Abstract
Retrograde transport from the Golgi to the ER is an essential process. Resident ER proteins that escape the ER and proteins that cycle between the Golgi and the ER must be retrieved. The interdependence of anterograde and retrograde vesicle trafficking makes the dissection of both processes difficult in vivo. We have developed an in vitro system that measures the retrieval of a soluble reporter protein, the precursor of the yeast pheromone α-factor fused to a retrieval signal (HDEL) at its COOH terminus (Dean, N., and H.R.B Pelham. 1990. J. Cell Biol. 111:369–377). Retrieval depends on the HDEL sequence; the α-factor precursor, naturally lacking this sequence, is not retrieved. A full cycle of anterograde and retrograde transport requires a simple set of purified cytosolic proteins, including Sec18p, the Lma1p complex, Uso1p, coatomer, and Arf1p. Among the membrane-bound v-SNAP receptor (v-SNARE) proteins, Bos1p is required only for forward transport, Sec22p only for retrograde trafficking, and Bet1p is implicated in both avenues of transport. Putative retrograde carriers (COPI vesicles) generated from Golgi-enriched membranes contain v-SNAREs as well as Emp47p as cargo.
Keywords: retrograde transport, Golgi apparatus, endoplasmic reticulum, COPI, SNARE
Proteins designated for secretion are translocated into the endoplasmic reticulum (ER), packaged into transport vesicles, and delivered to the cell surface via the Golgi apparatus. At the same time, endocytosed proteins may travel from the plasma membrane via the Golgi to the ER. Thus, anterograde and retrograde transport occur simultaneously and yet achieve a balance that retains the individual character of each participating membrane.
Transport between different compartments is mediated by coated vesicles. Vesicles involved in early transport steps are either coated with coatomer, a multimeric protein complex of ∼700 kD with the small GTPase Arf1p, or COPII consisting of two heterodimers (Sec23/24 and Sec13/31 complex) and the small GTPase Sar1p (Orci et al., 1986; Barlowe et al., 1994). Each coat has been implicated in forward transport; however, retrograde transport seems exclusively to be dependent on coatomer (Cosson and Letourneur, 1994; Bednarek et al., 1995, 1996; Orci et al., 1997). Coatomer was shown to be enriched on the cis-face of the Golgi apparatus (Duden et al., 1991), whereas a number of conditional mutants in coatomer components display defects in anterograde transport. The effect of coatomer mutations may be explained by an indirect effect in which a failure to retrieve essential SNAP receptor (SNARE)1 proteins eliminates anterograde transport.
At least a part of the selectivity achieved in vesicle targeting is attributed to the interaction of cognate vesicle or v-SNAREs and target or t-SNAREs. A cycle between the ER and the Golgi could, in principle, be achieved by two v- and two t-SNAREs, one pair directing anterograde targeting and the other directing retrograde targeting. Given that SNAREs are themselves localized by the secretory pathway, one challenge has been to understand how they may be regulated to express targeting activity in the correct location. The same consideration applies to other proteins that shuttle between the ER and the Golgi. In yeast, Golgi proteins such as Emp47p and ER proteins such as Emp24p continuously cycle yet maintain a concentration in one or the other organelle (Schimmöller et al., 1995; Schröder et al., 1995).
Many stable ER proteins undergo slow anterograde transport to the Golgi, from which they are retrieved by a receptor-mediated process. The best known of those receptors is Erd2p, which is responsible for retrieval of HDEL/KDEL-terminated soluble ER proteins (Lewis and Pelham, 1990; Semenza et al., 1990; Elmendorf and Haldar, 1993; Lee et al., 1993; Tang et al., 1993; Sanchez-Lopez et al., 1997). In addition, ER membrane proteins containing a KKXX motif at their COOH terminus are retrieved from the Golgi by direct interaction with a subcomplex of coatomer (COPI) consisting of α, β′, and ε-COP (Fiedler et al., 1996). Other less well-characterized mechanisms are known. For example, in Saccharomyces cerevisiae, the retrieval of Sec12p, the nucleotide exchange factor of Sar1p at the ER, is dependent on Rer1p, a Golgi-resident protein (Sato et al., 1995, 1997).
Progress in defining the exact contribution of coat proteins and SNAREs to anterograde and retrograde processes has been limited by the interdependence of the events in vivo. Here we describe a cell-free reaction using membranes isolated from gently lysed yeast spheroplasts and a set of pure cytosolic proteins to reconstitute a full cycle of transport and retrieval. The reaction has been used to define unique roles in transport to two v-SNAREs.
Materials and Methods
Yeast Strains, Media, and Yeast Transformation
Yeast strains used in this study are summarized in Table I. Yeast cells were grown in yeast extract, peptone, and dextrose growth medium (YPD). Yeast strains were transformed as described by Schiestl and Gietz (1989).
Table I.
Strains Used in This Paper
| Strain | Genotype | Source | ||
|---|---|---|---|---|
| RSY445 | MATα ura3-52 leu2-3, 112 trp1-289 pep4::URA3 prb1 his4-579 | Schekman strain collection | ||
| RSY955 | MATα leu2-3, 112 sec32-1 (bos1-1) | Schekman strain collection | ||
| RSY945 | MATα his4-619 bet1-1 | Schekman strain collection | ||
| RSY1289 | MATα leu2-3, 112 trp1-Δ901 lys2-801 suc2-Δ9 ura3-52 his3-Δ200 sed5-1 | Schekman strain collection | ||
| RSY1288 | MATα leu2-3, 112 trp1-Δ901 lys2-801 suc2-Δ9 ura3-52 his3-Δ200 sft1::LEU2 psft1-1 * | Banfield et al. (1995) | ||
| RSY1163 | MATa leu2-3, 112 ura3-53 ade2-101 kar2-133 | Schekman strain collection | ||
| RSY1169 | MATa leu2-3, 112 ura3-53 pep4::URA3 gls1-1 § | Schekman strain collection | ||
| RSY1218 | MATα ura3-52 leu2-3, 112 his4-619 suc2-Δ9 erd2-B36 | Schekman strain collection | ||
| RSY279 | MATα ura3-52 his4-619 sec22-3 | Schekman strain collection | ||
| MLY103 | MATα ura3-52 trp1-1 his3-11, -15 ufe1::TRP1 pep4::HIS3 pUT1 ‡ | Lewis and Pelham (1996) | ||
| EGY021c | MATα trp1 leu2 lys2 suc2Δ9 sec21::HIS3 pRS416-Sec21.2 | Gaynor and Emr (1997) | ||
| YAS31 | MATα ura3-52 leu2-3, 112 trp1-289 pep4::URA3 prb1 his4-579 | This study | ||
| LEU2-EMP47-MYC TRP1-OCH1-HA |
psft1-1 carries a ts-allele of sft1 in pRS313.
pUT1 carries ufe1-1 on pRS314.
The gene bearing the gls1-1 mutation has been cloned and named CWH41 (Simons et al., 1998).
YAS31:RSY445 was transformed with plasmids pAS2 and p44, which were linearized with XhoI or BstXI, respectively. pAS2 contains HA-tagged OCH1, and p44 bears a myc-tagged EMP47 controlled by their own promoters.
Preparation of Perforated Yeast Spheroplasts and Cytosol
Perforated yeast spheroplasts (semi-intact cells) were prepared as described by Rexach et al. (1994).
For cytosol, yeast cells were grown to early to mid-log phase in YPD. Wild-type and ts-mutant strains were grown at 30 and 23°C, respectively. The cells were harvested by centrifugation and washed twice with water. The cell pellet was resuspended in a minimal volume of buffer B88 (20 mM Hepes, pH 6.8, 250 mM sorbitol, 150 mM KOAc, 5 mM Mg(OAc)2 and pipetted into liquid nitrogen. The cell beads were ground up under liquid nitrogen in a blender (Worthington Biochemical Corp., Freehold, NJ) for large-scale preparations or in a mortar for small-scale preparations. The cell powder was thawed in an ice-water bath, and ATP and DTT each at a final concentration of 1 mM as well as protease inhibitors were added. The lysate was centrifuged (5 min, 3,000 g; 15 min, 20,000 g; 1 h, 100,000 g) and the 100,000-g supernatant was collected, carefully avoiding the pellet and the lipids which floated to the top.
Antibodies
Antibodies directed against c-myc (Evan et al., 1985); Sec21p (Hosobuchi et al., 1992); Sec22p (Bednarek et al., 1995); Sec23p (Barlowe et al., 1994); Bet1p, Bos1p, and coatomer (Rexach et al., 1994); and α-1,6 linked mannose (Baker et al., 1988) have been described. Affinity-purified antibodies against Sec18p and Ykt6p were generous gifts of W. Wickner (Dartmouth Medical School, Hanover, NH) and J. McNew (Sloan-Kettering Cancer Center, New York), respectively.
