Abstract
While Drosophila female meiosis is anastral, both meiotic divisions in Drosophila males exhibit prominent asters. We have identified a gene we call asterless (asl) that is required for aster formation during male meiosis. Ultrastructural analysis showed that asl mutants have morphologically normal centrioles. However, immunostaining with antibodies directed either to γ tubulin or centrosomin revealed that these proteins do not accumulate in the centrosomes, as occurs in wild-type. Thus, asl appears to specify a function required for the assembly of centrosomal material around the centrioles.
Despite the absence of asters, meiotic cells of asl mutants manage to develop an anastral spindle. Microtubules grow from multiple sites around the chromosomes, and then focus into a peculiar bipolar spindle that mediates chromosome segregation, although in a highly irregular way.
Surprisingly, asl spermatocytes eventually form a morphologically normal ana–telophase central spindle that has full ability to stimulate cytokinesis. These findings challenge the classical view on central spindle assembly, arguing for a self-organization of this structure from either preexisting or newly formed microtubules. In addition, these findings strongly suggest that the asters are not required for signaling cytokinesis.
Keywords: centrosome, spindle assembly, cytokinesis, male meiosis, Drosophila
Chromosome segregation during both mitosis and meiosis is mediated by the spindle, a complex bipolar structure consisting of microtubules and associated proteins. Although the basic structure of the spindle is similar in all cell types of all higher eukaryotes, the routes through which the spindle assembles can be substantially different (reviewed by Rieder et al., 1993; Merdes and Cleveland, 1997; Waters and Salmon, 1997).
In animal mitotic cells, spindle formation is mediated by the centrosomes. During prophase, duplicated centrosomes, while moving to the opposite poles of the cell, nucleate radial arrays of microtubules called the asters. After the breakdown of the nuclear envelope, the plus ends of astral microtubules are captured and stabilized by the kinetochores, allowing the formation of a bipolar spindle.
In contrast, higher plant cells and female meiotic cells of several animal species such as Caenorhabditis, Drosophila, or Xenopus, do not contain centrosomes (Smirnova and Bajer, 1992; Albertson and Thompson, 1993; Theurkauf and Hawley, 1992; Gard, 1992). In these systems, microtubules grow from multiple sites around the chromosomes and progressively self-organize into a bipolar spindle. Studies on Drosophila female meiosis and in vitro spindle assembly from Xenopus egg extracts have shown that microtubule focusing into spindle poles is mediated by minus-end–directed motor proteins. In Drosophila, the assembly and maintenance of a bipolar meiotic spindle requires the action of Ncd, a minus-end directed kinesin motor protein (Hatsumi and Endow, 1992; Matthies et al., 1996; Endow and Komma, 1997). Similarly, in Xenopus egg extracts spindle pole formation is mediated by cytoplasmic dynein, another minus-end–directed motor protein (Heald et al., 1996; Heald et al., 1997). Dynein forms a complex with NuMA (nuclear/mitotic apparatus protein) and dynactin, both of which are also necessary for proper microtubule focusing at the spindle poles (Merdes et al., 1996; Merdes and Cleveland, 1997). Thus, in acentrosomal spindles the minus-ends of the microtubules that grow around the chromatin move and converge towards the poles through the action of minus-end microtubule-based motors and their associated proteins.
Recent studies have shown that cells without centrosomes and cells with centrosomes share common mechanisms of spindle pole assembly (reviewed by Merdes and Cleveland, 1997). Inhibition of cytoplasmic dynein by a dynein-specific antibody disrupts spindle pole formation in both centrosome-free and centrosome-containing spindles (Gaglio et al., 1997; Heald et al., 1997). Furthermore, in the latter systems dynein depletion results in the detachment of centrosomes from the spindle poles (Gaglio et al., 1995; Echeverri et al., 1996). These observations, and the finding that most interpolar microtubules are not connected to the centrosomes (Mastronarde et al., 1993), suggested a model for pole formation in centrosome-containing spindles (Gaglio et al., 1997; Heald et al., 1997). It has been proposed that a substantial fraction of the microtubules nucleated by the centrosomes is released from these structures during the prometaphase search and capture process (Kirschner and Mitchison, 1986). The free minus-ends of these microtubules are then focused at the spindle poles through the action of the same structural and motor proteins that mediate pole formation in acentrosomal systems. The translocation of the microtubule minus-ends towards the spindle poles, coupled with microtubule elongation at the plus-ends and microtubule shortening at the minus-ends, would then create a poleward microtubule flux that exerts force through the spindle (Waters et al., 1996; Waters and Salmon, 1997). In addition, lateral interactions between the astral microtubules and the free minus ends of poleward-migrating microtubules would tether the centrosomes to the spindle poles, restoring the connection between these organelles and the rest of the spindle.
