Abstract
Polyunsaturated fatty acids (PUFAs) exert immunosuppressive effects, but the molecular alterations leading to T cell inhibition are not yet elucidated. Signal transduction seems to involve detergent-resistant membrane domains (DRMs) acting as functional rafts within the plasma membrane bilayer with Src family protein tyrosine kinases being attached to their cytoplasmic leaflet. Since DRMs include predominantly saturated fatty acyl moieties, we investigated whether PUFAs could affect T cell signaling by remodeling of DRMs. Jurkat T cells cultured in PUFA-supplemented medium showed a markedly diminished calcium response when stimulated via the transmembrane CD3 complex or glycosyl phosphatidylinositol (GPI)- anchored CD59. Immunofluorescence studies indicated that CD59 but not Src family protein tyrosine kinase Lck remained in a punctate pattern after PUFA enrichment. Analysis of DRMs revealed a marked displacement of Src family kinases (Lck, Fyn) from DRMs derived from PUFA-enriched T cells compared with controls, and the presence of Lck in DRMs strictly correlated with calcium signaling. In contrast, GPI-anchored proteins (CD59, CD48) and ganglioside GM1, both residing in the outer membrane leaflet, remained in the DRM fraction. In conclusion, PUFA enrichment selectively modifies the cytoplasmic layer of DRMs and this alteration could underlie the inhibition of T cell signal transduction by PUFAs.
Keywords: fatty acids, membrane lipids, lymphocytes, signal transduction, protein-tyrosine kinases
Antigen-specific immune responses are initiated by activation of T cells. Apart from stimulation via the T cell receptor (TCR)1–CD3 complex, costimulatory signals are required to result in full activation or anergy, respectively (Schwartz, 1990). A group of costimulatory molecules that are attached to the plasma membrane by a glycosyl phosphatidylinositol (GPI)-anchor (Robinson, 1991; Brown, 1993) is clustered in distinct detergent-resistant membrane domains (DRMs; Melkonian et al., 1995). Since a significant amount of Src family protein tyrosine kinases that play an essential role in T cell activation (Chan and Shaw, 1995) are concentrated on the cytoplasmic side of DRMs (Stefanova et al., 1991; Cinek and Hořejší, 1992), signaling via GPI-anchored proteins strictly depends on their localization in membrane domains (van den Berg et al., 1995). However, recent studies revealed that also signaling via transmembrane proteins is based on DRMs since critical phosphorylation events stimulated via the platelet-derived growth factor receptor (Liu et al., 1997) and the high-affinity IgE receptor (Field et al., 1997) occur in such membrane domains. Thus, DRMs appear to represent functional rafts (Simons and Ikonen, 1997) allowing spatially confined signaling events in general.
DRMs are insoluble in nonionic detergents due to their high content of cholesterol and sphingolipids (Brown and Rose, 1992; Schroeder et al., 1994; Hanada et al., 1995). These lipids self-associate by hydrophobic interactions involving predominantly saturated acyl chains to constitute DRMs as a liquid-ordered environment (Ahmed et al., 1997). Interestingly, many proteins associate with DRMs by fatty acyl moieties, e.g., by a GPI-anchor or by NH2-terminal myristoylation and palmitoylation, as it is the case with Src family protein tyrosine kinases (Rodgers et al., 1994; Shenoy-Scaria et al., 1994; Kabouridis et al., 1997). However, since sphingolipids are exclusively situated in the outer leaflet of the plasma membrane, no clear model exists on the lipid composition of the inner leaflet of DRMs.
Polyunsaturated fatty acids (PUFAs) modulate immune response particularly by affecting T cell function (Zurier, 1993). Accordingly, dietary PUFA supplementation modifies lymphocyte membrane phospholipids and function (Brouard and Pascaud, 1990; Valette et al., 1991; Rossetti et al., 1997). Notably, PUFAs affect T cells independent of eicosanoid production (Santoli and Zurier, 1989; Calder et al., 1992; Fowler et al., 1993). Therefore, PUFAs particularly of the (n-3) series have found clinical application not only for the prevention of atherosclerosis (Schmidt and Dyerberg, 1994) but also for the treatment of various inflammatory diseases (Belluzzi et al., 1996; Cappelli et al., 1997) and as adjuvant immunosuppressive agents (van der Heide et al., 1993). However, the underlying molecular and cellular mechanisms of PUFA-induced T cell inhibition have not yet been elucidated in detail.
Since lipid interactions are crucial for the formation of DRMs, the immunosuppressive effects of PUFAs could be due to changes in DRM structure and composition. Here we can show, that inhibition of T cell signaling by PUFAs is strictly paralleled their ability to remove Src family kinases from DRMs. Thus, our data provide evidence for a functionally important modification of DRMs by PUFAs thereby postulating a molecular mechanism for PUFA-mediated suppression of T cell activation.
Materials and Methods
Antibodies
Mouse mAbs MEM-43 (IgG2a), MEM-43/5 (IgG2b, both CD59), MEM-102 (IgG1, CD48), and lck-01 (IgG1, Lck) were all provided by Dr. V. Hořejší (Institute of Molecular Genetics, Prague, Czech Republic) and partly obtained from Monosan (Uden, The Netherlands). Other antibodies were purchased as follows: OKT3 (IgG2a, CD3) from Ortho Pharmaceuticals (Raritan, NJ); mAbs for Western blotting of Lck (clone 28, IgG2a) and Fyn (clone 25, IgG2b) from Transduction Laboratories (Lexington, KY); CD3ζ (8D3, IgG1) from PharMingen (San Diego, CA); F(ab′)2-fragments of goat anti–mouse (GAM)-IgG from Sigma Chemical Co. (St. Louis, MO) or Jackson ImmunoResearch Laboratories Inc. (West Grove, PA); HRP-labeled GAM-IgG from Bio-Rad Laboratories (Hercules, CA); FITC-labeled F(ab′)2-fragments of GAM-Ig from Dako (Glostrup, Denmark); TRITC-labeled F(ab′)2-fragments of GAM-IgG from Accurate (Westbury, NY).
Cell Culture and Lipid Modifications
The human T cell line Jurkat E6-1 (American Type Culture Collection, Rockville, MD) was grown under standard conditions in RPMI 1640 medium supplemented with 10% heat-inactivated bovine calf serum (HyClone, Logan, UT), penicillin/streptomycin (50 U/ml and 50 μg/ml, respectively; GIBCO BRL, Gaithersburg, MD), and 2 mM glutamine (GIBCO BRL) at 37°C in humidified atmosphere in presence of 5% CO2. For modifications of cellular lipids, 2 × 105 cells/ml were incubated for 2 d in serum-free Iscove's modified Dulbecco's medium (GIBCO BRL), supplemented with 0.4% (wt/vol) BSA (fraction V, containing less than 3 μM total fatty acids) purchased from Sigma Chemical Co. as all other reagents unless stated otherwise, 1 mg/l transferrin, 8.1 mg/l monothioglycerol, and glutamine and antibiotics as above (Stulnig et al., 1997). Free fatty acids of highest available quality (Sigma Chemical Co.) were added at 12.5–50 μM from stock solutions in ethanol (EtOH, final concentration ≤ 0.5%) into prewarmed and vigorously stirred medium before addition to cells. Control cultures always included ethanol at the appropriate concentration, and the solvent itself had no effect on signal transduction or biochemical analyses. In some experiments 10 μM butylated hydroxy-toluene (BHT) was included. Cell viability was >90% as determined by trypan blue exclusion.
Lipid Analyses
Cell lipids were extracted with chloroform/methanol (Bligh and Dyer, 1959), dried under nitrogen, transmethylated with methanolic hydrochloric acid and separated by gas chromatography (HP 5890A; Hewlett-Packard, Palo Alto, CA) as detailed previously (Raederstorff et al., 1991). In brief, a fused silica capillary column (50-m length, 0.25-mm inner diam, 0.1-mm layer thickness) was used with the following conditions: injection at 55°C, 10°C/min from 55–177°C, 1°C/min from 177–218°C, 4°C/min from 218-270°C. C-17 methylester was used as internal standard, and fatty acids were quantified by using commercial methylester standards. Unesterified cholesterol was quantified according to Heider and Boyett (1978). Data are presented as means ± SD and were compared by unpaired two-tailed Student's t test.