Monoclonal anti-HA (12CA5) antibodies were purchased from Berkeley Antibody Co. (Richmond, CA). Donkey anti–rabbit and sheep anti– mouse secondary antibodies coupled to horseradish peroxidase were obtained from Amersham Corp. (Arlington Heights, IL).
Antibodies against Arf1p were raised in rabbits using purified N-myristylated yeast Arf1p. The specificity of the antiserum was tested by immunoblot on total cell lysates using an ECL-kit (Amersham Corp.).
Purification of Coatomer, COPII, Uso1p, Sec18p, N-myristylated Yeast Arf1p, and N-myristylated Yeast Arf1Q71Lp
Coatomer was purified according to Hosobuchi et al. (1992). The purification of Sar1p, Sec23/24 complex, and Sec13/31 complex were performed as described by Barlowe et al. (1994) and Salama et al. (1993), respectively. A myc-tagged Uso1p was purified from yeast cytosol according to Barlowe (1997). Bacterially expressed Sec18p-6His was purified as previously described (Whiteheart et al., 1994), but after isolation, the peak fractions were pooled and dialyzed against B88 that contained 0.1 mM DTT, 0.1 mM PMSF, 0.1 mM ATP, and 15% glycerol. Escherichia coli BL21(DE3) strains, which coexpress yeast N-myristoyl transferase and either wild-type or dominant-activated (Q71L) yeast Arf1p, were provided by R. Kahn (National Institutes of Health, Bethesda, MD). N-myr-yArf1p and N-myr-yArf1Q71Lp were purified as described previously (Kahn et al., 1995). The Lma1p complex was a generous gift of Zuoyu Xu (Dartmouth Medical School) and was prepared as described (Xu et al., 1997).
In Vitro Round-Trip Retrieval Assay
Reagents used for the in vitro round-trip assay were prepared as described by Baker et al. (1988) unless indicated otherwise.
Stage I: Translocation.
The translocation reaction using [35S]ppα-factor-myc-HDEL (Dean and Pelham, 1990), [35S]ppα-factor-myc-EDLN (Dean and Pelham, 1990), or [35S]ppα-factor and gls1-1 mutant donor membranes was performed as described by Rexach et al. (1994), except that the scale was increased twofold and after the high-salt treatment, and incubation of the membranes in 2.5 M urea in B88 (20 mM Hepes, pH 6.8, 150 mM KOAc, 5 mM Mg(OAc)2, 250 mM sorbitol) for 10 min at 4°C was included. The membranes were then washed twice in B88 and resuspended to 220 μl in B88.
Stage II: Budding.
To the membranes of the stage I reaction, we added 25 μg/ml Sar1p, 25 μg/ml Sec23/24 complex, 75 μg/ml Sec13/31 complex, 50 μM GTP, and an ATP regeneration system (without GDP-mannose; Baker et al., 1988). The reaction mixture was incubated for 30 min at 20°C, chilled for 5 min on ice, and subjected to a medium-speed centrifugation (12,000 g, 30 s), which retained COPII vesicles in the supernatant fraction. An aliquot of the medium-speed supernatant (5 μl) was saved to determine the efficiency of the retrograde transport. COPII vesicle–enclosed gpαF in this sample was set as 100% for quantification purposes.
Stage III: Fusion.
The medium-speed supernatant from stage II was supplemented with an ATP-regenerating system (without GDP-mannose), 50 μM GTP, 1 μg/ml Lma1p complex, 1 μg/ml Sec18p, 1.5 μg/ml Uso1p, and 600 μg/ml perforated spheroplast membranes from a GLS1 strain, which were washed twice with B88 or washed with 2.5 M urea and B88 before addition. Fusion was allowed to take place for 15 min at 20°C.
Stage IV: Retrieval.
Cytosol was added to a final concentration of 2 mg/ ml or the same volume of B88 was added in a noncytosol control. Arf1p (12 μg/ml) or Arf1Q71Lp (12 μg/ml) and coatomer (37.5 μg/ml) instead of cytosol were added where indicated. Reactions (100 μl) were incubated for 30 min at 30°C. The reaction mixture was chilled on ice for 5 min, and the acceptor ER was sedimented by centrifugation at 12,000 g for 30 s. The pellet was washed once with 2.5 M urea in B88 for 10 min on ice and once with B88. Membranes in the pellet were resuspended to 100 μl with B88. Fusion with the acceptor ER was measured by precipitation of protease-protected [35S]gpαF-HDEL with concanavalin A–Sepharose (Rexach and Schekman, 1991) followed by separation of untrimmed [35S]gpαF-HDEL from trimmed [35S]gpαF-HDEL by SDS-PAGE. The amount of retrieved [35S]gpαF-HDEL was correlated to the total amount of [35S]gpαF-HDEL present in the medium-speed supernatant of the stage II reaction.
In Vitro Forward Transport Assay
Stage I: Translocation.
The translocation reaction using [35S]ppαF was performed as described above except that the high-salt and urea treatments were omitted.
Stage II: Transport.
An aliquot (10 μl) of the stage I membranes was incubated with an ATP regeneration system (Baker et al., 1988), 50 μM GTP, 40 μM MnCl2, and 2.5 mg/ml cytosol for 1 h at 20 or 30°C. Each reaction was performed in quadruplicate, and the final volume of each reaction was 50 μl. After the incubation, the reactions were chilled on ice for 5 min and centrifuged for 30 s at 12,000 g. 30 μl of the supernatant collected from the meniscus was treated with trypsin followed by trypsin inhibitor as described previously (Rexach and Schekman, 1991). An equal volume of 2% SDS was added, and the reactions were heated to 95°C for 5 min. For each set of experiments, two reactions were precipitated with concanavalin A–Sepharose or antibodies directed against α-1,6 linked mannose modifications and protein A–Sepharose according to Baker et al. (1988). Washed immunoprecipitates were quantified in a liquid scintillation counter (Beckman Instruments, Fullerton, CA). The budding efficiency was determined by comparing the amount of protease-protected [35S]gpαF in the supernatant to the total amount of [35S]gpαF translocated into the ER in the stage I reaction precipitated with concanavalin A–Sepharose.
Golgi Budding Assay
Yeast cells were grown to early to mid-log phase and lysed in liquid nitrogen as described above for the preparation of cytosol. The cell lysate was subjected to a low-speed centrifugation (5 min at 3,000 g). The resulting supernatant was centrifuged twice at 15,000 g to remove the ER. The membranes in the postnuclear supernatant were sedimented for 15 min at 50,000 g onto a 60% sucrose (wt/wt) cushion in 20 mM Hepes, pH 6.8, 5 mM Mg(OAc)2. Membranes at the interface were diluted to the starting volume with B88 and sedimented again on an identical cushion. Washed membranes were collected and stored in aliquots at −80°C.
For the Golgi budding reaction, we incubated the membranes with 0.1 mM GTP, GTP-γ-S, or GDP-β-S, coatomer (250 μg/ml), COPII-components (288 μg/ml Sec13/31p, 128 μg/ml Sec23/24p), Arf1p (80 μg/ml), and Sar1p (140 μg/ml) as indicated at 20°C for 30 min in a total volume of 200 μl. After chilling on ice, we loaded the samples on top of a Ficoll-sucrose gradient consisting of 0.4 ml 60% (wt/wt) sucrose, 0.8 ml 7.5% (wt/wt) Ficoll, 1 ml 5% (wt/wt) Ficoll, 1 ml 4% (wt/wt) Ficoll, 1 ml 3% (wt/wt) Ficoll, and 0.8 ml 2% (wt/wt) Ficoll in 15% (wt/wt) sucrose 20 mM Hepes, pH 6.8, 5 mM Mg(OAc)2. The vesicles were separated from the Golgi apparatus by centrifugation for 2 h at 35,000 rpm (model SW55 rotor; Beckman Instruments). Fractions (400 μl) were collected from the top, and small aliquots were analyzed for GDPase activity and refractive index. The remainder of the samples (380 μl) were precipitated with TCA, resolved on SDS-PAGE, and analyzed by immunoblot. The immunoblots were developed using the ECL-kit or the Vista-kit from Amersham Corp.
For flotation analysis, fractions 6–8 of the gradients were pooled, mixed with an equal volume of 80% Nycodenz in B88* (20 mM Hepes, pH 6.8, 150 mM KOAc, 5 mM Mg(OAc)2), and overlaid with 600 μl of 30, 25, 20, and 15% and 400 μl 10% Nycodenz in B88*. The gradients were centrifuged for 16 h at 40,000 rpm (model SW55 rotor; Beckman Instruments). Fractions (300 μl) were collected from the top and TCA-precipitated, and the top 11 fractions were analyzed as described above.
