20 min after start of CTX–K63 uptake, we observed in ∼60% of the cells an accumulation of CTX-A–K63 in perinuclear structures and an inhibition of translocation of CTX-A–K63 from the Golgi to the ER (Table I). When anti-Erd2p Fab fragments were injected 5 min before start of CTX uptake, CTX-A–K63 became also retained in Golgi-like structures (Fig. 7) in 12 out of 15 randomly chosen intact microinjected cells. Microinjection of BSA or preimmune IgGs did not have a significant effect under the same conditions (Table II). The CTX-K63–induced translocation of Erd2p as well as that of p53 was also strongly inhibited by microinjected anti-Erd2p Fab fragments (Fig. 8).
Figure 7.
Fab fragments directed against the cytoplasmic tail of Erd2p inhibit retrograde transport of CTX-A–K63 from the Golgi to the ER in Vero cells. The Fab fragments were microinjected 10–15 min before uptake of CTX– K63 was initiated as given in the Materials and Methods section. The cells were further incubated for 120 min. The cells were then fixed and immunostained for CTX-A (a and c). The microinjected cells (a and b, arrows) were identified by the immunofluorescence of Cy2-labeled anti–Erd2p Fab fragments that had been mixed at a 1:3 ratio with non-labeled anti– Erd2p Fab fragments (b and d). Note the retention of CTX-A–K63 in perinuclear structures in cells microinjected with the anti–Erd2p Fab fragments. a and b show the results of laser scan microscopy; c and d show the results of conventional immunofluorescence microscopy. Bar, 10 μm.
Figure 8.
Antibodies against Erd2p inhibit the CTX-K63–induced dispersion of Erd2p and of p53. The experiment was performed as in Fig. 7. 50 min after start of CTX– K63 uptake, the cells were fixed and processed for staining with anti-Erd2p and anti-p53 (triple immunofluorescence). (a) microinjected cell stained by microinjected Cy2-labeled anti–Erd2p Fab fragments; (b) staining for Erd2p after fixation and permeabilization with Cy3-labeled anti-Erd2p antibodies; (c) staining for p53 using mouse anti-p53 and an AMCA-labeled secondary anti–mouse IgG antibody. Note the strong reduction of dispersion of Erd2p and p53 in the anti-Erdp–injected cell. Bar, 10 μm.
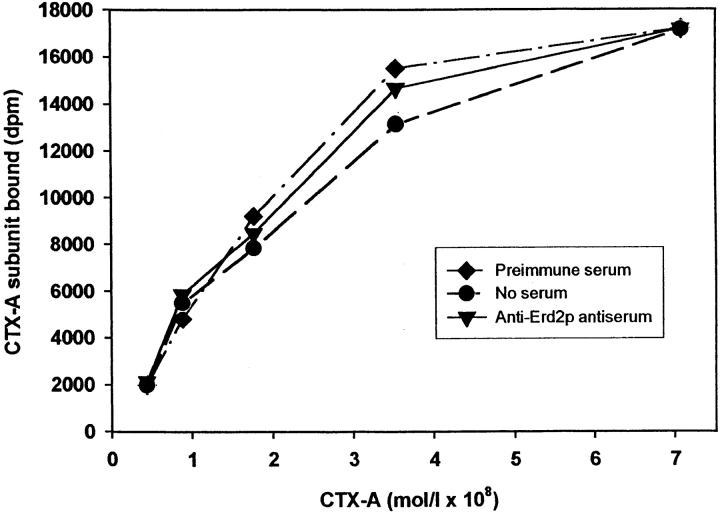
To exclude the possibility that the anti-Erd2p–induced inhibition of retrograde transport of CTX-A–K63 from the Golgi to the ER had resulted from a decreased binding of CTX-A–K63 to Erd2p, we measured the effect of the anti-Erd2p antibodies on the binding of 125I-labeled CTX-A to permeabilized Golgi vesicles from Sf9 cells overexpressing human Erd2p. The antibody binding was performed at pH 7.2, the binding of CTX-A to Erd2p at pH 6. At pH 6 the binding of CTX-A to Erd2p was still sufficiently high (∼60% of the binding observed at pH 5, unpublished results). Preincubation of the permeabilized Golgi membranes with anti-Erd2p antibodies did not affect binding of CTX-A to Erd2p (Fig. 9). Binding of CTX-A was specific as CTX-A could be displaced from the membranes by low concentrations of KDEL peptides but not by peptides without a COOH-terminal KDEL sequence (results not shown here). Therefore, inhibition of retrograde transport of CTX-A from the Golgi by microinjected anti-Erd2p antibodies results from an impaired sorting of the occupied receptor into COPI-coated vesicles or from an impaired vesicle budding rather than from a decreased binding of CTX-A to Erd2p.
Figure 9.
Binding of CTX-A to Erd2p of Golgi membranes from Sf9 cells is not affected by antibodies directed against the cytoplasmic tail of Erd2p. Erd2p was overexpressed in Sf9 cells using Bacculovirus infection. Golgi membranes from overexpressing Sf9 cells were prepared and permeabilized as given in the Materials and Methods section and incubated at pH 7.2 with preimmune serum, antiserum directed against the cytoplasmic tail of Erd2p, or without serum. The pH was then adjusted to 6.0 and the binding of increasing concentrations of 125I–CTX-A was measured. Blanks without membranes (ranging from ∼200 dpm to ∼1,800 dpm for the different concentrations of labeled CTX-A used) were subtracted. The binding curves obtained after the three different preincubation conditions show only minor (<5%) differences.