Quantitation of Calcium Response
The stimulated rise in cytoplasmic calcium concentration was determined as described (Stulnig et al., 1997). In brief, Jurkat cells were labeled with the fluorescent Ca2+-indicator INDO-1 (Molecular Probes, Eugene, OR), primed with mAb MEM-43 or washing buffer (HBSS including 10 mM Hepes, pH 7.4) containing 1% BSA for 20 min, and were finally stimulated by cross-linking with F(ab′)2-fragments of GAM-IgG. The specificity of the reaction was elaborated previously (Stulnig et al., 1997). For CD3 stimulation, OKT3 was added directly without extra priming or cross-linking. Thapsigargin was added at 0.2 μM. Measurement of [Ca2+]i by flow cytometry was performed on a FACStarplus® (Becton Dickinson, San Jose, CA; excitation 333–363 nm, detection at 530 nm for calcium-free INDO-1 and at 395 nm for calcium-bound form) at 37°C constant temperature. The ratio of the fluorescence intensity at both wavelengths was computed as a direct estimate of the cytoplasmic calcium concentration (Grynkiewicz et al., 1985), and results were expressed as percent of the solvent control as described (Stulnig et al., 1997).
Protein Tyrosine Phosphorylation
Protein tyrosine phosphorylation was analyzed on 2 × 107 Jurkat T cells stimulated by OKT3 (5 μg/ml) for 2 min at 37°C or left unstimulated. The reaction was stopped by addition of ice-cold washing buffer and pelleting by brief centrifugation (30 s, 13,000 g, 4°C). Cells (5 × 107/ml) were immediately lysed for 30 min in ice-cold TBS (20 mM Tris-HCl, pH 8.2, 140 mM NaCl) containing 1% NP-40 (Pierce Chemical Co., Rockford, IL), 1 mM sodium orthovanadate, 10 mM NaF, 5 mM EDTA, and protease inhibitors (1 μM pepstatin, 10 μg/ml aprotinin [Bayer, Leverkusen, Germany], 5 mM iodoacetamide, 10 μg/ml leupeptin and 0.4 mM pefabloc [Boehringer Mannheim, Mannheim, Germany]). Nuclei were removed by brief centrifugation and proteins were analyzed by reducing SDS-PAGE and immunoblotting using HRP-labeled anti-phosphotyrosine antibody 4G10 (1:4,000; Upstate Biotechnology, Lake Placid, NY).
Analysis of DRMs
Jurkat T cells (4 × 107) were washed three times in washing buffer and incubated in 1 ml hypotonic buffer (42 mM KCl, 5 mM MgCl2, 10 mM Hepes, pH 7.4) for 15 min on ice in the presence of protease inhibitors. Cells were broken by passing 10 times through a 26-G needle and checked by microscopy. Post-nuclear supernatants were prepared (200 g, 10 min, 4°C) and plasma membranes were enriched by centrifugation at 10,000 g for 10 min at 4°C (Cerny et al., 1996). Membrane pellets were lysed for 30 min on ice in 0.5 ml lysis buffer consisting of TBS including 1% Brij-58 (Pierce Chemical Co.) and protease inhibitors (Stulnig et al., 1997). Lysates were adjusted to 40% (wt/vol) sucrose, placed under a 5–20% linear sucrose gradient in TBS with protease inhibitors and ultracentrifuged (345,000 g, 18 h, 4°C; SW60Ti; Beckman Instruments, High Wycombe, Buckinghamshire, UK). 400-μl fractions were collected from the top. In some experiments, 100-μl aliquots of fractions were diluted 10× with TBS and insoluble particles were spun down at 400,000 g for 1 h and pellets redissolved in SDS sample buffer. Proteins were separated by nonreducing SDS-PAGE (Laemmli, 1970) and blotted onto nitrocellulose membrane (Hybond ECL, Amersham, UK). Membranes were developed according to standard Western blotting procedures, and detection was performed by chemiluminescence (Boehringer Mannheim). For quantitative comparisons, films (X-omat AR; Eastman Kodak Co., Rochester, NY) were analyzed by densitometric scanning. Protein content was quantified by the BCA-assay using BSA standard (Pierce Chemical Co.).
Detection of GM1
Ganglioside GM1 was detected on dot blots of fractions onto nitrocellulose membrane, after blocking with 3% BSA in phosphate-buffered saline (pH 7.4), and binding of 2 ng/ml HRP-labeled cholera toxin B subunit (Calbiochem-Novabiochem, La Jolla, CA) in blocking buffer (adapted from Ilangumaran et al., 1996). Blots were washed 4× in TBS containing 0.05% Tween-20 (Pierce Chemical Co.) and detected by chemiluminescence.
Immunofluorescence Analysis
Cell surface expression of CD3 and CD59 on unfixed cells was detected by indirect immunofluorescence using FITC-labeled second antibody and quantified by flow cytometry. For microscopic examination of CD59, cells were either fixed as usual in 4% formaldehyde for 20 min followed by 0.1 M glycine, or by prolonged fixation (1 h) as proposed by Mayor et al. (1994) to prevent antibody-induced clustering. Lck was detected on cytoslides fixed in ice-cold acetone for 5 min using mAb lck-01 and all antigens were visualized by TRITC-labeled GAM-Ig.
Results
Enrichment of PUFAs in T Cells
Fatty acid composition of Jurkat T cells was modified by two day culture in medium supplemented with distinct fatty acids (Table I). Addition of any PUFA markedly increased their proportion in parallel with a decrease in monounsaturated fatty acids. Moreover, at least half of the originally added PUFA was elongated to C20 and C22 fatty acids with concomitant further unsaturation up to pentaenes (Marzo et al., 1995), but hexaenes (22:6) were not synthesized by the cells (Chow et al., 1991). Membrane cholesterol content was not diminished by PUFA enrichment, ruling out that cholesterol lowering might underlie inhibition of signal transduction by GPI-anchored proteins (Stulnig et al., 1997).
Table I.