GDPase Enzyme Assay
The GDPase assay was performed as described by Yanagisawa et al. (1990). Samples (5 μl) were mixed with 50 μl 20 mM imidazole-HCl, pH 7.4, 2 mM CaCl2, 0.1% Triton X-100, and 5 mM GDP or CDP as control and incubated for 20 min at 30°C. Reactions were terminated by the addition of 10 μl 6.7% SDS. Inorganic phosphate was detected by adding 200 μl of 0.35% ammonium molybdate, 0.15% ascorbic acid in 0.86 N H2SO4. The assay was allowed to develop for 20 min at 42°C and quantified by reading the absorbance at 750 nm in a plate reader (model MR5000; DYNEX Technologies, Inc., Chantilly, VA).
Results
Features of the In Vitro Round-Trip Assay
We established an in vitro system that allowed us to follow the retrieval of the precursor of the pheromone α-factor to which an HDEL sequence had been fused ([35S]gpαF-HDEL; Dean and Pelham, 1990). This reporter molecule was chosen because the precursor of α-factor is a useful tool in monitoring early steps in transport, such as translocation into the ER, budding from the ER, and fusion with the Golgi apparatus (Baker et al., 1988; Wuestehube and Schekman, 1992; Rexach et al., 1994).
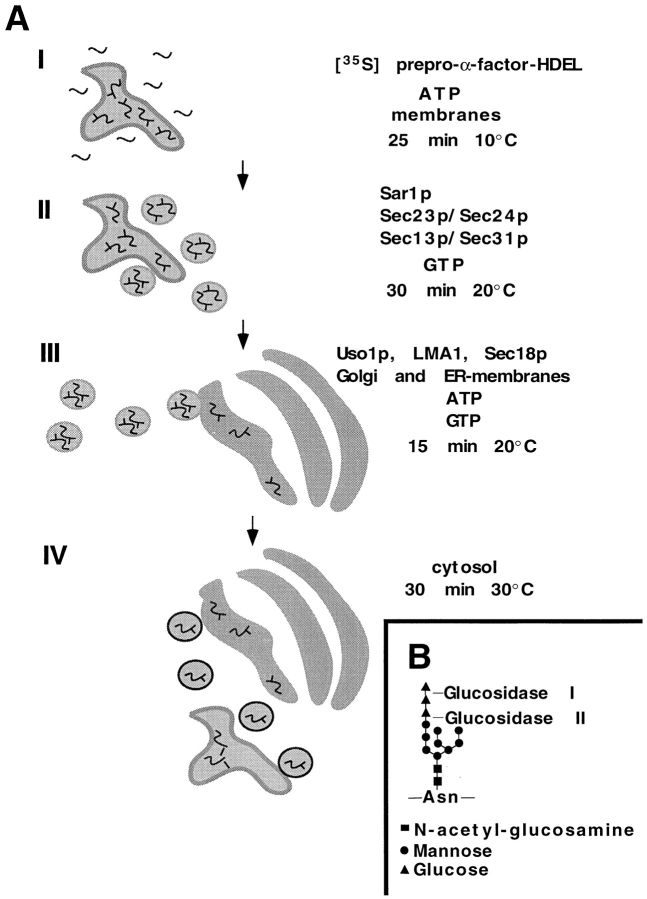
The assay is divided into four stages (Fig. 1 A). In the first step [35S]ppαF-HDEL is translocated into the lumen of the ER, where it is N-glycosylated. In the second stage, ER-enclosed [35S]gpαF-HDEL is packaged into COPII transport vesicles using purified proteins. Because proteins required for fusion are missing, the COPII vesicles do not fuse with the Golgi apparatus. Transport vesicles are separated from the donor membranes by centrifugation. In the third step, purified Sec18p, the Lma1p complex, and Uso1p as well as acceptor membranes are added to the vesicle fraction. These proteins are necessary and sufficient to allow the fusion of COPII vesicles with the cis-Golgi membrane (Barlowe, 1997). Finally, cytosol is added to the reaction, providing the proteins necessary for retrograde transport. Retrieval depends upon the recognition of the HDEL sequence of [35S]gpαF-HDEL by Erd2p, the HDEL receptor. This recognition may either occur in the cis-Golgi or in a compartment formed by the fusion of COPII vesicles containing at least Erd2p, possibly the yeast equivalent of an intermediate compartment. However, since an intermediate compartment has not yet been identified in yeast, we refer to the -HDEL sorting membrane as Golgi. Retrograde transport vesicles capturing [35S]gpαF-HDEL bound to Erd2p must reach their destination and fuse with the acceptor ER to complete the reaction.
Figure 1.
Schematic drawing of the retrieval assay. (A) The retrieval assay is divided into four steps: (I) posttranslational translocation of reporter molecules into ER membranes, (II) budding of the reporter in COPII vesicles from ER membranes, (III) fusion of anterograde vesicles with a Erd2p-containing compartment (pre-Golgi or cis-Golgi) and delivery of the cargo, and (IV) budding of retrograde transport vesicles from the Erd2p-containing compartment and fusion of these vesicles with the acceptor ER. For details, see text and Materials and Methods. (B) Trimming of the terminal glucose residues of the N-glycan by glucosidase I and glucosidase II.
To measure the amount of retrieved [35S]gpαF-HDEL, we took advantage of the SDS-PAGE mobility difference between gpαF in ER membranes from glucosidase wild-type (GLS1) and mutant (gls1-1) cells (Latterich and Schekman, 1994). Glucosidase I initiates trimming of an N-linked oligosaccharide by removing a terminal glucose residue on glycoproteins in the ER (Fig. 1 B). Glucosidase II removes the two remaining glucose residues. gls1-1 prevents the trimming of the terminal sugar residue and thus the action of glucosidase II. This mutation does not affect the transport efficiency of [35S]gpαF-HDEL (data not shown). We used perforated spheroplasts (semi-intact cells) from a gls1-1 strain as donor membranes. Glucose trimming should occur if [35S]gpαF-HDEL is retrieved to a GLS1 acceptor ER membrane. Trimming results in a higher electrophoretic mobility species of the reporter molecule. We refer to a complete cycle of anterograde and retrograde transport as a round-trip.
We performed a round-trip in vitro assay using acceptor membranes from either a GLS1 or a gls1-1 strain (Fig. 2 A). As predicted, a higher mobility form of [35S]gpαF-HDEL was detected only when glucosidase I activity was present in the acceptor membranes. The trimmed species was absent when gls1-1 acceptor membranes were used (Fig. 2 A, compare lanes 2 and 3). A species migrating at the position of untrimmed [35S]gpαF-HDEL was present in all lanes. This form most likely represents [35S]gpαF-HDEL retained in COPII vesicles that were unable to fuse with the Golgi, in the Golgi, or in retrograde transport vesicles derived from the Golgi.
Figure 2.

Features of the retrieval assay. (A) The detected signal is trimmed gpαF-HDEL. In stage III of the assay, either membranes derived from wild-type (lanes 1 and 2) or gls1-1 (lane 3) were added, and retrieval was allowed to occur. In the control (lane 1), cytosol was omitted. After the reaction was completed, the samples were subjected to trypsin digestion, concanavalin A–Sepharose precipitation, and SDS-PAGE. The bands were visualized on a PhosphorImager (model STORM 860; Molecular Dynamics, Sunnyvale, CA). The amount of retrieved reporter was normalized to the amount of reporter molecules present in the vesicle fraction of the stage II reaction. (B) Retrieval is dependent on the HDEL sequence. Either gpαf-HDEL (lanes 1–3) or gpαF (lanes 4–6) were used as reporter molecules in a retrieval assay. In lanes 1 and 4, the reporter was translocated into wild-type ER. Cytosol was omitted in lanes 2 and 5. Complete reactions are shown in lanes 3 and 6. (C) Retrieval is dependent on functional Erd2p. A retrieval was performed using either wild-type (WT) or erd2-B36 acceptor membranes. The quantification was performed as described in A.
More than 1% of the [35S]gpαF-HDEL present in the COPII vesicle fraction (stage II) was trimmed in the final stage of a typical round-trip reaction. Considering the complexity of the reaction, this low efficiency is not surprising. A final signal of about 1.6% would result if each budding and targeting step in a round-trip assay achieved 25% efficiency.
We were concerned that fragments of the donor ER might contaminate the vesicle fraction such that subsequent homotypic membrane fusion with the acceptor ER could account for the trimmed signal. To rule out this possibility, we used acceptor membranes from a kar2-133 strain. This mutant is deficient in homotypic ER membrane fusion at 30°C even when only one of the fusion partners is mutant (Latterich and Schekman, 1994). Wild-type and the kar2-133 mutant acceptor membranes incubated at 30°C produced the same signal of trimmed [35S]gpαF-HDEL. We conclude that the trimming is not due to homotypic ER membrane fusion.