IgGs or Fab Fragments Directed Against β-COP (Anti-EAGE) Inhibit the Retrograde Transport of CTX-A–K63 from the Golgi to the Intermediate Compartment and the ER
It has been shown previously (Pepperkok et al., 1993) that a specific anti–β-COP antibody (anti-EAGE; Duden et al., 1991; Pepperkok et al., 1993) inhibits the anterograde transport of the VSV-G protein from the ER to the Golgi. If the occupied Erd2p would undergo retrograde transport from the Golgi to the ER via COPI-coated vesicles, anti-EAGE antibodies should also inhibit this transport step. When affinity-purified antibodies were injected before CTX–K63 uptake the transport of CTX-A–K63 to the Golgi as measured 30 min after start of CTX uptake was not impaired (Table I). When antibodies were injected ∼20 min after start of CTX–K63 uptake and subsequently incubated for another 80 min before fixation and analysis by immunofluorescence CTX-A–K63 was mostly retained in compact perinuclear structures similar to those stained with antibodies against Erd2p, indicating that CTX-A–K63 was retained in the Golgi (Table II) in contrast to non-injected or mock-injected cells where CTX-A–K63 at this time point was observed in reticular structures, sometimes also in structures resembling the intermediate compartment (p53 compartment) (Table II). When the antibodies were injected later, namely 45–60 min after start of CTX uptake, only very little or no inhibition of transport of CTX-A–K63 from the Golgi to the IC and the ER was observed (results not shown here), indicating that once coatomer recruitment and vesicle budding had occurred, the anti–β-COP antibody was without effect.
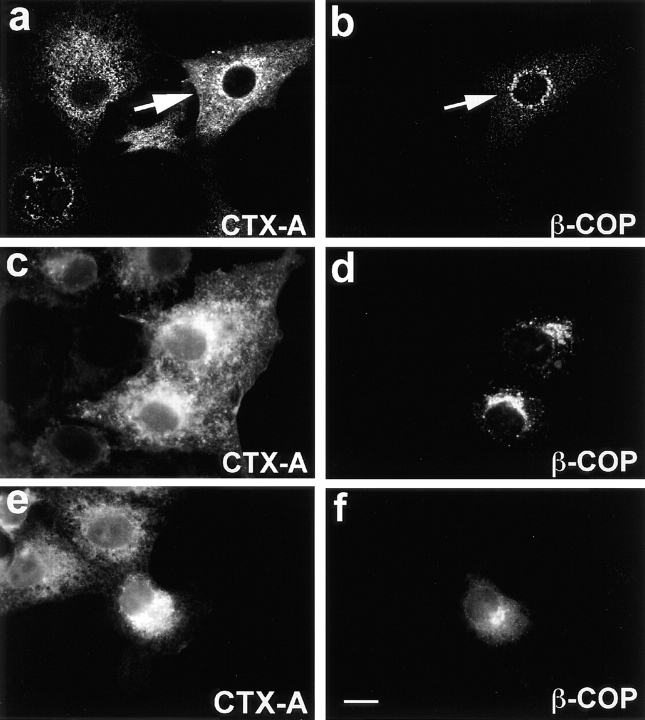
An inhibitory effect of anti–β-COP IgGs due to unspecific “cross-linking” could be excluded as anti-EAGE Fab fragments injected 5 min before the start of CTX uptake, inhibited also the translocation of CTX-A–K63 from the Golgi to the ER in 16 out of 20 randomly chosen intact microinjected cells (Fig. 10).
Figure 10.
Fab fragments from IgGs directed against the EAGE peptide of β-COP inhibit the translocation of CTX-A–K63 from the Golgi to the ER in Vero cells. Fab fragments were microinjected 10–15 min before starting the uptake of CTX– K63 as in Fig. 2. Incubation of the cells was continued for another 120 min. The cells were then fixed and processed for double immunofluorescence. Microinjected cells are identified by immunofluorescence of Cy2-labeled anti–β-COP Fab fragments that had been mixed with the unlabeled anti–β-COP Fab fragments at a 1:3 ratio. a, c, and e show CTX-A–K63; b, d, and f β-COP. a and b were obtained by laser scan microscopy (microinjected cell indicated by arrow); c–f were obtained by conventional immunofluorescence microscopy. Bar, 10 μm.