Modification of T Cell Fatty Acid Composition
| EtOH‡ | 18:0 | 18:1 (n-9) | 18:2 (n-6) | 18:3 (n-3) | 20:4 (n-6) | 20:5 (n-3) | 22:6 (n-3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14:0 | 1.8 ± 0.2§ | 1.1 ± 0.3 | 1.0 ± 0.2 | 1.3 ± 0.2 | 1.7 ± 0.3 | 1.5 ± 0.3 | 1.6 ± 0.1 | 1.7 ± 0.3 | ||||||||
| 16:0 | 26.4 ± 0.7 | 17.0 ± 1.4** | 19.0 ± 1.9** | 22.1 ± 2.3 | 29.6 ± 2.3 | 24.7 ± 2.1 | 24.3 ± 1.0* | 28.3 ± 1.5 | ||||||||
| 16:1 | 7.0 ± 0.7 | 2.6 ± 0.6 | 2.0 ± 0.5 | 2.1 ± 1.0** | 4.1 ± 1.5* | 2.1 ± 0.9* | 2.1 ± 0.7** | 3.4 ± 0.6** | ||||||||
| 18:0 | 10.8 ± 0.4 | 14.5 ± 1.4 | 12.7 ± 1.7 | 10.1 ± 0.3 | 10.6 ± 2.5 | 10.2 ± 0.5 | 10.8 ± 1.0 | 12.8 ± 0.2** | ||||||||
| 18:1 (n-9) | 24.7 ± 0.0 | 39.8 ± 0.5** | 41.9 ± 4.4* | 8.7 ± 1.2** | 14.1 ± 4.5 | 9.1 ± 0.9** | 10.0 ± 0.3** | 13.6 ± 0.6** | ||||||||
| 18:1 (n-7) | 9.0 ± 1.0 | 4.9 ± 0.6** | 4.3 ± 0.6** | 2.5 ± 0.6** | 3.3 ± 0.8** | 2.3 ± 0.3** | 2.4 ± 0.1** | 3.2 ± 0.3** | ||||||||
| 18:2 (n-6) | 1.9 ± 0.4 | 1.4 ± 0.2 | 1.2 ± 0.2 | 11.5 ± 0.8** | 4.4 ± 3.0 | 1.4 ± 0.4 | 1.7 ± 0.4 | 1.8 ± 0.3 | ||||||||
| 18:3 (n-6) | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 1.8 ± 0.5 | 0.1 ± 0.0 | 0.3 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 | ||||||||
| 18:3 (n-3) | 0.3 ± 0.1 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 6.1 ± 2.1* | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | ||||||||
| 18:4 (n-3) | 0.2 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 1.0 ± 0.3 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | ||||||||
| 20:0 | 0.2 ± 0.1 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | ||||||||
| 20:1 (n-9) | 2.0 ± 0.1 | 4.0 ± 0.1** | 4.5 ± 0.5* | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.0 | 1.0 ± 0.1 | 1.2 ± 0.1 | ||||||||
| 20:2 (n-6) | 0.4 ± 0.1 | 0.3 ± 0.0 | 0.3 ± 0.1 | 2.6 ± 0.1 | 1.2 ± 0.7 | 0.4 ± 0.0 | 0.5 ± 0.1 | 0.5 ± 0.1 | ||||||||
| 20:3 (n-9) | 2.6 ± 0.2 | 3.6 ± 0.2 | 3.0 ± 0.2 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | ||||||||
| 20:3 (n-6) | 1.9 ± 0.3 | 1.6 ± 0.2 | 1.1 ± 0.2 | 16.5 ± 3.3* | 2.0 ± 0.5 | 2.7 ± 0.0 | 1.7 ± 0.1 | 2.0 ± 0.2 | ||||||||
| 20:4 (n-6) | 4.0 ± 0.4 | 2.9 ± 0.2 | 2.1 ± 0.3 | 7.3 ± 0.5** | 1.5 ± 0.4 | 19.1 ± 2.5** | 1.3 ± 0.0 | 2.2 ± 0.0 | ||||||||
| 20:5 (n-3) | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 4.5 ± 1.4* | 0.1 ± 0.0 | 12.9 ± 1.5** | 2.0 ± 0.2 | ||||||||
| 22:0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.0 | ||||||||
| 22:1 (n-9) | 0.6 ± 0.2 | 0.7 ± 0.0 | 0.7 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | ||||||||
| 22:4 (n-6) | 2.9 ± 0.4 | 1.7 ± 0.1 | 2.1 ± 0.3 | 8.6 ± 0.2** | 1.1 ± 0.3 | 20.6 ± 0.9** | 1.1 ± 0.1 | 1.2 ± 0.0 | ||||||||
| 22:5 (n-6) | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.6 ± 0.1 | 0.2 ± 0.1 | 0.9 ± 0.2 | 0.1 ± 0.0 | 0.1 ± 0.0 | ||||||||
| 22:5 (n-3) | 0.6 ± 0.1 | 0.4 ± 0.0 | 0.4 ± 0.1 | 0.4 ± 0.0 | 10.1 ± 3.4* | 0.5 ± 0.0 | 24.2 ± 1.8** | 2.0 ± 0.1 | ||||||||
| 22:6 (n-3) | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.6 ± 0.2 | 0.1 ± 0.0 | 0.7 ± 0.3 | 21.0 ± 0.8** | ||||||||
| 24:0 | 0.6 ± 0.4 | 0.9 ± 0.3 | 1.0 ± 0.2 | 1.2 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.3 ± 0.5 | 0.9 ± 0.2 | ||||||||
| 24:1 | 0.7 ± 0.0 | 1.0 ± 0.1 | 1.1 ± 0.1 | 0.5 ± 0.0 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.0 | ||||||||
| Saturated | 40.3 ± 1.2 | 34.2 ± 1.1** | 34.4 ± 6.1 | 35.1 ± 2.3* | 43.7 ± 1.6* | 38.0 ± 1.4 | 38.6 ± 0.1 | 44.2 ± 2.1 | ||||||||
| Monounsaturated | 44.0 ± 1.8 | 53.0 ± 1.2** | 54.5 ± 5.0 | 15.0 ± 2.9** | 23.3 ± 6.4* | 15.2 ± 1.9** | 16.4 ± 0.8** | 22.1 ± 1.6** | ||||||||
| Polyunsaturated | 15.7 ± 1.9 | 12.8 ± 1.2 | 11.0 ± 1.2* | 49.9 ± 5.0** | 33.0 ± 6.2* | 46.8 ± 2.9** | 45.0 ± 0.7** | 33.7 ± 0.5** | ||||||||
| Unsaturation index | 1.0 ± 0.1‖ | 1.0 ± 0.0 | 0.9 ± 0.1 | 1.7 ± 0.1** | 1.5 ± 0.3 | 2.0 ± 0.1** | 2.3 ± 0.0** | 2.0 ± 0.0** | ||||||||
| Cholesterol | 7.4 ± 0.4 ¶ | 8.0 ± 1.1 | 7.3 ± 0.5 | 8.5 ± 0.7 | ND | ND | 9.0 ± 0.2 | 7.6 ± 0.3 |
Significant differences to the solvent control are indicated by asterisks (
P < 0.05,
P < 0.01,
P < 0.001) for fatty acids exceeding 4.0 mol%.
Jurkat T cells were cultured for 48 h in medium supplemented with 25 μM of fatty acids or solvent (EtOH) as indicated.
Fatty acids are expressed in mol%; means ± SD from three independent determinations.
The unsaturation index is calculated as the mean number of double bonds per fatty acid.
μg/mg protein.
Enrichment with PUFAs Inhibits T Cell Signaling
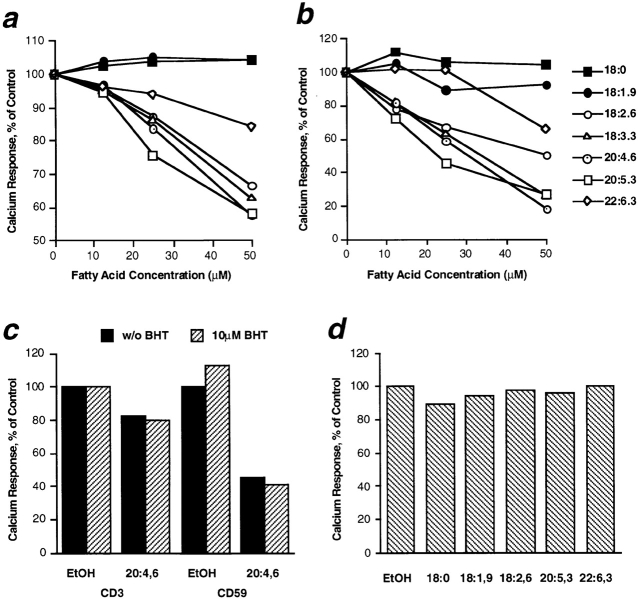
Fatty acid–modified T cells were stimulated via the transmembrane TCR–CD3 complex and GPI-anchored CD59 and analyzed for the rise in cytoplasmic calcium concentration. Whereas enrichment with saturated and monounsaturated fatty acids did not change calcium response in T cells, PUFAs dose-dependently inhibited signaling via both CD3 and CD59 (Fig. 1, a and b). Signal transduction via CD59 was more heavily affected than CD3 signaling, and at 50 μM fatty acid concentration calcium response was decreased to 20% and 60%, respectively, of the solvent control. Cell surface expression of CD3 and CD59 was essentially unaltered in all instances, except for cells treated with 50 μM of 16:0 which showed a decrease in CD59 expression to 88% of control, and those treated with maximal concentrations of 18:2 (n-6) and 20:4 (n-6) that overexpressed CD3 to 162% and 143% of control levels, respectively (data not shown). Addition of the antioxidant BHT to the culture did not reverse PUFA-mediated inhibition of calcium signaling ruling out that fatty acid oxidation was responsible for this effect (Fig. 1 c). Moreover, maximal rise in cytoplasmic calcium concentration as induced by incubation with the endoplasmic reticulum [Ca2+]-ATPase inhibitor thapsigargin was unaltered in PUFA-enriched T cells compared with controls indicating that calcium influx itself was not affected by the treatment (Fig. 1 d). Thus, enrichment of T cell lipids with PUFAs inhibits very early signaling events.