The HDEL Sequence at the COOH Terminus Is Necessary and Sufficient for Retrieval In Vitro
If our round-trip reaction requires retrieval, then glucose trimming should depend upon the HDEL signal. We compared [35S]gpαF and [35S]gpαF-HDEL as substrates and found trimming to be signal dependent (Fig. 2 B, compare lanes 3 and 6). The gpαF-HDEL we used in our studies also contained a myc-epitope tag. To ensure that the tag was not responsible for the trimmed signal, we compared the gpαF-HDEL to a gpαF where the HDEL sequence had been removed, but the myc-tag was still present. This resulted in EDLN at the COOH terminus (gpαF-EDLN; Dean and Pelham, 1990). No retrieval was detected for gpαF-EDLN (data not shown). All three proteins behaved similarly in a forward transport reaction with respect to budding from the ER and fusion with the Golgi apparatus (data not shown). These results demonstrate that the HDEL sequence is necessary and sufficient to retrieve a protein normally destined for secretion back to the ER in vitro.
Another prediction for the assay is that retrieval of the reporter should be dependent on functional Erd2p. To test this, we prepared acceptor membranes from an erd2-B36 mutant strain, in which the retrieval of HDEL-containing proteins is strongly impaired in vivo (Semenza et al., 1990). When acceptor membranes from the erd2-B36 mutant were used in the assay, the amount of trimmed gpαF-HDEL was reduced 3-fold compared with wild-type (Fig. 2 C). Taken together, these results demonstrate that the round-trip in vitro assay is capable of recapitulating the retrieval of soluble HDEL-containing proteins, enabling a biochemical investigation of the mechanisms by which retrieval and retrograde transport are achieved.
Functional Coatomer Is Required for Retrieval In Vitro
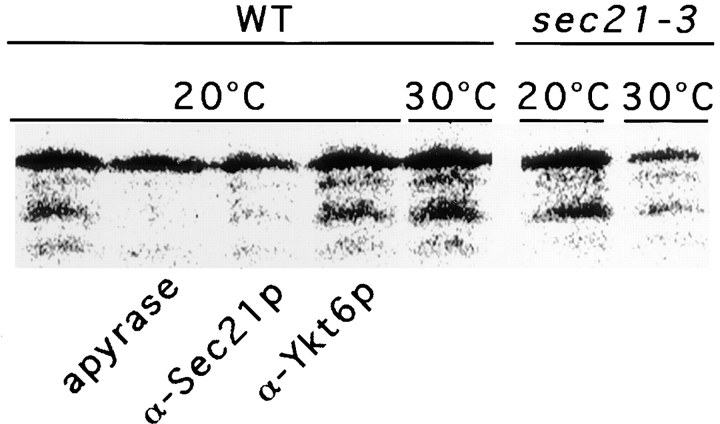
Recent genetic studies suggest a role for coatomer in retrieval (Letourneur et al., 1994; Lewis and Pelham, 1996; Gaynor and Emr, 1997). However, biochemical data showing that retrograde Golgi to ER vesicles are COPI coated is lacking. Therefore, we used our round-trip assay to detect a role for coatomer. SEC21 encodes the γ-subunit of coatomer (Hosobuchi et al., 1992), and the sec21-3 mutant is defective for retrieval in vivo (Gaynor and Emr, 1997). We prepared cytosol and acceptor membranes from either wild-type or sec21-3 strains grown at a permissive temperature. The cytosol and membrane fractions were then used in a stage IV incubation at either 20 or 30°C. The transport defect could not be investigated at 37°C because of membrane lysis (data not shown).
At 20°C, reactions containing wild-type and mutant components promoted significant levels of retrieval (Fig. 3, compare lanes WT 20°C and sec21-3 20°C). Retrieval was enhanced at 30°C in the wild-type but not in the mutant reaction (Fig. 3, compare lanes WT 30°C and sec21-3 30°C). In addition, affinity-purified anti-Sec21p antibodies blocked retrieval at the permissive temperature, whereas affinity-purified anti-Ykt6p antibodies had no effect on retrograde transport (Fig. 3). Ykt6p is a SNARE that is thought to be essential for ER-to-Golgi transport (McNew et al., 1997). Because Sec21p is part of the coatomer complex, we suggest that retrieval is mediated by COPI in vitro.
Figure 3.
Functional coatomer is required for retrograde transport. Retrograde transport assays were performed as described in Materials and Methods except that stages III and IV were combined by the addition of cytosol and acceptor membranes to the vesicle fraction. Wild-type cytosol and membranes (WT) or mutant acceptor membranes and cytosol (sec21-3) were used. The samples were either incubated at 20 or 30°C during stage III/IV. Apyrase, affinity-purified rabbit α-Sec21p antibodies, or affinity-purified rabbit α-Ykt6p antibodies were added where indicated.
Cytosol Can Be Replaced by Coatomer and Arf1p to Support Retrieval In Vitro
The assembly and disassembly of coats require the action of small GTPases (for review see Salama and Schekman, 1995). Arf1p is the GTPase necessary for coatomer-dependent budding from the Golgi. Because coatomer and Arf1p are sufficient to promote the formation of COPI vesicles, we performed a retrieval assay where cytosol was replaced with purified myristylated Arf1p and coatomer (Fig. 4 A). Arf1p or coatomer alone stimulated some retrieval of the gpαF-HDEL (Fig. 4 A, compare lanes 1 to 2 and 3). Although endogenous Arf1p and coatomer were detected in the Golgi fraction, addition of both proteins stimulated retrieval to almost the level obtained with cytosol (Fig. 4 A, compare lanes 1 and 4). In contrast, the addition of COPII resulted in background retrieval of gpαF-HDEL (Fig. 4 A, compare lane 5 to lanes 1 and 4).
Figure 4.
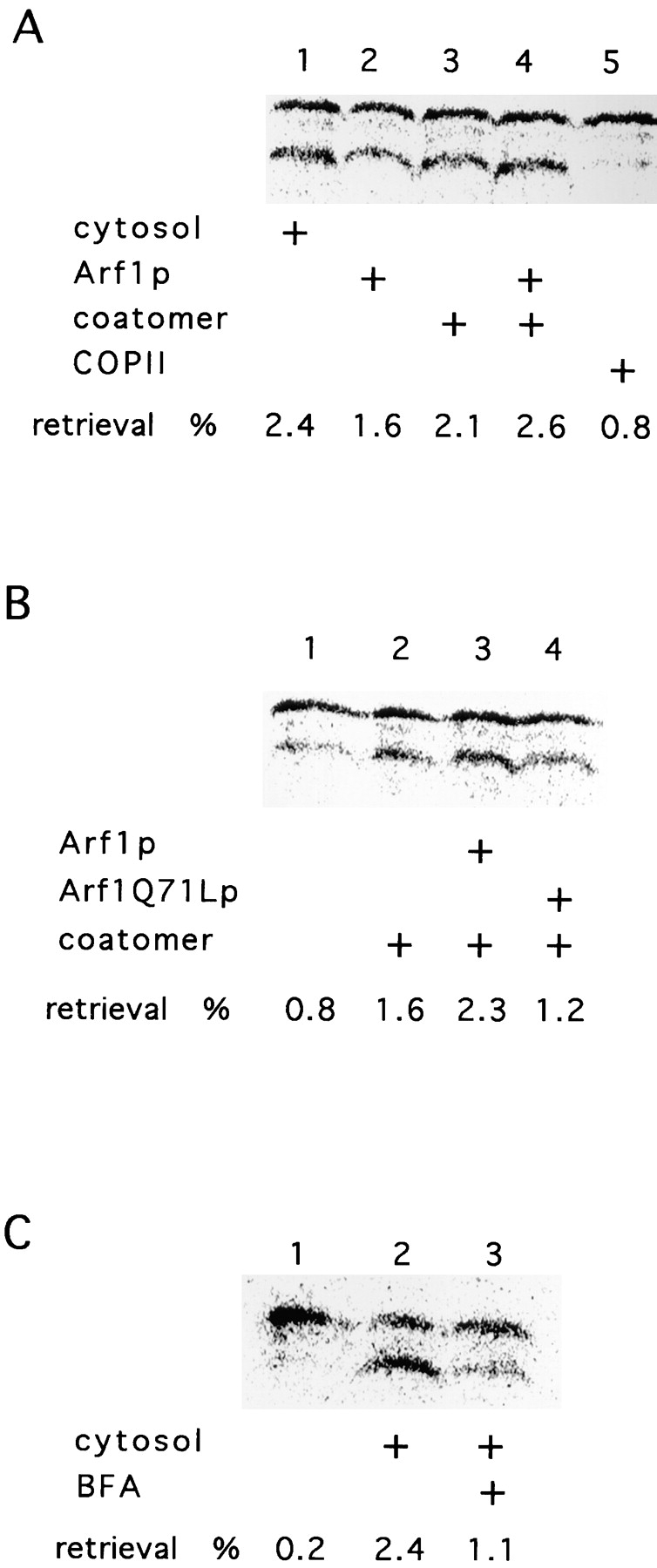
Involvement of coatomer and Arf1p in retrieval. (A) Cytosol is replaced by coatomer and Arf1p. In stage IV of the retrieval assay, cytosol, coatomer, Arf1p, or COPII components were added as indicated. The samples were treated as described in Fig. 2 A. The percentage of retrieved gpαf-HDEL is indicated. (B) Dominant-active Arf1Q71Lp blocks retrograde transport. Cytosol, Arf1p, Arf1Q71Lp, and coatomer were added to the retrograde transport reaction (stage IV) as shown. (C) Retrograde transport is sensitive to BFA. Retrograde transport was assessed in the absence (lane 2) or the presence (lane 3) of 100 μM BFA in stage IV of the assay. The sample in lane 2 contained the same concentration of EtOH (0.4%) as that in lane 3. In lane 1, cytosol was omitted.