IgGs and Fab Fragments Directed Against p23 Inhibit Retrograde Transport of CTX-A–K63 from the Golgi, But Not Its Internalization
Recruitment of COPI proteins to the Golgi membrane seems to require not only ADP ribosylating factor (ARF) GTP, but also their direct interaction with the cytoplasmically oriented COOH termini of either transmembrane cargo proteins or of proteins of the p24 protein family (Stamnes et al., 1995; Fiedler et al., 1996; Sohn et al., 1996). It appears that members of the p24 protein family are particularly involved in the recruitment of COP proteins and thus in the budding of transport vesicles (for a review see Gaynor et al., 1998). Recently, Blum et al. (1996), Sohn et al. (1996), and Rojo et al. (1997) have described a new member of this family, designated p23. Sohn et al. (1996) have demonstrated that the interaction of p23 with coatomers does not only depend on its COOH-terminal KKLIE sequence but also on two sequential phenylalanines positioned 8 and 9 amino acids upstream from the COOH-terminal glutamic acid. They have proposed that p23 might be particularly involved in vesicular transport from the Golgi back to the ER (Sohn et al., 1996). This was supported by the observation that p23 was enriched in Golgi-derived COPI-coated vesicles (Sohn et al., 1996). As the role of p23 in retrograde transport of KDEL proteins from the Golgi to the ER has not been studied so far, we have analyzed the effects of microinjection of antibodies directed against the COOH terminus of p23 on the transport of CTX–K63. When injected before uptake of CTX– K63, no significant effect of the anti-p23 IgGs on transport of CTX-A–K63 from the plasma membrane to Golgi-like structures was observed (Table I), a result similar to that observed with anti–β-COP antibodies. This indicates that neither β-COP nor p23 are involved in a rate-limiting step of transport of CTX–K63 to the Golgi compartment.
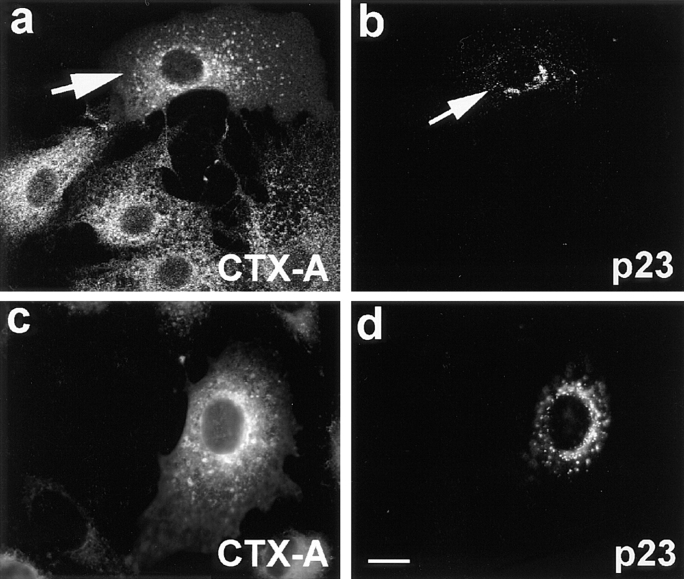
In further experiments, we microinjected anti-p23 IgGs ∼15–20 min after start of CTX–K63 uptake, when a considerable amount of CTX-A–K63 had already reached the Golgi. Cells were then incubated for another 80 min and subsequently analyzed for the distribution of CTX-A–K63. As a result, a significant inhibition of the translocation of CTX-A from the Golgi to the intermediate compartment and to the ER occurred (Table II). CTX-A–K63 accumulated in structures overlapping with the Golgi staining by anti-p23 antibodies. In view of the high concentration of p23 in Golgi or IC membranes (Sohn et al., 1996; Rojo et al., 1997) cross-linking of p23 by the anti-p23 IgGs had to be considered as the reason for an unspecific effect on the retrograde transport of CTX–K63. However, microinjection of anti–p23 Fab fragments led also to an inhibition of the translocation of CTX-A–K63 from the Golgi to the ER in 13 out of 15 randomly selected intact microinjected cells (Fig. 11). The distribution of CTX-A–K63 in the presence of anti-p23 IgGs or Fab fragments resembled that observed with anti–β-COP antibodies.
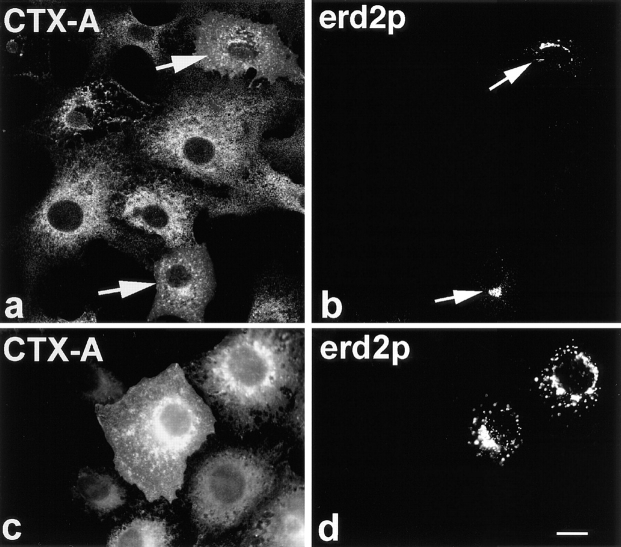
Figure 11.