Figure 1.
Calcium signaling in T cells enriched with different fatty acids. Jurkat T cells were cultured in medium supplemented with distinct fatty acids as indicated, and stimulated for calcium response via CD3 or GPI-anchored CD59. The maximal rise in cytoplasmic calcium concentration was monitored and is given as percent of the solvent control (EtOH; Stulnig et al., 1997). (a and b) Cells incubated with various amounts of fatty acids were stimulated via CD3 (a) or CD59 (b). Means from three independent analyses are given for each fatty acid. (c) T cells were cultured with or without 25 μM polyunsaturated arachidonic acid (20:4 (n-6)) in presence or absence of the antioxidant BHT (10 μM) were stimulated via CD3 or CD59 as indicated. (d) T cells cultured with 25 μM of different fatty acids or EtOH were incubated with thapsigargin for induction of maximal calcium influx.
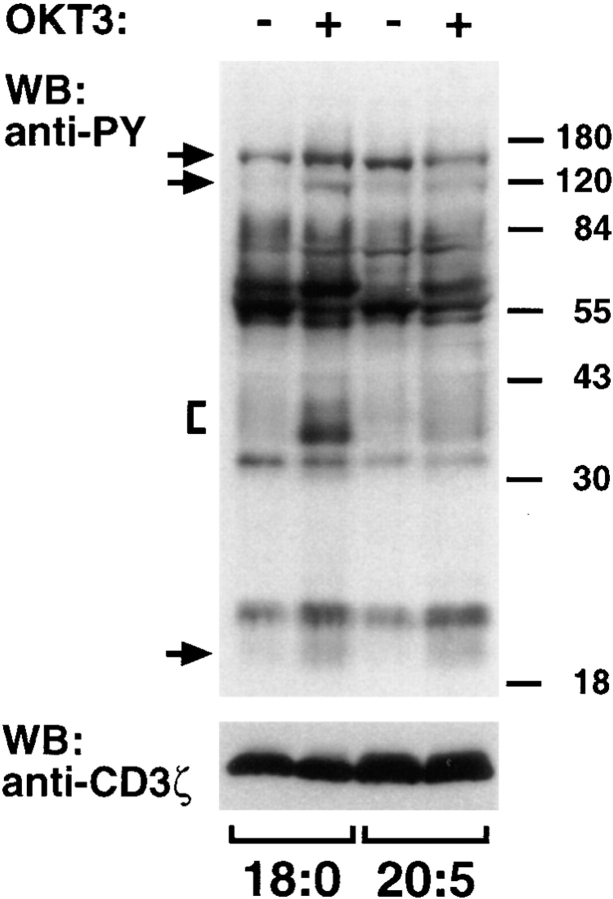
Calcium signaling requires generation of inositol 1,4,5-trisphosphate by phospholipid breakdown that by itself could be disturbed by membrane lipid alterations. However, also CD3 signaling independent of phospholipid cleavage, as is the induction of protein tyrosine phosphorylation, was markedly reduced in PUFA-enriched T cells (20:5) compared with the control (18:0) without apparent changes in the pattern of phosphoproteins (Fig. 2). Thus, inhibition of T cell signaling by PUFA enrichment involves protein tyrosine phosphorylation.
Figure 2.
Stimulation of protein tyrosine phosphorylation in PUFA-enriched T cells. Jurkat T cells were cultured in medium supplemented with 50 μM eicosapentaenoic (20:5 (n-3)) or stearic acid (18:0) which served as control. Cells were either left unstimulated or stimulated by mAb OKT3 for 2 min as indicated. Lysates from 4 × 105 cells per lane (21.6 and 21.2 μg cell protein for 18:0 and 20:5-treated cells, respectively) were analyzed by Western blotting for tyrosine phosphorylated proteins (PY), and for CD3ζ to control for equal loading. Note that the phosphorylation of typical phosphoproteins as indicated on the left was markedly abolished in stimulated PUFA-enriched T cells compared with the control. Molecular mass of marker proteins are given in thousands on the right.
The increase in the proportion of PUFAs and particularly the unsaturation index of fatty acid–modified T cells was associated with inhibition of T cell signaling, but not in case of the very long chain docosahexaenoic acid (22:6 (n-3); see Table I, Fig. 1, a and b) that only moderately inhibited calcium response. Thus, not fatty acid unsaturation by itself but molecular changes differently induced by distinct PUFAs might underlie inhibition of T cell calcium response.
Localization of Membrane Proteins in PUFA-enriched T Cells
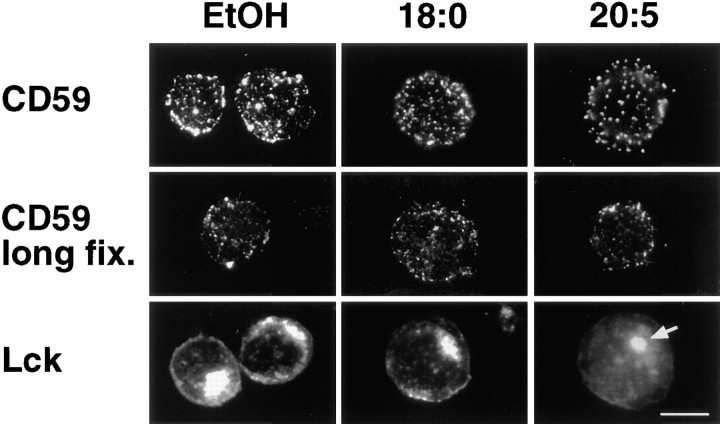
Lipid–lipid interactions are crucial for the formation of membrane domains containing GPI-anchored proteins and Src family protein tyrosine kinases, thereby suggesting a role of membrane domains in signal transduction (Robinson, 1991; Stefanova et al., 1991; Brown, 1993). Membrane domains may be detected by immunofluorescence, though protein clustering could be antibody-induced unless cells have been extensively fixed (Mayor et al., 1994). Even on profoundly fixed cells, a clustered pattern of GPI-anchored CD59 was observed irrespective of whether cells have been cultured with PUFAs or noninhibitory fatty acids as exemplified by eicosapentaenoic (20:5 (n-3)) and stearic acid (18:0) in Fig. 3. Src family protein tyrosine kinase Lck, which from confocal analysis (Ley et al., 1994) is known to be located at the plasma membrane apart from its association with pericentrosomal vesicles (Fig. 3, arrow), showed a fine-grained distribution only in cells cultured under noninhibitory conditions (Fig. 3). In contrast to control cells, most of the presumed plasma membrane Lck was diffusely dispersed in PUFA-enriched T cells. Thus, immunofluorescence analyses provided a hint that organization of plasma membrane domains could be disturbed in PUFA-enriched T cells.
Figure 3.
Distribution of GPI-anchored CD59 and Src family kinase Lck in fatty acid–modified T cells. Jurkat T cells cultured in the presence of either no fatty acid (EtOH), 25 μM saturated stearic acid (18:0) or polyunsaturated eicosapentaenoic acid (20:5 (n-3)) were fixed with paraformaldehyde and analyzed for the cell surface distribution of CD59 by immunofluorescence. CD59 was detected on cells fixed by conventional formaldehyde fixation (CD59; mAb MEM-43), or by prolonged fixation that was proposed to prevent antibody-induced clustering of surface proteins (Mayor et al., 1994), but markedly interfered with immunodetection (CD59 long fix.; mAb MEM-43/5). For detection of Lck, fatty acid–modified cells were fixed in acetone before immunofluorescence staining. According to Ley et al. (1994), Lck was localized on the plasma membrane and in pericentrosomal vesicles (arrow). Compared with the clustered pattern of Lck in untreated (EtOH) and 18:0-treated cells, Lck was rather diffusely distributed in PUFA-enriched T cells but without discrimination of the nucleus suggesting membrane localization of the kinase. Bar, 5 μm.