To further establish the role of Arf1p in our retrieval system, we substituted Arf1p with a dominant-active mutant form of the protein (Arf1Q71Lp). This mutant protein is predominantly in its GTP-bound form, and the hydrolysis of GTP is diminished (Kahn et al., 1995). Upon addition of Arf1p and coatomer, a slight stimulation of the retrieval signal could be observed compared with the cytosol experiment (Fig. 4 B, compare lanes 2 and 3). Replacement of Arf1p with Arf1Q71Lp resulted in a reduction of trimmed gpαF-HDEL (Fig. 4 B, compare lane 4 to lanes 2 and 3). The residual signal is again most likely due to endogenous Arf1p bound to the Golgi membrane. Alternatively, a reduced level of GTP hydrolysis by mutant Arf1p may allow residual transport.
Brefeldin A (BFA) inhibits nucleotide exchange on Arf1p and blocks COPI vesicle formation (Donaldson et al., 1992). Thus, the addition of brefeldin A to the retrograde transport reaction should inhibit retrieval. Indeed, addition of 100 μM brefeldin A to stage IV of the round-trip reaction significantly reduced the formation of trimmed [35S]gpαF-HDEL (Fig. 4 C, compare lanes 2 and 3). The formation of retrieved gpαF-HDEL was at least partially restored in the presence of BFA in reactions supplemented with Arf1p preequilibrated with GTP (data not shown).
The Involvement of SNAREs in Retrograde Transport
It has not been possible to assess directly the role of v-SNAREs in retrograde transport because mutants exhibit a defect in anterograde ER-to-Golgi transport. The round-trip reaction permitted us to examine the role of SNAREs in retrieval. We prepared membranes from different SNARE mutant strains grown at a permissive temperature. Stage IV of the assay was performed at 30°C, a semipermissive temperature, which reduced but did not abolish the formation of trimmed [35S]gpαF-HDEL in mutant membranes (Fig. 5 A). As controls, assays of anterograde vesicle budding (Fig. 5 B) and ER-to-Golgi transport (Fig. 5 C) were conducted with mutant and wild-type membranes.
Figure 5.

Involvement of SNAREs in retrograde transport. (A) The role of different SNAREs in retrieval was assessed by using mutants as acceptor membranes. Mutant membranes were prepared from cells grown at a permissive temperature and were added to the stage III of the retrieval assay. Stage IV was performed at 30°C. kar2-133 acceptor membranes were used as a positive control. The treatment of the samples and the quantification was done as described in Fig. 2 A. In another experiment, the following retrieval was obtained: control, 0.7%; WT, 2.4%; ufe1-1, 0.4%; sed5-1, 2.1%; sft1-1, 1.8%; sec22-3, 0.9%; bet1-1, 1.2%; and bos1-1, 3.2%. (B and C) The behavior of SNAREs in anterograde transport. A forward transport assay was performed at 30°C except for “bet1-1 20°C.” The amount of budded gpαF was determined by concanavalin A–Sepharose precipitation and normalized to the amount of gpαF translocated into the ER. The arrival of gpαF in the Golgi was monitored by precipitation with rabbit α-1,6 mannose antibodies and protein A–Sepharose. The amount of gpαF precipitated by rabbit α-1,6 mannose antibodies was normalized to the amount of budded gpαF from the ER. All samples were treated with trypsin before solubilization of the membranes and precipitation.
Membranes from three t-SNARE mutants were examined in all three transport assays. UFE1 encodes a t-SNARE in the ER membrane thought to participate exclusively in docking of retrograde vesicles. As expected, membranes from ufe1-1 mutant cells were not deficient in budding of ER vesicles or in ER-to-Golgi transport (Fig. 5, B and C). However, the signal in the retrieval reaction was reduced to background (Fig. 5 A). SED5 encodes the t-SNARE on the cis-Golgi involved in anterograde transport from the ER. A stage III incubation of sed5-1 acceptor membranes at 20°C followed by a stage IV incubation at 30°C produced no defect in retrograde transport (Fig. 5 A). However, ER-to-Golgi transport, but not ER budding, was blocked at the higher temperature (Fig. 5, compare B and C). SFT1 encodes a putative medial Golgi t-SNARE. sft1-1 showed no defect in any of the assays (Fig. 5, A–C). Likewise, tlg2 null mutant membranes, deficient in trafficking at or after the trans-Golgi (Holthuis et al., 1998), showed no defect in our in vitro reactions (data not shown).
The requirement for three v-SNAREs was also examined. Here the exact roles of these molecules have been difficult to distinguish because of the interdependence of the anterograde and retrograde processes. bos1-1 mutant membranes were defective in ER-to-Golgi transport, but not in ER budding, when incubations were conducted at 30°C (Fig. 5, compare B and C). However, no defect in retrieval was observed with bos1-1 mutant membranes incubated at 20°C in stage III followed by 30°C in stage IV (Fig. 5 A). This result suggests that Bos1p acts specifically in anterograde targeting. The converse was found for sec22-3 mutant membranes, which displayed normal ER-to-Golgi and ER budding at 30°C, but were defective in a stage IV retrieval incubation at this temperature (Fig. 5, compare A with B and C). By this analysis, we suggest that Sec22p serves as a retrograde v-SNARE. In yet another contrast, bet1-1 mutant membranes were defective both in retrieval and in ER-to-Golgi transport at 30°C (Fig. 5, A and B). Thus, Bet1p may serve as a v-SNARE in both directions.
Sec18p Is Required for Vesicle Fusion with the ER
Anterograde vesicle targeting requires the NSF ortholog Sec18p, whereas homotypic ER fusion requires the NSF homologue, Cdc48p. To examine which ATPase is responsible for retrograde vesicle fusion with the ER, we performed a retrieval assay including affinity-purified anti-Sec18p antibodies in stage IV. The production of trimmed [35S]gpαF-HDEL was reduced from 2.3 to 0.4% and was restored to 1.7% when excess recombinant Sec18p (15 μg) was preincubated with the antibody. Antibodies against Sec17p (yeast α-SNAP) also blocked retrograde transport. Furthermore, cdc48-3 mutant membranes displayed no retrieval defect. Thus, at least a part of the targeting machinery for anterograde and retrograde vesicle docking is shared. However, although fusion of anterograde vesicles with the cis-Golgi requires Ca2+, the retrieval reaction (stage IV) was unaffected by up to 8 mM EGTA (data not shown).
Generation of COPI-coated Vesicles from the Golgi Apparatus In Vitro
Transport of gpαF-HDEL from the Golgi to the ER was stimulated by components of the COPI coat (Fig. 4). To monitor directly the uptake of membrane cargo into COPI vesicles, we developed a vesicle budding assay using enriched Golgi membranes as a donor.
We used myc-tagged Emp47p, a Golgi protein that continuously recycles through the ER, as a marker for the formation of retrograde transport vesicles. To follow Golgi membranes in subsequent experiments, we introduced HA-tagged Och1p (a marker of the yeast cis-Golgi membrane), to create strain YAS31, which contains two copies of OCH1 and EMP47 controlled by their own promoters. This strain had a normal growth rate and no apparent morphological abnormalities at temperatures between 23 and 37°C (data not shown).