Fab fragments from IgGs directed against the COOH-terminal tail of p23 inhibit the retrograde transport of CTX-A–K63 from the Golgi to the ER in Vero cells. The Fab fragments were microinjected 10–15 min before starting CTX–K63 uptake. The cells were further incubated for 120 min as in Fig. 2 followed by fixation and double-immunofluorescence microscopy. (a and c) CTX-A–K63; (b and d) p23. Microinjected cells are identified using Cy2-labeled anti–p23 Fab fragments that mixed with unlabeled anti-p23 at a 1:3 ratio. a and b were obtained by laser scan microscopy (microinjected cell indicated by arrows); c and d were obtained by conventional immunofluorescence microscopy. Bar, 10 μm.
p24 shares with p23 the di-phenylalanine motif in the COOH terminus. It does not possess a COOH-terminal KKXX or KKXXX motif (Stamnes et al., 1995), but appears to bind coatomer (Fiedler et al., 1996). The cytoplasmic tail of p23 (Nickel et al., 1997) but not that of p24 (Fiedler et al., 1996) is able to retrieve the corresponding fusion proteins with CD8 (CD8-p23, CD8-p24) from post-ER compartments to the ER. Therefore, we have compared the effects of anti-p23 antibodies with those of anti-p24 antibodies on the retrograde transport of CTX-A–K63 from the Golgi to the ER. When antibodies raised against a peptide comprising the 12 COOH-terminal amino acids of p24 were injected into Vero cells about 20 min after the start of CTX–K63 uptake, transport of CTX-A–K63 from the Golgi to the ER was not significantly impaired (result from 20 randomly evaluated microinjected cells) although the binding of the antibodies to Golgi-like structures occurred (Fig. 12). Injection of anti-p23 into cells from the same coverslips was clearly inhibitory (Fig. 12).
Figure 12.
Comparison of effects of antibodies directed against the COOH termini of p23 and p24 on retrograde transport of CTX-A–K63 from the Golgi to the ER. (a and b) Vero cells that were neither treated with CTX– K63 nor microinjected, were fixed, permeabilized, and then immunostained for p24 or p23 using Cy5- and Cy2- labeled primary antibodies, respectively. Both antibodies stain similar structures, mainly representing Golgi membranes. (c and d) Vero cells were loaded with CTX–K63 as given in Fig. 2. About 20 min after the start of CTX– K63 uptake, antibodies against p24 were microinjected. As control, cells from the same coverslip were injected with antibodies directed against the COOH terminus of p23 (inset). Cells were then incubated for another 80 min, fixed, and then analyzed for the distribution of CTX-A–K63 (c) or p24 (d) or p23 (inset in d) by laser scan immunofluorescence microscopy. The cell microinjected with the anti-p24 antibodies is marked by an asterisk. Bar, 10 μm.
Discussion
After uptake of CTX, its A subunit is dissociated and transported from the Golgi to the ER as an individual protein (Majoul et al., 1996). Specificity of this transport is conferred by the COOH-terminal sequence KDEL that is recognized within the lumen of the Golgi by Erd2p. The demonstration of Erd2p in Golgi-derived COPI vesicles (Sönnichsen et al., 1996; Orci et al., 1997) has indicated that the retrograde transport of Erd2p could occur via COPI-coated vesicles. However, the direct involvement of COPI vesicles in the retrograde transport of a KDEL protein from the Golgi to the ER has shown here for the first time in a functional assay using the retrograde transport of a KDEL protein. Microinjected GTP-γ-S inhibited the transport of CTX-A from the plasma membrane to the Golgi as well as the transport from the Golgi to the ER.
To date it is unclear whether transport steps of the early secretory pathway are constitutive or regulated, e.g., stimulated by an increased occupancy of receptor molecules involved. The transient translocation of Erd2p after uptake of CTX–K63 indicates that the increased occupancy of Erd2p enhances the retrograde transport of the occupied receptor. The fact that giantin did not participate in this translocation but kept its Golgi-like distribution underlines the specificity of the sorting of Erd2p into retrograde vesicles under the conditions of our experiment.
Whereas before internalization of CTX–K63, Erd2p was located mainly in Golgi-like structures that clearly differed from the punctated structures characterized by mSEC13, the arrival of CTX-A–K63 in the Golgi induced a preferential colocalization of Erd2p with mSEC13 (Fig. 6). Some of the punctated structures harboring Erd2p as well as mSEC13 may represent COPII vesicles transporting Erd2p from the IC back to the cis-Golgi.
Townsley et al. (1993) have shown previously that mutations in very different positions of Erd2p inhibit or abolish the translocation of the overexpressed Erd2p from the Golgi to the ER. Here we show for the first time that IgGs and the corresponding Fab fragments directed against the COOH-terminal part of the cytoplasmic tail of Erd2p inhibit the retrograde transport of a KDEL ligand. Interestingly, anti–Erd2p Fab fragments inhibited or abolished not only the CTX-K63–induced dispersion of Erd2p itself, but also that of p53, a protein known to participate in the binding of COPI proteins (Tisdale et al., 1997). This indicates that the increased occupancy of Erd2p does not simply induce an increased sorting of the occupied Erd2p into COPI-coated vesicles but leads also to an increased formation of these vesicles. How Erd2p might participate in the recruitment of coatomer to the Golgi membrane or in vesicle budding remains to be established. It may be that the interaction of the antibodies with the COOH terminus of Erd2p interferes with the homo-oligomerization of the occupied Erd2p. Such a homo-oligomerization has been proposed by Townsley et al. (1993), but has not yet been demonstrated. Our binding studies with 125I–CTX-A make it unlikely that the inhibition could have resulted from a decreased binding of our cargo KDEL protein (CTX-A–K63) to Erd2p.