PUFA Enrichment Selectively Modifies the Cytoplasmic Leaflet of DRMs
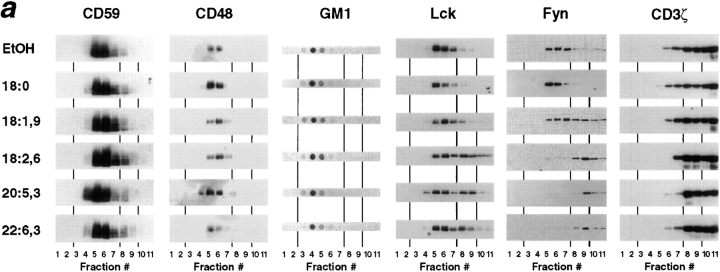
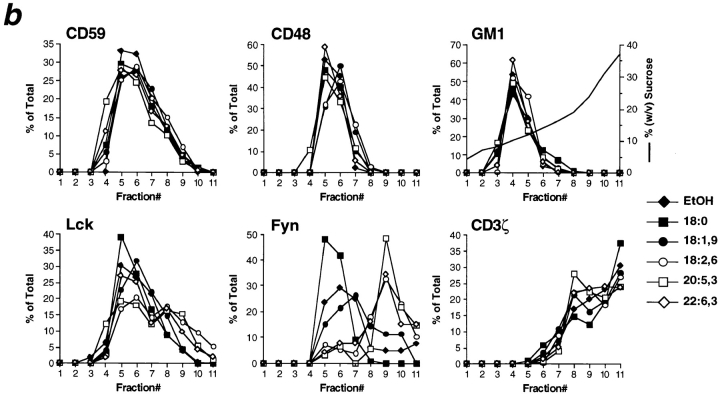
DRMs consist of a lipid bilayer with GPI-anchored proteins and sphingolipids, e.g., GM1, residing in the exoplasmic leaflet and Src family protein tyrosine kinases attached to the cytoplasmic leaflet. Since the enhanced unsaturation of fatty acyl moieties in PUFA-enriched cells could disturb lipid–lipid interactions in DRMs, we analyzed fatty acid–treated T cells for the location of these marker components in DRMs by floating in density gradients. As shown in Fig. 4, nearly all GPI-anchored CD59 and CD48 from cells treated with saturated or monounsaturated fatty acids floated up to DRM fractions 4–7 containing 9–17% sucrose (buoyant density 1.034–1.070 g/cm3), though these fractions together comprised only about 1% of total protein. Similar to controls, GPI-anchored proteins from PUFA-enriched T cells were recovered almost exclusively in DRM fractions 4–7. In addition to GPI-anchored proteins, most of the ganglioside GM1 was located in DRM fractions irrespective of fatty acid enrichment. Thus, the outer leaflet of DRMs as detected by GPI-anchored proteins and sphingolipids was not altered in T cells enriched with PUFAs.
Figure 4.
Density gradient distribution of membrane constituents from fatty acid–modified T cells after lysis with nonionic detergent. (a) Membranes of T cells treated with 50 μM fatty acids were solubilized in lysis buffer containing 1% Brij-58 and fractionated by sucrose density gradient centrifugation. Floating fractions 4–7 indicate DRMs, fractions 8 and 9 were named intermediate, whereas most soluble proteins were localized at the bottom in fractions 10 and 11. Typical distribution of GPI-anchored proteins CD59 and CD48, ganglioside GM1, Src family kinases Lck and Fyn, and CD3ζ are shown from a single experiment from a total of 3–8 for each antigen. CD3ζ-blots were overexposed to illustrate its occurrence in DRM fractions. (b) Densitometric analysis of films shown in panel (a) with data from individual fractions given in percent of the whole gradient. The shape of the sucrose gradient is illustrated by the plain line within the GM1 histogram.
Src family kinase Lck is critically involved in the initiation of TCR signal transduction (Weiss and Littman, 1994). Lck is required for phosphorylation and hence activation of PLCγ1, which links the kinase cascade to inositol 1,4,5-trisphosphate generation and calcium signaling, whereas the role of Src family kinase Fyn in this signaling pathway is less well documented. Src family kinases p56lck and p59fyn were predominantly located in DRM fractions 4–7 only when cells were cultured with saturated or monounsaturated fatty acids (Fig. 4). Particularly with 18:0, an additional p60lck was detected that is known to be due to Lck serine phosphorylation and not to enhance catalytic activity of the kinase (Veillette et al., 1988). In contrast to saturated and monounsaturated fatty acids, membranes from T cells enriched with polyunsaturated linoleic (18:2 (n-6)) or eicosapentaenoic acid (20:5 (n-3)) showed a strong shift of Lck from DRMs toward higher density fractions, and Fyn was almost totally displaced from DRMs in PUFA-enriched T cells. A small proportion (∼10%) of CD3ζ was also found in DRMs, and this proportion was moderately reduced in PUFA-enriched cells (Fig. 4). Since the transmembrane CD3ζ molecule largely consists of a cytoplasmic domain, interactions with palmitoylated proteins, as are Src family kinases, might occur that would not only explain its localization in DRMs but also its displacement from DRMs in PUFA-enriched T cells. Notably, in contrast to other PUFAs, T cells enriched with the highly unsaturated docosahexaenoic acid (22:6 (n-3)), which only moderately inhibited calcium response (Figs. 1 and 2), displaced Src family kinase Lck from DRMs to a considerably minor extent. In conclusion, PUFA enrichment selectively altered the cytoplasmic layer of DRMs particularly by displacing Src family protein tyrosine kinases from DRMs to other membrane regions.
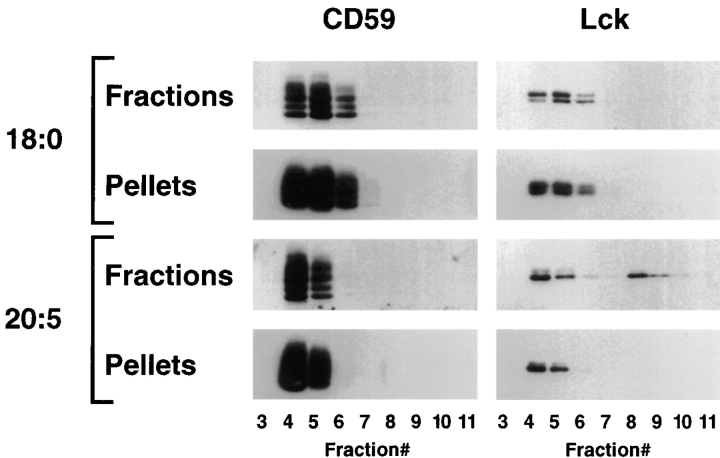
Notably, part of Src family kinases from PUFA-enriched T cells, particularly from cells treated with eicosapentaenoic acid (20:5 (n-3)), was recovered from intermediate fractions 8 and 9 containing 18–27% sucrose (buoyant density 1.070–1.115 g/cm3; Fig. 4). This intermediate fraction contained ∼10% of total protein and was clearly separated from DRMs in fractions 4–7 and the mass of soluble proteins (89%) in fractions 10 and 11. In contrast to GPI-anchored proteins and Src-family kinases from DRM fractions 4–7, Lck from intermediate fractions could be pelleted only in trace amounts (Fig. 5), suggesting that Lck originally located in detergent-soluble parts of the membrane was recovered in this fraction.
Figure 5.
Detergent-insolubility of gradient fractions in fatty acid– treated T cells. Membranes of Jurkat T cells treated with 50 μM stearic acid (18:0) or polyunsaturated eicosapentaenoic acid (20:5 (n-3)) were fractionated by density gradient centrifugation as described in Fig. 4. Aliquots of individual fractions were diluted and pelleted to test for detergent-insoluble components. CD59 and Lck were detected in fractions and pellets by Western blotting.
Correlation of Signal Transduction in PUFA-enriched T Cells with the Presence of Src Family Kinase Lck in DRMs
The presence of Src family protein tyrosine kinase Lck in DRMs strictly correlated with calcium response via CD3 and CD59 in fatty acid–treated cells and could also explain the attenuated inhibition by docosahexaenoic acid (22:6 (n-3)) as illustrated in Fig. 6. The correlation obtained with Fyn was less apparent (data not shown). The correlation with Lck further emphasized that the displacement of this Src family kinase from DRMs represents a functionally important alteration leading to inhibition of signal transduction in PUFA-enriched T cells.