Membranes from a postnuclear pellet were incubated with buffer alone, COPII (including Sar1p and GTP-γ-S), or COPI (including Arf1p and GTP-γ-S). Slowly sedimenting vesicles were separated from Golgi membranes by velocity sedimentation on a sucrose density gradient. Fractions of the gradient were analyzed by immunoblot. In the buffer control, some Sec21p, the γ-subunit of coatomer, was at the top of the gradient. This residual protein may represent endogenous protein associated with Golgi membranes (Fig. 6). Likewise, residual Sec23p, a COPII subunit, was present in the load fractions of the gradient. Golgi membrane sedimentation to the bottom of the gradient was confirmed by measuring the distribution of the marker enzyme, GDPase. Other Golgi marker proteins, such as myc-Emp47p and Och1p-HA, were also largely in the more rapidly sedimenting membrane (Fig. 6). No change in the distribution of these proteins was observed in incubations with COPII proteins, although a notable amount of Sec23p migrated to the bottom of the gradient (Fig. 6). In contrast, COPI liberated a fraction of myc-Emp47p and Och1p-HA, which sedimented more slowly (fractions 6–8). This distribution was coincident with Sec21p, suggesting that fractions 6–8 represented COPI-coated vesicles derived from the Golgi apparatus. These vesicles could represent carriers involved in intra-Golgi transport supported by COPI. Och1p, for example, is known to cycle between cis- and trans-Golgi (Harris and Waters, 1996). Alternatively, the vesicles may represent retrograde vesicles involved in a continuous recycling of cis-Golgi proteins. Of course, the vesicle fraction could contain a mixture of anterograde and retrograde COPI vesicles.
Figure 6.

Emp47p, Bos1p, Sec22p, and Och1p are cargo of Golgi-derived COPI vesicles. Golgi-budding assays from postnuclear membranes were performed either without addition of coat proteins (buffer) or in the presence of COPI or COPII. The reaction was loaded on a Ficoll/sucrose gradient (see Materials and Methods for details) and centrifuged for 2 h at 100,000 g. Fractions were collected from the top, separated by SDS-PAGE, and analyzed by immunoblot with antibodies directed against Sec21p, Sec23p, Sec22p, Bos1p, the HA-epitope (OCH1-HA), and the myc-epitope (myc-EMP47). The arrows indicate the direction of the gradient from the top to the bottom.
If v-SNAREs involved in anterograde transport are recycled, one would predict that they should be found in the COPI-coated retrograde transport vesicles derived from the Golgi fraction. Most of the Bos1p and Sec22p signal was found in the rapidly sedimenting membrane fraction (Fig. 6). Whereas no vesicular release of Bos1p and Sec22p was observed in the presence of COPII, a fraction of Bos1p and Sec22p sedimented more slowly in incubations containing coatomer, Arf1p, and GTP-γ-S (Fig. 6). This observation supports the assignment of a retrograde role for the COPI vesicles formed in this incubation.
To extend these results, we pooled fractions 6–8 of gradients described above and conducted a second fractionation based on coated membrane buoyant density. Again, Golgi membranes incubated without coat proteins did not give rise to a signal, indicating that no fragmentation of the membranes occurred during the procedures (data not shown). The pool from gradients of incubations with coatomer, Arf11p, and GTP-γ-S produced vesicles in which SNAREs and coatomer co-fractionated (Fig. 7; COPI, compare Bos1p and Bet1p to Ret1p). Incubations with COPII proteins and Sar1p instead of coatomer and Arf1p resulted in no signal of SNAREs or COPII components (Fig. 7; COPII). The Sec23p signal in the load of the flotation gradient is most likely due to trailing from the top of the velocity gradient; the blot was heavily overexposed to enhance the detection of COPII vesicles. This confirms the formation of retrograde COPI-coated transport vesicles from the Golgi-enriched membranes.
Figure 7.
Enrichment of COPI-coated vesicles derived from the Golgi. Fractions 6–8 of the gradients of a Golgi budding reaction performed in the presence of either COPI or COPII and GTP-γ-S were pooled and floated on a Nycodenz gradient as described in Material and Methods. Fractions were collected from the top, separated by SDS-PAGE, and analyzed by immunoblot with antibodies direct against Ret1p (or coatomer), Sec23p, Bos1p, and Bet1p. The arrows indicate the direction of movement of lipid particles within the gradient.
Discussion
Extensive genetic and morphological evidence has implicated COPI in retrograde vesicular traffic from the Golgi apparatus to the ER. Unfortunately, because the processes of anterograde and retrograde transport are intimately linked in a cycle, it has been difficult to assign roles to certain proteins unambiguously to one or the other limb of the cycle. For this reason, we developed a cell-free system that reproduces both events.
Previously, we established a transport assay that measures the vesicular traffic of yeast α-factor precursor from the ER to the Golgi (Baker et al., 1988). Dean and Pelham (1990) showed that the yeast retrieval signal, HDEL, appended to the COOH terminus of the α-factor precursor is sufficient to promote the retrieval of intact, glycosylated precursor (gpαF), to the ER in vivo. We combined our cell-free reaction with the use of an HDEL retrieval signal to produce a convenient and reliable tracer of the round-trip reaction. Completion of retrograde transport was monitored by the conversion of a glucosylated form of the gpαF-HDEL, formed in the ER of a strain deficient in glucose trimming of N-linked glycans, to a trimmed species in the ER of a glucosidase proficient strain. Retrieval in vitro was shown to depend on the HDEL signal and on the receptor for this signal, Erd2p.
To focus on those requirements unique to retrograde transport, we incorporated the observations of Barlowe (1997), who showed that COPII vesicles dock and fuse with a crude Golgi membrane fraction in the presence of Sec18p, the Lma1p complex, and Uso1p. Other requirements for anterograde transport were supplied by the membrane fraction. Once in the Golgi membrane, [35S]gpαF-HDEL was retrieved to an acceptor ER fraction in the presence of cytosol. The requirements for cytosol in the retrograde event were satisfied by pure yeast coatomer and myristylated Arf1p. Other obvious requirements for retrieval, such as the Arf1p nucleotide exchange proteins Gea1p and Gea2p (Chardin et al., 1996; Peyroche et al., 1996), and possibly an Arf1p GTPase-activating protein, most likely are provided as peripheral membrane components of the Golgi fraction. Retrieval was inhibited by BFA, and this inhibition was relieved by including Arf1p-GTP in the incubation. Thus, the requirement for Arf1p nucleotide exchange is recapitulated in the reaction.
Arf1p has been suggested to promote COPI vesicle budding by activating phospholipase D (PLD), which hydrolyzes PC to create phosphatidic acid, an acidic phospholipid that attracts coatomer to synthetic liposomes (Ktistakis et al., 1996). However, we saw no evidence of this pathway in our round-trip reaction. Membranes isolated from spo14, a yeast mutant missing the standard PLD, displayed normal retrieval in vitro (Waksman et al., 1996). An independent Ca2+-dependent PLD in yeast (Waksman et al., 1997) is unlikely to serve as an alternative in retrograde transport because the retrieval reaction sustained by spo14 mutant membranes was unaffected by excess EGTA. Other targets of Arf1p include phosphatidylinositol-specific kinases. PIP2 produced by these kinases may enhance the action of PLD (Martin et al., 1996). However, wortmannin, an inhibitor of this class of kinases, had no effect on our retrieval reaction.
We used the round-trip reaction to distinguish roles for three v-SNAREs that have been implicated in ER-to-Golgi traffic: Bos1p, Bet1p, and Sec22p. Our results confirm a role for Bos1p in anterograde targeting, and we suggest that Sec22p serves the equivalent role in retrograde targeting. Surprisingly, Bet1p was required in both directions. Bet1p may potentiate SNARE interactions among anterograde and retrograde partners (Stone et al., 1997) and may replace Sec22p, which is not essential in vivo, on the retrograde limb. In parallel, we have confirmed the roles of Sed5p and Ufe1p as t-SNAREs in anterograde and retrograde traffic, respectively.
Targeting/fusion of retrograde transport vesicles with the ER was blocked by Sec18p antibody. Thus, whereas both directions in the ER-to-Golgi cycle require Sec18p, its homologue, Cdc48p, acts in its place to promote homotypic fusion of ER membranes (Latterich et al., 1995). Surprisingly, although these two ATPases act to promote distinct fusion reactions involving the ER, both use the same t-SNARE, Ufe1p (Patel et al., 1998).