On the basis of microinjection experiments with IgGs against β-COP it had been concluded, that COPI-coated vesicles were involved in anterograde transport at some step between ER and Golgi (Pepperkok et al., 1993). On the other hand, inhibition of COPI-directed ER–cis-Golgi transport in yeast is cargo selective, whereas inhibition of COPII-directed transport is not (Gaynor and Emr, 1997). This could indicate that the inhibition of anterograde ER– Golgi transport had resulted indirectly from an inhibition of COPI-dependent retrograde transport from the cis-Golgi to the ER leading to a depletion in the ER of factors necessary for sorting and/or packaging of cargo into COPII-coated vesicles. Here we show for the first time that antibodies or Fab fragments directed against β-COP inhibit indeed a retrograde transport step from the Golgi towards the ER. The fact that anti–β-COP antibodies had little or no inhibitory effect on CTX-A–K63 transport when applied after most of CTX-A–K63 had left the Golgi (45–60 min after start of CTX–K63 uptake), indicates that the antibodies act most likely by inhibition of either coatomer recruitment or the budding process itself.
Although a function of COPI proteins, notably β-COP in early endocytosis, has been described (Guo et al., 1994; Daro et al., 1997), we did not observe an inhibition of transport of CTX-A–K63 from the plasma membrane to the Golgi by anti-EAGE antibodies. This would be in line with CTX uptake occurring via caveolae (Orlando and Fishman, 1998; Wolf et al., 1998) rather than via early endosomes.
p23, a member of the p24 protein family (Sohn et al., 1996) was proposed to function as a coatomer receptor in the Golgi, because the protein is strikingly enriched in COPI vesicles when compared with their donor Golgi membranes and its cytoplasmic domain binds to coatomer (Sohn et al., 1996). Therefore, inhibition of retrograde Golgi–ER transport of CTX-A–K63 by microinjected antibodies directed against the cytoplasmic tail of p23 results most likely from inhibition of coatomer recruitment or of budding of COPI vesicles. The role of p23 in the recruitment of coatomers has been questioned by Rojo et al. (1997) who describe a de-enrichment of p23 in COPI-coated buds and vesicles and no inhibition of coatomer binding to p23-enriched membranes by antibodies directed against the COOH terminus of p23. However, under the conditions applied by Rojo et al. (1997) one might expect a decreased binding of anti-p23 antibodies to buds and COPI-coated vesicles when analyzed by immuno-EM as the binding of coatomer to p23 most likely competes with the binding of antibodies to p23. Nevertheless, these authors observed an inhibition of the anterograde transport of VSV-G by this anti-p23 antibody.
In contrast to the inhibition of VSV-G transport by anti-p23 IgGs, Rojo et al. (1997) could not observe a transport inhibition by Fab fragments of the same antibodies. From that they concluded that part of the transport inhibition by anti-p23 IgGs might have resulted from a physical change in the IC membranes because of a cross-linking of p23 molecules by the bivalent antibodies. In our experiments, however, we observed a clear-cut inhibitory effect of anti– p23 Fab fragments on CTX-A–K63 transport. The discrepancy may have technical reasons.
p24, another member of the p24 protein family which is found under steady-state conditions mainly associated with cis-Golgi structures but also with COPI vesicles, seems likewise to be able to bind coatomer (Stamnes et al., 1995; Fiedler et al., 1996; Dominguez et al., 1998) although it possesses a COOH-terminal RRVV instead of a KKXX motif. In our experiments, microinjection of antibodies directed against the cytoplasmic domain of p24, in contrast to p23, did not inhibit translocation of the retrograde KDEL cargo protein, although the antibodies bound efficiently to p24 (Fig. 12). We raised these antibodies against the 10 COOH-terminal amino acids of the cytoplasmic tail of p24 that consists of only 12 amino acid residues, expecting that their binding in vivo to p24 should compete with any physiological function associated with the COOH-terminal tail of p24. However, we have to consider the possibility that the discrepancies between the actions of anti-p23 and anti-p24 antibodies had simply resulted from an insufficient binding or neutralizing action of the anti-p24 antibodies. Further experiments are needed before it can be concluded that p23, but not p24 is involved in COPI-mediated retrograde transport from the Golgi.
Acknowledgments
The authors wish to thank R. Duden, Cambridge, UK for extensive and helpful discussions; and H.P. Hauri, Basel, Switzerland and B.L. Tang, Singapore for the generous gift of antibodies against giantin and p53, and against mSEC13, respectively.
This work was supported by grants by of the Deutsche Forschungsgemeinschaft (grant No. 43/56-1) and the Fonds der Chemischen Industrie given to H.-D. Söling.