Figure 6.
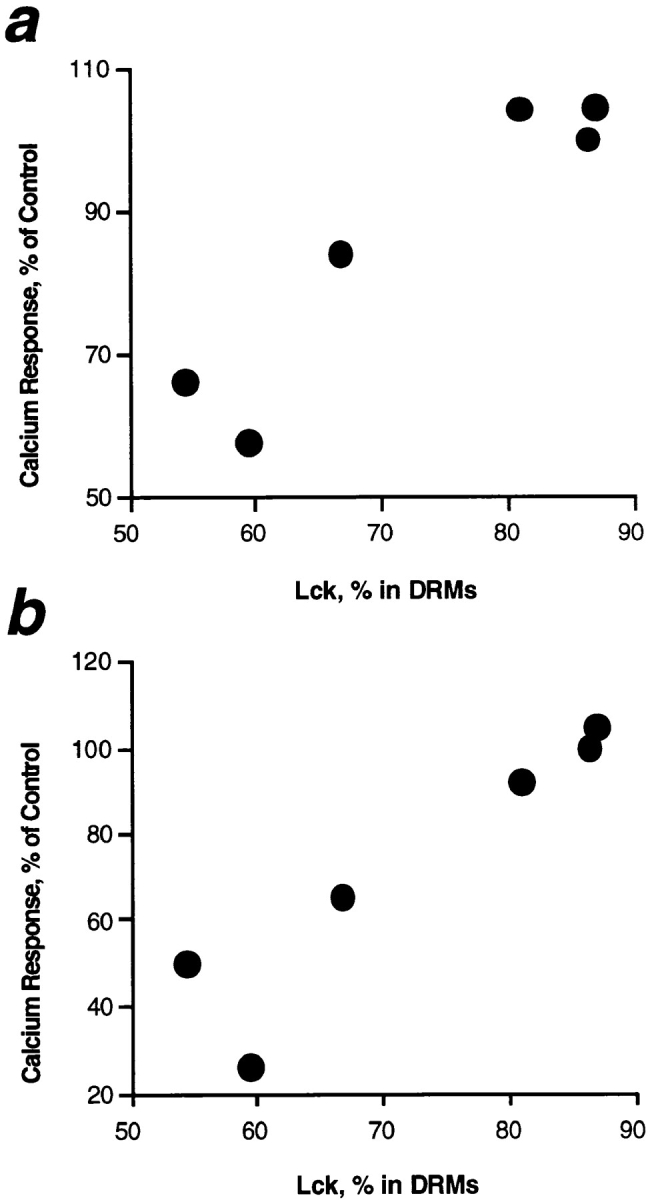
Correlation of T cell calcium response with the presence of Lck in DRMs. T cells were treated with 50 μM of individual fatty acids or ethanol alone as given in Fig. 4. The proportion of Src family kinase Lck recovered in DRM fractions 4–7 as shown in Fig. 4 is given on the abscissa, and means of calcium responses via CD3 (a) or GPI-anchored CD59 (b) are displayed on the ordinate. Note the close correlation between both parameters in all fatty acid treatments.
Discussion
PUFAs exert strong inhibitory effects on T cells by yet unknown molecular mechanisms. The data presented here provide evidence that the PUFA-induced inhibition of T cell activation is due to displacement of Src family kinase Lck from the cytoplasmic layer of DRMs. These findings not only demonstrate the possibility to selectively alter parts of the DRM lipid bilayer but also emphasize a possible role of DRMs in pathophysiologic or pharmacologic processes.
DRMs are membrane domains and thus consist of two lipid layers (Brown and Rose, 1992). However, only lipid constituents of the outer layer, sphingolipids, and cholesterol, are known to mediate the formation of an liquid-ordered state that confers detergent insolubility and separates DRMs from other membrane parts that are in a liquid-crystalline phase (Ahmed et al., 1997; Schroeder et al., 1998). The concentration of Src family protein tyrosine kinases (Stefanova et al., 1991; Shenoy Scaria et al., 1992; Bohuslav et al., 1993) and G-protein α-subunits (Solomon et al., 1996) in the inner layer clearly shows that the organization of DRMs is not confined to the outer layer. However, the nature of the inner leaflet and its interaction with the outer layer is still under debate (Brown and London, 1997; Harder and Simons, 1997). The raft hypothesis (Simons and Ikonen, 1997) suggests that very long chain fatty acyl moieties from sphingolipids may interdigitate partially into the cytoplasmic lipid leaflet. It was speculated that this interference may favor inner leaflet phospholipids with saturated acyl chains to be concentrated at the cytoplasmic layer of DRMs (Harder and Simons, 1997). Thus, Src family protein tyrosine kinases, which are attached to the cytoplasmic layer usually by saturated acyl moieties (myristoyl, palmitoyl) may be forced into DRMs. According to this model, enhancing the degree of unsaturation within DRM lipids could disturb the inner lipid layer and, consequently, exclude Src family protein tyrosine kinases from membrane domains. The less predominant effect of very long chain docosahexaenoic acid (22:6 (n-3)), which did not lead to accumulation of PUFAs of 20 carbon chain length or less, could be due to partial exclusion of 22 carbon fatty acids from the inner layer of DRMs. It would be highly interesting, whether PUFAs exert similar effects on DRM composition and signal transduction in caveolin-expressing cells or whether the presence of this protein could abolish PUFA-induced functional and/or biochemical effects.
On the other hand, so-called protein palmitoylation, which mediates the localization of Src family kinases and other proteins on the cytoplasmic leaflet of DRMs, may not be restricted to palmitoyl moieties but may also attach even unsaturated fatty acids (Mumby, 1997). Therefore, the PUFA-induced dislocation of Src family kinases could be due to altered protein acylation. However, regardless of whether unsaturated membrane lipids or altered protein acylation or both may underlie the displacement of Src family protein tyrosine kinases out of DRMs after PUFA enrichment, this finding revealed that the composition of the outer and the inner leaflet of DRMs can be modified independently of each other.
Src family kinases displaced from DRMs by PUFA enrichment occurred in a fraction of intermediate density. However, Lck from this fraction could not be pelleted, thereby distinguishing the intermediate fraction from previously published DRMs of higher density (Parkin et al., 1996). The lack of sedimentability could suggest that the kinase was no longer attached to detergent-insoluble complexes. Alternatively, DRMs may be altered so that the detergent-insoluble complexes derived from them loose sedimentability, e.g., due to reduced size (Naslavsky et al., 1997). With respect to the latter hypothesis, Src family kinases recovered from intermediate fractions could have been located in structurally altered membrane domains from which, in contrast to the study by Naslavsky et al. (1997), typical DRM marker molecules residing in the external membrane leaflet were excluded. In contrast to Lck, CD3ζ from intermediate and high-density fractions could be pelleted irrespective of fatty acid enrichment (data not shown) which is presumably due to its known cytoskeletal association (Caplan et al., 1995). Thus, the intermediate fraction seems to contain a mixture of complexes, and the exact nature of membranes to which Src family kinases localize in PUFA-enriched T cells has yet to be elucidated.
Our data emphasize the importance of Src family kinase Lck to be located in DRMs for signal transduction. Interestingly, a transmembrane Lck mutant lacking acylation sites is excluded from DRMs in transfected T cells and does no longer transduce signals when stimulated via CD3 alone (Kabouridis et al., 1997). In addition, differences in the activity of Lck localized in various membrane domains have been described (Arni et al., 1996; Rodgers and Rose, 1996). Moreover, the integrity of DRMs has most recently been demonstrated to be critical for efficient T cell signaling (Xavier et al., 1998). According to these studies, the dislocation of Src family protein tyrosine kinase Lck as shown here could be the primary mechanism underlying PUFA-mediated T cell inhibition.