We have shown previously that COPII vesicles package the full set of v-SNAREs required for both directions of targeting in the ER-to-Golgi cycle (Rexach et al., 1994). Although these vesicles in principle have the complete apparatus of targeting/fusion to both the Golgi and the ER membranes, they do so only to the former (Rexach et al., 1994). Clearly, some other element, possibly protein or lipid, contributes to the directionality and specificity of this process. Two candidates are Tip20p and Sec20p, proteins that have been shown genetically to interact with Sec22p (Cosson et al., 1997; Lewis et al., 1997). Sec20p is a type II integral membrane protein that contains a lumenal HDEL sequence that ensures its recycling to the ER. Tip20p is a peripheral protein that anchors to the membrane in association with Sec20p. Perhaps Sec22p only becomes activated as a v-SNARE when it partners in the Golgi with Sec20p and Tip20p on their way back to the ER. How could this interaction be limited to the Golgi and not occur in the ER from which all three proteins will be recycled into the anterograde path?
Sec22p interacts with Ufe1p, whereas Bet1p does not (Lewis et al., 1997). Yet they are both required for retrograde transport. Bet1p and Sec22p may form a cis-SNARE complex in transport vesicles. Upon interaction with Tip20p and Sec20p, this complex may dissociate releasing Sec22p to engage in a trans-SNARE complex with Ufe1p. Bet1p may be the chaperone for Sec22p in retrograde transport and interact with Ufe1p only in the absence of Sec22p. In the anterograde direction, Bet1p and Bos1p would partner, forming the cis-SNARE complex and engaging in a trans-SNARE complex with Sed5p.
Regulated Ca2+ flux may provide a crucial distinction between targeting/fusion in the anterograde and retrograde directions. Although COPII vesicle targeting/fusion to the Golgi membrane requires Ca2+, the retrograde and homotypic fusion events do not (Rexach et al., 1994; Latterich et al., 1995). Syntaxin, the synaptic plasma membrane t-SNARE, is associated with and may regulate a Ca2+ channel to provide Ca2+ for fusion of synaptic vesicles (Leveque et al., 1994; Bezprozvanny et al., 1995). Likewise, Sec18p-mediated priming and docking of Bos1p and Sed5p may promote Ca2+ flux to activate fusion of COPII vesicles with the cis-Golgi membrane in yeast. Perhaps this Ca2+ flux acts to reconfigure the Sec22p–Sec20p– Tip20p complex in preparation for the packaging of an active, retrograde v-SNARE into COPI-coated retrieval vesicles. On return to the ER, this complex would be consumed and not reactivated until the proteins reappear at the Golgi membrane. This speculation makes obvious predictions that could be tested by comparing Sec22p complexes found in COPII and COPI vesicles formed in the round-trip reaction described here.
Acknowledgments
We are grateful to W. Wickner, Z. Xu, J. McNew, H.R. Pelham, S. Schöder-Köhne, J. Gerst, and C. Barlowe for reagents. J. Chuang, J. Herrmann, K. Matsuoka, and M. Ziman are acknowledged for critical reading of the manuscript. We thank present and past members of the Schekman lab for stimulating discussions.
This work was supported by a long-term fellowship of the European Molecular Biology Organization (EMBO) to A. Spang.
Abbreviations used in this paper
- Arf1p
ADP ribosylation factor
- BFA
brefeldin A
- PLD
phospholipase D
- SNARE
SNAP receptor
Footnotes
Address all correspondence to Randy Schekman, Department of Molecular and Cell Biology and Howard Hughes Medical Institute, 401 Barker Hall, University of California, Berkeley, CA 94720. Tel.: (510) 642-5686. Fax: (510) 642-7846. E-mail: schekman@uclink4.berkeley.edu
References
- Baker D, Hicke L, Rexach M, Schleyer M, Schekman R. Reconstitution of SECgene product-dependent intercompartmental protein transport. Cell. 1988;54:335–344. doi: 10.1016/0092-8674(88)90196-1. [DOI] [PubMed] [Google Scholar]
- Banfield DK, Lewis ML, Pelham HRB. A SNARE-like protein required for traffic through the Golgi complex. Nature. 1995;375:806–809. doi: 10.1038/375806a0. [DOI] [PubMed] [Google Scholar]
- Barlowe C. Coupled ER to Golgi transport reconstituted with purified cytosolic proteins. J Cell Biol. 1997;139:1097–1108. doi: 10.1083/jcb.139.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola B, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Bednarek S, Ravazzola M, Hosobuchi M, Amherdt M, Perrelet A, Schekman R, Orci L. COPI- and COPII-coated vesicles bud directly from the endoplasmic reticulum in yeast. Cell. 1995;83:1183–1196. doi: 10.1016/0092-8674(95)90144-2. [DOI] [PubMed] [Google Scholar]
- Bednarek SY, Orci L, Schekman R. Traffic COP's and formation of vesicle coats. Trends Cell Biol. 1996;6:468–473. doi: 10.1016/0962-8924(96)84943-9. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Scheller RH, Tsien RW. Functional impact of syntaxin on gating of N-type and Q-type calcium channels. Nature. 1995;378:623–626. doi: 10.1038/378623a0. [DOI] [PubMed] [Google Scholar]
- Chardin P, Paris S, Antonny B, Robineau S, Beraud-Dufour S, Jackson CL, Chabre M. A human exchange factor for ARF contains Sec7- and pleckstrin-homology domains. Nature. 1996;384:481–484. doi: 10.1038/384481a0. [DOI] [PubMed] [Google Scholar]
- Cosson P, Letourneur F. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science. 1994;263:1629–1631. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- Cosson P, Schröder-Köhne S, Sweet DS, Demollière C, Hennecke S, Frigerio G, Letourneur F. The Sec20/Tip20p complex is involved in ER retrieval of dilysine-tagged proteins. Eur J Cell Biol. 1997;73:93–97. [PubMed] [Google Scholar]
- Dean N, Pelham HRB. Recycling of proteins from the Golgi compartment to the ER in yeast. J Cell Biol. 1990;111:369–377. doi: 10.1083/jcb.111.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG, Cassel D, Kahn RA, Klausner RD. ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein β-COP to Golgi membranes. Proc Natl Acad Sci USA. 1992;89:6408–6412. doi: 10.1073/pnas.89.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duden R, Griffiths G, Frank R, Argos P, Kreis TE. β-COP, a 110 kd protein associated with non-clathrin-coated vesicles and the Golgi complex shows homology to β-adaptin. Cell. 1991;64:649–665. doi: 10.1016/0092-8674(91)90248-w. [DOI] [PubMed] [Google Scholar]
- Elmendorf HG, Haldar K. Identification and localization of ERD2 in the malaria parasite Plasmodium faciparum: separation from sites of sphingomyelin synthesis and implications for organization of the Golgi. EMBO (Eur Mol Biol Organ) J. 1993;12:4763–4773. doi: 10.1002/j.1460-2075.1993.tb06165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K, Veit M, Stamnes MA, Rothman JE. Bimodal interaction of coatomer with the p24 family of putative cargo receptors. Science. 1996;273:1396–1399. doi: 10.1126/science.273.5280.1396. [DOI] [PubMed] [Google Scholar]
- Gaynor EC, Emr S. COPI-independent anterograde transport: cargo-selective ER to Golgi protein transport in yeast COPI mutants. J Cell Biol. 1997;136:789–802. doi: 10.1083/jcb.136.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SL, Waters MG. Localization of a yeast early Golgi mannosyltransferase, Och1p, involves retrograde transport. J Cell Biol. 1996;132:985–998. doi: 10.1083/jcb.132.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis JCM, Nichols BJ, Dhruvakumar S, Pelham HRB. Two syntaxin homologues in the TGN/endosomal system of yeast. EMBO (Eur Mol Biol Organ) J. 1998;17:113–126. doi: 10.1093/emboj/17.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosobuchi M, Kreis T, Schekman R. SEC21is a gene required for ER to Golgi protein transport that encodes a subunit of a yeast coatomer. Nature. 1992;360:603–605. doi: 10.1038/360603a0. [DOI] [PubMed] [Google Scholar]
- Kahn RA, Clark J, Rulka C, Stearns T, Zhang C, Randazzo PA, Terui T, Cavenagh M. Mutational analysis of Saccharomyces cerevisiae ARF1. . J Biol Chem. 1995;270:143–150. doi: 10.1074/jbc.270.1.143. [DOI] [PubMed] [Google Scholar]