Abbreviations used in this paper
- BFA
brefeldin A
- COPI and COPII
coat protein I and II
- CTX
cholera toxin
- CTX-A
cholera toxin A subunit
- IC
intermediate compartment
References
- Barlowe C, d'Enfert C, Schekman R. Purification and characterization of SAR1p, a small GTP-binding protein required for transport vesicle formation from the endoplasmic reticulum. J Biol Chem. 1993;268:873–879. [PubMed] [Google Scholar]
- Barthel A, Nickel W, Tonko C, Söling HD. Sorting and budding of constitutive secretory vesicles in hepatocytes and hepatoma cells. Adv Enzyme Regul. 1995;35:283–292. doi: 10.1016/0065-2571(94)00021-t. [DOI] [PubMed] [Google Scholar]
- Bastiaens PIH, Majoul IV, Verveer PJ, Söling HD, Jovin TM. Imaging the intracellular trafficking and state of the AB5 quarternary structure of cholera toxin. EMBO (Eur Mol Biol Organ) J. 1996;15:4243–4248. [PMC free article] [PubMed] [Google Scholar]
- Belden WJ, Barlowe C. Erv25p, a component of COP II-coated vesicles, forms a complex with emp24p that is required for efficient endoplasmic reticulum to Golgi transport. J Biol Chem. 1996;271:26939–26946. doi: 10.1074/jbc.271.43.26939. [DOI] [PubMed] [Google Scholar]
- Blum R, Feick P, Puype M, Vanderkerckhove J, Klengel R, Mystainczyk W, Schulz I. Tmp21 and p24A. Two type I proteins enriched in pancreatic microsomal membranes, are members of a family involved in vesicular trafficking. J Biol Chem. 1996;27:17183–17189. doi: 10.1074/jbc.271.29.17183. [DOI] [PubMed] [Google Scholar]
- Cosson P, Letourneur F. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science. 1994;263:1629–1631. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- Daro E, Sheff D, Gomez M, Kreis T, Mellman I. Inhibition of endosome function in CHO cells bearing a temperature-sensitive defect in the coatomer COPI component ε-COP. J Cell Biol. 1997;139:1747–1759. doi: 10.1083/jcb.139.7.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez M, Dejgaard K, Füllekrug J, Dahan S, Fazel A, Paccaud J-P, Thomas DY, Bergeron JJM, Nilsson T. gp25L/emp24/p24 protein family members of the cis-Golgi network bind both COPI and II coatomer. J Cell Biol. 1998;140:751–765. doi: 10.1083/jcb.140.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duden R, Griffiths G, Frank R, Argos P, Kreis TE. β-COP, a 110 kd protein associated with non-clathrin-coated vesicles and the Golgi complex, shows homology to β-adaptin. Cell. 1991;64:649–665. doi: 10.1016/0092-8674(91)90248-w. [DOI] [PubMed] [Google Scholar]
- Elrod-Erickson MJ, Kaiser CA. Genes that control fidelity of endoplasmic reticulum to Golgi transport identified as suppressors of vesicle budding mutations. Mol Biol Cell. 1996;7:1043–1058. doi: 10.1091/mbc.7.7.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K, Veit M, Stamnes MA, Rothman JE. Bimodal interaction of coatomer with the p24 family of putative cargo receptors. Science. 1996;273:1396–1399. doi: 10.1126/science.273.5280.1396. [DOI] [PubMed] [Google Scholar]
- Fontana MR, Manetti R, Gianelli V, Magagnoli C, Marchini A, Olivieri R, Domenghini M, Rappuoli R, Pizza M. Construction of nontoxic derivatives of cholera toxin and characterization of the immunological response against the A subunit. Infect Immun. 1995;63:2356–2360. doi: 10.1128/iai.63.6.2356-2360.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor EC, Emr SD. COPI-independent anterograde transport: cargo-selective ER to Golgi protein transport in yeast COPI mutants. J Cell Biol. 1997;136:789–802. doi: 10.1083/jcb.136.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor, E.C., T.R. Graham, and S.D. Emr. 1998. COPI in ER/Golgi and intra-Golgi transport: do yeast COPI mutants point the way? Biochim. Biophys. Acta. In press. [DOI] [PubMed]
- Guo Q, Vasile E, Krieger M. Disruptions in Golgi structure and membrane traffic in a conditional lethal mammalian cell mutant are corrected by ε-COP. J Cell Biol. 1994;125:1213–1224. doi: 10.1083/jcb.125.6.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Ericsson M, Krijnse-Locker J, Nilsson T, Goud B, Söling HD, Tang BL, Wong SH, Hong W. Localization of the Lys,Asp,Glu,Leu tetrapeptide receptor to the Golgi complex and the intermediate compartment in mammalian cells. J Cell Biol. 1994;127:1557–1574. doi: 10.1083/jcb.127.6.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo J, Muniz M, Velasco A. Trimeric G proteins regulate the cytosol-induced redistribution of Golgi enzymes into the endoplasmic reticulum. J Cell Sci. 1995;108:1805–1815. doi: 10.1242/jcs.108.4.1805. [DOI] [PubMed] [Google Scholar]