GPI-anchored proteins particularly depend on DRMs for signal transduction since these membrane domains mediate the association with Src family protein tyrosine kinases (Stefanova et al., 1991; Brown, 1993; Malek et al., 1994; van den Berg et al., 1995). Apart from inhibition of TCR–CD3 signaling (Chow et al., 1991; Vassilopoulos et al., 1997), PUFA enrichment affected stimulation via GPI-anchored CD59 to an even greater extent (Fig. 1). The stronger abrogation of signal transduction via CD59 could be due to the fact that DRMs are the only means for GPI-anchored proteins to interact with Src family kinases. Our previous results, showing that cholesterol lowering selectively inhibits signaling via GPI-anchored proteins but not via CD3 in T cells, demonstrated that pathways involved in CD59 and CD3 signaling can be differentiated by means of their lipid requirements (Stulnig et al., 1997). In contrast to PUFA enrichment, signal transduction inhibition by cholesterol depletion was not accompanied by detectable changes in DRM composition (Stulnig et al., 1997 and unpublished data). Thus, lipids may modulate T cell signaling by different mechanisms and provide a means for influencing various signaling pathways in common or separately from each other.
In conclusion, the partial disruption of DRMs by PUFA leading to exclusion of Src family protein tyrosine kinases attached to the cytoplasmic side revealed a dynamic interference between both lipid layers within membrane domains whose modification leads to pronounced functional alterations. Since dietary PUFAs have been shown to be effectively incorporated into leukocyte membrane lipids with concomitant functional changes (Brouard and Pascaud, 1990; Valette et al., 1991; Rossetti et al., 1997) these subcellular alterations appear to be also of clinical importance.
Acknowledgments
We are grateful to Dr. V. Hořejší for discussion and kind provision of antibodies, and to Dr. L. Wagner for discussion and help in immunofluorescence studies.
This work was supported by the Austrian Science Foundation, project no. P11404-Med, and the Austrian National Bank project no. 7196 (T.M. Stulnig), the ICP Programme for Molecular Medicine of the Austrian Ministry for Research and Transport (W. Waldhäusl and T.M. Stulnig), and the SFB005 from the Austrian Science Foundation (H. Stockinger).
Abbreviations used in this paper
- BHT
butylated hydroxy-toluene
- EtOH
ethanol
- DRM
detergent-resistant membrane domain
- GAM
goat anti–mouse antibody
- GPI
glycosyl phosphatidylinositol
- PUFA
polyunsaturated fatty acid
- TCR
T cell receptor
Footnotes
Address all correspondence to T.M. Stulnig, Dept. of Internal Medicine III, Division of Endocrinology and Metabolism, University of Vienna, Währinger Gürtel 18-20, A-1090 Vienna, Austria. Tel.: 43 1 40400 4319. Fax: 43 1 40400 4845. E-mail: thomas.stulnig@akh-wien.ac.at
References
- Ahmed SN, Brown DA, London E. On the origin of sphingolipid/ cholesterol rich detergent insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent insoluble, liquid ordered lipid phase in model membranes. Biochemistry. 1997;36:10944–10953. doi: 10.1021/bi971167g. [DOI] [PubMed] [Google Scholar]
- Arni S, Ilangumaran S, van Echten G, Deckert, Sandhoff K, Poincelet M, Briol A, Rungger E, Brandle, Hoessli DC. Differential regulation of Src-family protein tyrosine kinases in GPI domains of T lymphocyte plasma membranes. Biochem Biophys Res Commun. 1996;225:801–807. doi: 10.1006/bbrc.1996.1254. [DOI] [PubMed] [Google Scholar]
- Belluzzi A, Brignola C, Campieri M, Pera A, Boschi S, Miglioli M. Effect of an enteric-coated fish-oil preparation on relapses in Crohn's disease. N Engl J Med. 1996;334:1557–1560. doi: 10.1056/NEJM199606133342401. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method for total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bohuslav J, Cinek T, Hoř ejší V. Large, detergent-resistant complexes containing murine antigens Thy-1 and Ly-6 and protein tyrosine kinase p56lck. Eur J Immunol. 1993;23:825–831. doi: 10.1002/eji.1830230409. [DOI] [PubMed] [Google Scholar]
- Brouard C, Pascaud M. Effects of moderate dietary supplementations with n-3 fatty acids on macrophage and lymphocyte phospholipids and macrophage eicosanoid synthesis in the rat. Biochim Biophys Acta. 1990;1047:19–28. doi: 10.1016/0005-2760(90)90255-v. [DOI] [PubMed] [Google Scholar]
- Brown D. The tyrosine kinase connection: how GPI-anchored proteins activate T cells. Curr Opin Immunol. 1993;5:349–354. doi: 10.1016/0952-7915(93)90052-t. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Structure of detergent resistant membrane domains: does phase separation occur in biological membranes. Biochem Biophys Res Commun. 1997;240:1–7. doi: 10.1006/bbrc.1997.7575. [DOI] [PubMed] [Google Scholar]
- Calder PC, Bevan SJ, Newsholme EA. The inhibition of T-lymphocyte proliferation by fatty acids is via an eicosanoid-independent mechanism. Immunology. 1992;75:108–115. [PMC free article] [PubMed] [Google Scholar]
- Caplan S, Zeliger S, Wang L, Baniyash M. Cell-surface-expressed T-cell antigen-receptor zeta chain is associated with the cytoskeleton. Proc Natl Acad Sci USA. 1995;92:4768–4772. doi: 10.1073/pnas.92.11.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelli P, DiLiberato L, Stuard S, Ballone E, Albertazzi A. N-3 polyunsaturated fatty acid supplementation in chronic progressive renal disease. J Nephrol. 1997;10:157–162. [PubMed] [Google Scholar]
- Cerny J, Stockinger H, Hoř ejší V. Noncovalent associations of T lymphocyte surface proteins. Eur J Immunol. 1996;26:2335–2343. doi: 10.1002/eji.1830261010. [DOI] [PubMed] [Google Scholar]
- Chan AC, Shaw AS. Regulation of antigen receptor signal transduction by protein tyrosine kinases. Curr Opin Immunol. 1995;8:394–401. doi: 10.1016/s0952-7915(96)80130-0. [DOI] [PubMed] [Google Scholar]
- Chow SC, Sisfontes L, Jondal M, Bjorkhem I. Modification of membrane phospholipid fatty acyl composition in a leukemic T cell line: effects on receptor mediated intracellular Ca2+increase. Biochim Biophys Acta. 1991;1092:358–366. doi: 10.1016/s0167-4889(97)90013-6. [DOI] [PubMed] [Google Scholar]
- Cinek T, Hoř ejší V. The nature of large noncovalent complexes containing glycosyl-phosphatidylinositol-anchored membrane glycoproteins and protein tyrosine kinases. J Immunol. 1992;149:2262–2270. [PubMed] [Google Scholar]
- Field KA, Holowka D, Baird B. Compartmentalized activation of the high affinity immunoglobulin e receptor within membrane domains. J Biol Chem. 1997;272:4276–4280. doi: 10.1074/jbc.272.7.4276. [DOI] [PubMed] [Google Scholar]
- Fowler KH, Chapkin RS, McMurray DN. Effects of purified dietary n-3 ethyl esters on murine T lymphocyte function. J Immunol. 1993;151:5186–5197. [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hanada K, Nishijima M, Akamatsu Y, Pagano RE. Both sphingolipids and cholesterol participate in the detergent insolubility of alkaline phosphatase, a glycosylphosphatidylinositol-anchored protein in mammalian membranes. J Biol Chem. 1995;270:6254–6260. doi: 10.1074/jbc.270.11.6254. [DOI] [PubMed] [Google Scholar]
- Harder T, Simons K. Caveolae, digs, and the dynamics of sphingolipid cholesterol microdomains. Curr Opin Cell Biol. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- Heider JG, Boyett RL. The picomole determination of free and total cholesterol in cells in culture. J Lipid Res. 1978;19:514–518. [PubMed] [Google Scholar]