- Ktistakis NT, Brown HA, Waters MG, Sternweis PC, Roth MG. Evidence that phospholipase D mediates ADP ribosylation factor– dependent formation of Golgi coated vesicles. J Cell Biol. 1996;134:295–306. doi: 10.1083/jcb.134.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latterich M, Schekman R. The karyogamy gene KAR2 and novel proteins are required for ER-membrane fusion. Cell. 1994;78:87–98. doi: 10.1016/0092-8674(94)90575-4. [DOI] [PubMed] [Google Scholar]
- Latterich M, Fröhlich KU, Schekman R. Membrane fusion and the cell cycle: Cdc48p participates in the fusion of ER membranes. Cell. 1995;82:885–893. doi: 10.1016/0092-8674(95)90268-6. [DOI] [PubMed] [Google Scholar]
- Lee HI, Newman TC, Raikhel NV. The Arabidopsis endoplasmic reticulum retention receptor functions in yeast. Proc Natl Acad Sci USA. 1993;90:11433–11437. doi: 10.1073/pnas.90.23.11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F, Gaynor EC, Hennecke S, Demoullière C, Duden R, Emr SD, Riezman H, Cosson P. Coatomer is essential for retrieval of di-lysine tagged proteins to the ER. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Leveque, C., O. el Far, N. Martin-Moutot, K. Sato, R. Kato, M. Takahashi, and M.J. Seagar. 1994. Purification of the N-type calcium channel associated with syntaxin and synaptotagmin. A complex implicated in synaptic vesicle exocytosis. J. Biol. Chem. 269:6306–6312. [PubMed]
- Lewis MJ, Pelham HR. A human homologue of the yeast HDEL receptor. Nature. 1990;348:162–163. doi: 10.1038/348162a0. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Pelham HR. SNARE-mediated retrograde traffic from the Golgi complex to the endoplasmic reticulum. Cell. 1996;85:205–215. doi: 10.1016/s0092-8674(00)81097-1. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Rayner JC, Pelham HR. A novel SNARE complex implicated in vesicle fusion with the endoplasmic reticulum. EMBO (Eur Mol Biol Organ) J. 1997;16:3017–3024. doi: 10.1093/emboj/16.11.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Brown FD, Hodgkin MN, Bradwell AJ, Cook SJ, Hart M, Wakelam MJO. Activation of phospholipase D and phosphatidylinositol 4-phosphate 5-kinase in HL60 membranes is mediated by endogenous Arf but not Rho. J Biol Chem. 1996;271:17397–17403. doi: 10.1074/jbc.271.29.17397. [DOI] [PubMed] [Google Scholar]
- McNew JA, Søgaard M, Lampen NM, Machida S, Ye RR, Lacomis L, Tempst P, Rothman JE, Söllner TH. Ykt6p, a prenylated SNARE essential for endoplasmic reticulum-Golgi transport. J Biol Chem. 1997;272:17776–17783. doi: 10.1074/jbc.272.28.17776. [DOI] [PubMed] [Google Scholar]
- Orci L, Glick BS, Rothman JE. A new type of coated vesicular carrier that appears not to contain clathrin: its possible role in protein transport within the Golgi stack. Cell. 1986;46:171–184. doi: 10.1016/0092-8674(86)90734-8. [DOI] [PubMed] [Google Scholar]
- Orci L, Stamnes M, Ravazzola M, Amherdt A, Perrelet A, Söllner TH, Rothman JE. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell. 1997;90:335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- Patel SK, Indig FE, Olivieri N, Levine ND, Latterich M. Organelle membrane fusion: a novel function for the syntaxin homolog Ufe1p in ER membrane fusion. Cell. 1998;92:611–620. doi: 10.1016/s0092-8674(00)81129-0. [DOI] [PubMed] [Google Scholar]
- Peyroche A, Paris S, Jackson CL. Nucleotide exchange on ARF mediated by yeast Gea1 protein. Nature. 1996;384:479–481. doi: 10.1038/384479a0. [DOI] [PubMed] [Google Scholar]
- Rexach MF, Schekman R. Distinct biochemical requirements for the budding, targeting and fusion of ER-derived transport vesicles. J Cell Biol. 1991;114:219–229. doi: 10.1083/jcb.114.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexach M, Latterich M, Schekman R. Characteristics of endoplasmic reticulum–derived transport vesicles. J Cell Biol. 1994;126:1133–1148. doi: 10.1083/jcb.126.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama NR, Schekman RW. The role of coat proteins in the biosynthesis of secretory proteins. Curr Opin Cell Biol. 1995;7:536–543. doi: 10.1016/0955-0674(95)80011-5. [DOI] [PubMed] [Google Scholar]
- Salama NR, Yeung T, Schekman RW. The Sec13p complex and reconstitution of vesicle budding for the ER with purified cytosolic proteins. EMBO (Eur Mol Biol Organ) J. 1993;12:4073–4082. doi: 10.1002/j.1460-2075.1993.tb06091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Lopez R, Castro SG, Lizardi PM, Alagon A. The secretory pathway of Entamoeba histolytica: characterization and expression of the ERD2 gene. Arch Med Res. 1997;28:59–61. [PubMed] [Google Scholar]
- Sato K, Nishikawa S, Nakano A. Membrane protein retrieval from the Golgi apparatus to the endoplasmic reticulum (ER): characterization of the RER1gene product as a component involved in ER localization of Sec12p. Mol Biol Cell. 1995;6:1459–1477. doi: 10.1091/mbc.6.11.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Sato M, Nakano A. Rer1p as common machinery for endoplasmic reticulum localization of membrane proteins. Proc Natl Acad Sci USA. 1997;94:9693–9698. doi: 10.1073/pnas.94.18.9693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl RH, Gietz RD. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Schimmöller F, Singer-Krüger B, Schröder S, Krüger U, Barlowe C, Riezman H. The absence of Emp24p, a component of ER-derived COPII-coated vesicles, causes a defect in transport of selected proteins to the Golgi. EMBO (Eur Mol Biol Organ) J. 1995;14:1329–1339. doi: 10.1002/j.1460-2075.1995.tb07119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder S, Schimmöller F, Singer-Krüger B, Riezman H. The Golgi-localization of yeast Emp47p depends on its di-lysine motif but is not affected by the ret1-1mutation in α-COP. J Cell Biol. 1995;131:895–912. doi: 10.1083/jcb.131.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza JC, Hardwick KG, Dean N, Pelham HRB. ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell. 1990;61:1349–1357. doi: 10.1016/0092-8674(90)90698-e. [DOI] [PubMed] [Google Scholar]
- Simons JF, Ebersold M, Helenius A. Cell wall 1,6-β glucan synthesis in Saccharomyces cerevisiaedepends on ER glucosidases I and II, and the molecular chaperone BIP/kar2. EMBO (Eur Mol Biol Organ) J. 1998;17:396–405. doi: 10.1093/emboj/17.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone S, Sacher M, Mao Y, Carr C, Lyons P, Quinn AM, Ferro-Novick S. Bet1p activates the v-SNARE Bos1p. Mol Biol Cell. 1997;8:1175–1181. doi: 10.1091/mbc.8.7.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang BL, Wong SH, Qi XL, Low SH, Hong W. Molecular cloning, characterization, subcellular localization and dynamics of p23, the mammalian KDEL receptor. J Cell Biol. 1993;120:325–328. doi: 10.1083/jcb.120.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman M, Eli Y, Liscovitch M, Gerst JE. Identification and characterization of a gene encoding phospholipase D activity in yeast. J Biol Chem. 1996;271:2361–2364. doi: 10.1074/jbc.271.5.2361. [DOI] [PubMed] [Google Scholar]
- Waksman M, Tang X, Eli Y, Gerst JE, Liscovitch M. Identification of a novel Ca2+-dependent, phosphatidylethanolamine-hydrolyzing phospholipase D in yeast bearing a disruption in PLD1. . J Biol Chem. 1997;272:36–39. doi: 10.1074/jbc.272.1.36. [DOI] [PubMed] [Google Scholar]
- Whiteheart SW, Rossnagel K, Buhrow SA, Brunner M, Jaenicke R, Rothman JE. N-ethylmaleimide-sensitive fusion protein: a trimeric ATPase whose hydrolysis of ATP is required for membrane fusion. J Cell Biol. 1994;126:945–954. doi: 10.1083/jcb.126.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuestehube LJ, Schekman RW. Reconstitution of transport from endoplasmic reticulum to Golgi complex using endoplasmic reticulum-enriched membrane fraction from yeast. Methods Enzymol. 1992;219:124–136. doi: 10.1016/0076-6879(92)19015-x. [DOI] [PubMed] [Google Scholar]
- Xu Z, Mayer A, Muller E, Wickner W. A heterodimer of thioredoxin and IB2 acts with Sec18p (NSF) to promote yeast vacuole inheritance. J Cell Biol. 1997;136:299–306. doi: 10.1083/jcb.136.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa K, Resnick D, Abeijon C, Robbins PW, Hirschberg CB. A guanosine diphosphatase enriched in Golgi vesicles of Saccharomyces cerevisiae. Purification and characterization. J Biol Chem. 1990;265:19351–19355. [PubMed] [Google Scholar]