- Jackson MR, Nilsson T, Peterson PA. Retrieval of transmembrane proteins to the endoplasmic reticulum. J Cell Biol. 1993;12:317–333. doi: 10.1083/jcb.121.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicot M, Desbuquois B. Fate of injected 125I-labeled cholera toxin taken up by rat liver in vivo. Generation of the active A1peptide in the endosomal compartment. Eur J Biochem. 1987;163:433–442. doi: 10.1111/j.1432-1033.1987.tb10816.x. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lingwood CA, Williams DB, Furuya W, Manolson MF, Grinstein S. Dynamic measurement of the pH of the Golgi complex in living cells using retrograde transport of the verotoxin receptor. J Cell Biol. 1996;134:1387–1399. doi: 10.1083/jcb.134.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CY, Cancedda F, Duffy LK. ADP-ribosyl transferase activity of cholera toxin polypeptide A1 and the effect of limited trypsinolysis. Biochem Biophys Res Commun. 1981;102:1021–1027. doi: 10.1016/0006-291x(81)91640-5. [DOI] [PubMed] [Google Scholar]
- Lencer WI, Constable C, Moe S, Jobling MG, Webb HM, Ruston S, Madara JL, Hirst TR, Holmes RK. Targeting of cholera toxin and Escherichia coliheat labile toxin in polarized epithelia: Role of COOH-terminal KDEL. J Cell Biol. 1995;131:951–962. doi: 10.1083/jcb.131.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F, Gaynor EC, Hennecke S, Demolliere C, Duden R, Emr RSD, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Pelham HRB. Ligand-induced redistribution of a human KDEL receptor from the Golgi complex to the endoplasmic reticulum. Cell. 1992;68:353–364. doi: 10.1016/0092-8674(92)90476-s. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Pelham HRB. SNARE-mediated retrograde traffic from the Golgi complex to the endoplasmic reticulum. Cell. 1996;85:205–215. doi: 10.1016/s0092-8674(00)81097-1. [DOI] [PubMed] [Google Scholar]
- Linstedt AD, Hauri HP. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol Biol Cell. 1993;4:679–693. doi: 10.1091/mbc.4.7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majoul IV, Bastaiens PIH, Söling HD. Transport of an external Lys-Asp-Glu-Leu (KDEL) protein from the plasma membrane to the endoplasmic reticulum: Studies with cholera toxin in Vero cells. J Cell Biol. 1996;133:777–789. doi: 10.1083/jcb.133.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melancon P, Glick BS, Malhotra V, Weidman PJ, Serafini T, Gleason ML, Orci L, Rothman JE. Involvement of GTP-binding “G” proteins in transport through the Golgi stack. Cell. 1987;51:1053–1062. doi: 10.1016/0092-8674(87)90591-5. [DOI] [PubMed] [Google Scholar]
- Miesenböck G, Rothman JE. The capacity to retrieve escaped ER proteins extends to the trans-most cisterna of the Golgi stack. J Cell Biol. 1995;129:309–319. doi: 10.1083/jcb.129.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Pelham HRB. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1979;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Nambiar MP, Oda T, Chen C, Kuwazuru Y, Wu HC. Involvement of the Golgi region in the intracellular trafficking of cholera toxin. J Cell Physiol. 1993;154:222–228. doi: 10.1002/jcp.1041540203. [DOI] [PubMed] [Google Scholar]
- Nickel W, Sohn T, Bünning C, Wieland FT. p23, a major COPI-vesicle membrane protein, constitutively cycles though the early secretory pathway. Proc Natl Acad Sci USA. 1997;94:11393–11398. doi: 10.1073/pnas.94.21.11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, Nakano A. Inhibition of GTP hydrolysis by Sar1p causes accumulation of vesicles that are a functional intermediate of the ER-to-Golgi transport in yeast. J Cell Biol. 1994;124:425–434. doi: 10.1083/jcb.124.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Stamnes M, Ravazzola M, Amherdt M, Perrelet A, Söllner TH, Rothman JE. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell. 1997;90:335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- Orlandi PA, Curran PK, Fishman PH. Brefeldin A blocks the response of cultured cells to cholera toxin. Implications for intracellular traficking in toxin action. J Biol Chem. 1993;268:12010–12016. [PubMed] [Google Scholar]
- Orlandi PA, Fishman PH. Filipin-dependent inhibition of cholera toxin: Evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol. 1998;141:905–916. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham HRB. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1994;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Pepperkok R, Scheel J, Horstmann H, Hauri HP, Griffiths G, Kreis TE. β-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell. 1993;74:71–82. doi: 10.1016/0092-8674(93)90295-2. [DOI] [PubMed] [Google Scholar]
- Rojo M, Pepperkok R, Emery G, Kellner R, Stang E, Parton RG, Gruenberg J. Involvement of the transmembrane protein p23 in biosynthetic protein transport. J Cell Biol. 1997;139:1119–1135. doi: 10.1083/jcb.139.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K, Garred O, van Deurs B. Thapsigargin-induced transport of cholera toxin to the endoplasmic reticulum. Proc Natl Acad Sci USA. 1996;93:12339–12343. doi: 10.1073/pnas.93.22.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Niskikawa S, Nakano A. Membrane protein retrieval from the Golgi apparatus to the endoplasmic reticulum (ER): characterization of the RER1gene products as a component involved in ER localization of Sec12p. Mol Biol Cell. 1995;6:1459–1477. doi: 10.1091/mbc.6.11.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Sato K, Nakano A. Endoplasmic reticulum localization of Sec12p is activated by two mechanisms: Rer1p-dependent retrieval that requires the transmembrane domain and Rer1p-independent retention that involves the cytoplasmic domain. J Cell Biol. 1996;134:279–293. doi: 10.1083/jcb.134.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler J, Schwarzmann G, Knack I, Rohm K-H, Wiegandt H. Studies of ligand binding to cholera toxin; III, cooperativity of oligosaccharide binding. Hoppe-Seylers Z Physiol Chem. 1978;359:719–723. doi: 10.1515/bchm.1978.359.1.719. [DOI] [PubMed] [Google Scholar]