- Ilangumaran S, Arni S, Chicheportiche Y, Briol A, Hoessli DC. Evaluation by dot-immunoassay of the differential distribution of cell surface and intracellular proteins in glycosylphosphatidylinositol-rich plasma membrane domains. Analyt Biochem. 1996;235:49–56. doi: 10.1006/abio.1996.0090. [DOI] [PubMed] [Google Scholar]
- Kabouridis PS, Magee AI, Ley SC. S-acylation of lck protein tyrosine kinase is essential for its signalling function in T lymphocytes. EMBO (Eur Mol Biol Organ) J. 1997;16:4983–4998. doi: 10.1093/emboj/16.16.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ley SC, Marsh M, Bebington CR, Proudfoot K, Jordan P. Distinct intracellular localization of lck and fyn protein tyrosine kinases in human T lymphocytes. J Cell Biol. 1994;125:639–649. doi: 10.1083/jcb.125.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PS, Ying YS, Anderson RGW. Platelet-derived growth factor activates mitogen-activated protein kinase in isolated caveolae. Proc Natl Acad Sci USA. 1997;94:13666–13670. doi: 10.1073/pnas.94.25.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek TR, Fleming TJ, Codias EK. Regulation of T lymphocyte function by glycosylphosphatidylinositol(GPI)-anchored proteins. Semin Immunol. 1994;6:105–113. doi: 10.1006/smim.1994.1015. [DOI] [PubMed] [Google Scholar]
- Marzo I, Martinez MJ, Lorenzo, Anel A, Desportes P, Alava MA, Naval J, Pineiro A. Biosynthesis of unsaturated fatty acids in the main cell lineages of human leukemia and lymphoma. Biochim Biophys Acta. 1995;1257:140–148. doi: 10.1016/0005-2760(95)00064-j. [DOI] [PubMed] [Google Scholar]
- Mayor S, Rothberg KG, Maxfield FR. Sequestration of GPI-anchored proteins in caveolae triggered by cross-linking. Science. 1994;264:1948–1951. doi: 10.1126/science.7516582. [DOI] [PubMed] [Google Scholar]
- Melkonian KA, Chu T, Tortorella LB, Brown DA. Characterization of proteins in detergent-resistant membrane complexes from Madin-Darby canine kidney epithelial cells. Biochemistry. 1995;34:16161–16170. doi: 10.1021/bi00049a031. [DOI] [PubMed] [Google Scholar]
- Mumby SM. Reversible palmitoylation of signaling proteins. Curr Opin Cell Biol. 1997;9:148–154. doi: 10.1016/s0955-0674(97)80056-7. [DOI] [PubMed] [Google Scholar]
- Naslavsky N, Stein R, Yanai A, Friedlander G, Taraboulos A. Characterization of detergent insoluble complexes containing the cellular prion protein and its scrapie isoform. J Biol Chem. 1997;272:6324–6331. doi: 10.1074/jbc.272.10.6324. [DOI] [PubMed] [Google Scholar]
- Parkin ET, Turner AJ, Hooper NM. Isolation and characterization of two distinct low-density, Triton-insoluble, complexes from porcine lung membranes. Biochem J. 1996;319:887–896. doi: 10.1042/bj3190887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raederstorff D, Meier CA, Moser U, Walter P. Hypothyroidism and thyroxin substitution affect the n-3 fatty acid composition of rat liver mitochondria. Lipids. 1991;26:781–787. doi: 10.1007/BF02536158. [DOI] [PubMed] [Google Scholar]
- Robinson PJ. Phosphatidylinositol membrane anchors and T-cell activation. Immunol Today. 1991;12:35–41. doi: 10.1016/0167-5699(91)90110-F. [DOI] [PubMed] [Google Scholar]
- Rodgers W, Rose JK. Exclusion of CD45 inhibit activity of p56lck associated with glycolipid-enriched membrane domains. J Cell Biol. 1996;135:1515–1523. doi: 10.1083/jcb.135.6.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers W, Crise B, Rose JK. Signals determining protein tyrosine kinase and glycosyl-phosphatidylinositol-anchored protein targeting to a glycolipid enriched membrane fraction. Mol Cell Biol. 1994;14:5384–5391. doi: 10.1128/mcb.14.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti RG, Seiler CM, DeLuca P, Laposata M, Zurier RB. Oral administration of unsaturated fatty acids: effects on human peripheral blood T lymphocyte proliferation. J Leuk Biol. 1997;62:438–443. doi: 10.1002/jlb.62.4.438. [DOI] [PubMed] [Google Scholar]
- Santoli D, Zurier RB. Prostaglandin E precursor fatty acids inhibit human IL-2 production by a prostaglandin E-independent mechanism. J Immunol. 1989;143:1303–1309. [PubMed] [Google Scholar]
- Schmidt EB, Dyerberg J. Omega-3 fatty acids. Current status in cardiovascular medicine. Drugs. 1994;47:405–424. doi: 10.2165/00003495-199447030-00003. [DOI] [PubMed] [Google Scholar]
- Schroeder R, London E, Brown D. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc Natl Acad Sci USA. 1994;91:12130–12134. doi: 10.1073/pnas.91.25.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder RJ, Ahmed SN, Zhu YZ, London E, Brown DA. Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J Biol Chem. 1998;273:1150–1157. doi: 10.1074/jbc.273.2.1150. [DOI] [PubMed] [Google Scholar]
- Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Shenoy-Scaria AM, Kwong J, Fujita T, Olszowy MW, Shaw AS, Lublin DM. Signal transduction through decay-accelerating factor. Interaction of glycosyl-phosphatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn. J Immunol. 1992;149:3535–3541. [PubMed] [Google Scholar]
- Shenoy-Scaria AM, Dietzen DJ, Kwong J, Link DC, Lublin DM. Cysteine3 of src family protein tyrosine kinases determines palmitoylation and localization in caveolae. J Cell Biol. 1994;126:353–363. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Solomon KR, Rudd CE, Finberg RW. The association between glycosylphosphatidylinositol-anchored proteins and heterotrimeric G protein alpha subunits in lymphocytes. Proc Natl Acad Sci USA. 1996;93:6053–6058. doi: 10.1073/pnas.93.12.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova I, Hoř ejší V, Ansotegui IJ, Knapp W, Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991;254:1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- Stulnig TM, Berger M, Sigmund T, Stockinger H, Hoř ejší V, Waldhäusl W. Signal transduction via glycosyl phosphatidylinositol-anchored proteins in T cells is inhibited by lowering cellular cholesterol. J Biol Chem. 1997;272:19242–19247. doi: 10.1074/jbc.272.31.19242. [DOI] [PubMed] [Google Scholar]
- Valette L, Croset M, Prigent AF, Meskini N, Lagarde M. Dietary polyunsaturated fatty acids modulate fatty acid composition and early activation steps of concanavalin A-stimulated rat thymocytes. J Nutr. 1991;121:1844–1859. doi: 10.1093/jn/121.11.1844. [DOI] [PubMed] [Google Scholar]
- van den Berg CW, Cinek T, Hallett MB, Horejsi V, Morgan BP. Exogenus glycosyl phosphatidylinositol-anchored CD59 associates with kinases in membrane clusters on U937 cells and becomes Ca2+-signaling competent. J Cell Biol. 1995;131:669–677. doi: 10.1083/jcb.131.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heide JJ, Bilo HJ, Donker JM, Wilmink JM, Tegzess AM. Effect of dietary fish oil on renal function and rejection in cyclosporine-treated recipients of renal transplants. N Engl J Med. 1993;329:769–773. doi: 10.1056/NEJM199309093291105. [DOI] [PubMed] [Google Scholar]
- Vassilopoulos D, Zurier RB, Rossetti RG, Tsokos GC. Gammalinolenic acid and dihomogammalinolenic acid suppress the CD3 mediated signal transduction pathway in human T cells. Clin Immunol Immunopathol. 1997;83:237–244. doi: 10.1006/clin.1997.4343. [DOI] [PubMed] [Google Scholar]
- Veillette A, Horak ID, Horak EM, Bookman MA, Bolen JB. Alterations of the lymphocyte-specific protein tyrosine kinase (p56lck) during T-cell activation. Mol Cell Biol. 1988;8:4353–4361. doi: 10.1128/mcb.8.10.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Xavier R, Brennan T, Li QQ, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- Zurier RB. Fatty acids, inflammation and immune response. Prostaglandins Leukot Essent Fatty Acids. 1993;48:57–62. doi: 10.1016/0952-3278(93)90010-t. [DOI] [PubMed] [Google Scholar]