- Scheel J, Pepperkok R, Lowe M, Griffiths G, Kreis TE. Dissociation of coatomer from membranes is required for brefeldin A-induced transfer of Golgi enzymes to the endoplasmic reticulum. J Cell Biol. 1997;137:319–333. doi: 10.1083/jcb.137.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmöller F, Singer-Krüger B, Schröder S, Krüger U, Barlowe C, Riezman H. The absence of Emp24p, a component of ER-derived COPII-coated vesicles, causes a defect in transport of selected proteins to the Golgi. EMBO (Eur Mol Biol Organ) J. 1995;14:1329–1339. doi: 10.1002/j.1460-2075.1995.tb07119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder S, Schimmöller F, Singer-Krüger B, Riezman H. The Golgi localization of yeast Emp47p depends on its dilysine motif but is not affected by the ret-1 mutation in α-COP. J Cell Biol. 1995;131:895–912. doi: 10.1083/jcb.131.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutze MP, Peterson PA, Jackson MR. An N-terminal double-arginine motif maintains type II membrane proteins in the endoplasmic reticulum. EMBO (Eur Mol Biol Organ) J. 1994;13:1696–1705. doi: 10.1002/j.1460-2075.1994.tb06434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaninger R, Plutner H, Bockoch GM, Balch WE. Multiple GTP-binding proteins regulate vesicular transport from the ER to Golgi membranes. J Cell Biol. 1992;119:1077–1096. doi: 10.1083/jcb.119.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönnichsen B, Watson R, Clausen H, Misteli T, Warren G. Sorting of COPI-coated vesicles under interphase and mitotic conditions. J Cell Biol. 1996;134:1411–1425. doi: 10.1083/jcb.134.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofer A, Futerman AH. Cationic amphiphilic drugs inhibit the internalization of cholera toxin to the Golgi apparatus and the subsequent elevation of AMP. J Biol Chem. 1995;270:12117–12122. doi: 10.1074/jbc.270.20.12117. [DOI] [PubMed] [Google Scholar]
- Sohn K, Orci L, Ravazzola M, Amherdt M, Bremser M, Lottspeich F, Fiedler K, Helms JB, Wieland FT. A major transmembrane protein of Golgi-derived COPI-coated vesicles involved in coatomer binding. J Cell Biol. 1996;135:1239–1248. doi: 10.1083/jcb.135.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamnes MA, Craighead MW, Hoe MH, Lampen N, Geromanos S, Tempst P, Rothman JE. An integral membrane component of coatomer-coated transport vesicles defines a family of proteins involved in budding. Proc Natl Acad Sci USA. 1995;92:8011–8015. doi: 10.1073/pnas.92.17.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A, Bolscher J, Feltkamp C, Ploegh H. Retrograde transport from the Golgi region to the endoplasmic reticulum is sensitive to GTP-γ-S. J Cell Biol. 1992;116:1357–1367. doi: 10.1083/jcb.116.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TC, Kahn RA, Melancon P. Two distinct members of the ADP-ribosylation factor family of GTP-binding proteins regulate cell-free intra-Golgi transport. Cell. 1992;70:69–79. doi: 10.1016/0092-8674(92)90534-j. [DOI] [PubMed] [Google Scholar]
- Teasdale RD, Jackson MR. Signal-mediated sorting of membrane proteins between the endoplasmic reticulum and the Golgi apparatus. Annu Rev Cell Dev Biol. 1996;12:27–54. doi: 10.1146/annurev.cellbio.12.1.27. [DOI] [PubMed] [Google Scholar]
- Tisdale EJ, Plutner H, Matteson J, Balch WE. p53/58 binds COPI and is required for selective transport through the early secretory pathway. J Cell Biol. 1997;137:581–593. doi: 10.1083/jcb.137.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley FM, Wilson DW, Pelham HRB. Mutational analysis of the human KDEL-receptor: distinct structural requirements for Golgi retention, ligand binding and retrograde transport. EMBO (Eur Mol Biol Organ) J. 1993;12:2821–2829. doi: 10.1002/j.1460-2075.1993.tb05943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran D, Carpentier JL, Sawano F, Gorden P, Orci L. Ligands internalized through coated or noncoated invaginations follow a common intracellular pathway. Proc Natl Acad Sci USA. 1987;84:7957–7961. doi: 10.1073/pnas.84.22.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DW, Lewis MJ, Pelham HRB. pH-dependent binding of KDEL to its receptor in vitro. J Biol Chem. 1993;268:7465–7468. [PubMed] [Google Scholar]
- Wolf, A.A., M.G. Jobling, S. Wimer-Mackin, M. Ferguson-Maltzman, J.L. Madara, R.K. Holmes, and W.I. Lencer. Ganglioside structure dictates signal transduction by cholera toxin and association with caveolae-like membrane domains in polarized epithelia. J. Cell Biol. 141:917–928. [DOI] [PMC free article] [PubMed]