If the poles are assembled with similar mechanisms in both acentrosomal and centrosomal systems, centrosome-containing cells should be able to assemble a spindle even in the absence of centrosomes. In most cell types, however, this is not the case. For example, micromanipulation experiments carried out in echinoderm embryonic cells, vertebrate somatic cells, and grasshopper spermatocytes have clearly shown that removal of centrosomes from prophase cells prevents spindle formation (Sluder and Rieder, 1985; Sluder et al., 1986; Rieder and Alexander, 1990; Rieder et al., 1993; Zhang and Nicklas, 1995). However, if centrosomes are removed or lost during anaphase, the spindle poles remain focused, and chromosome segregation is not affected (for review see Waters and Salmon, 1997). On the other hand, experiments on spermatocytes of the crane fly Pales ferruginea indicate that in these cells spindle pole assembly is independent of the presence of centrosomes (Steffen et al., 1986). The reason why Pales spermatocytes can assemble spindle poles in the absence of centrosomes whereas the other systems cannot, is not understood. An intriguing possibility is that the requirement of centrosomes for spindle assembly simply reflects the fact that in some cell types these organelles are the only source of microtubule nucleation. Thus, in the absence of centrosomes, there would not be enough microtubules to be focused at the spindle poles, and spindle assembly would be prevented (Waters and Salmon, 1997).
In this paper we describe another centrosome-containing system that does not require centrosomes for spindle formation. While Drosophila female meiosis is anastral (Theurkauf and Hawley, 1992), both meiotic divisions in Drosophila males exhibit prominent asters (Cenci et al., 1994; see Fig. 2). We have genetically micromanipulated Drosophila male meiosis by means of mutations in asterless (asl), a gene required for centrosome assembly and aster formation. In asl spermatocytes, despite the absence of functional centrosomes, microtubules grow from multiple sites around the chromosomes, and self-organize into peculiar anastral spindles. These spindles manage to mediate chromosome segregation, although in a very irregular way. Surprisingly, asl spermatocytes develop a morphologically normal ana–telophase central spindle. The finding that asl mutants are completely devoid of asters and have normal central spindles gave us the opportunity to test the relative role of these structures in signaling cytokinesis. Our results show that central spindles are fully able to induce cytokinesis, indicating that asters are not required for the cytokinetic signal.
Figure 2.
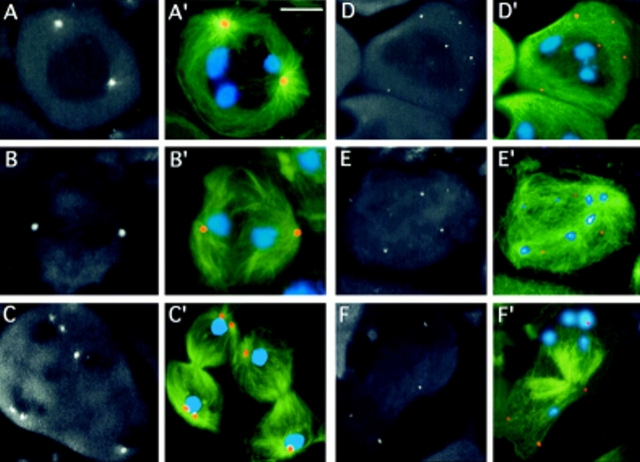
First meiotic division in wild-type (Oregon R) males. Cells were stained for tubulin (green) and DNA (by Hoechst 33258; blue). (A) Prometaphase I (stage M1; see Cenci et al.[1994] for stage designation). (B) metaphase I (stage M3). (C) Early anaphase I (stage M4a). (D) Telophase I (stage M5). Note the prominent asters in all phases of meiotic cell division. Bar, 10 μm.
Materials and Methods
Drosophila Stocks and Mutagenesis
To isolate the asl2 and asl3 mutant alleles we mutagenized es ca males with a 25-mM ethyl methane sulfonate (EMS)1 solution (Lewis and Bacher, 1968) and mated them with Oregon-R virgin females. The F1 es ca/++ males were crossed individually to asl1 es/TM6C, Sb e Tb ca females, and their es ca/asl1 es male progeny were tested for fertility. The es ca/TM6C brothers of the sterile males were then mated to apXa/TM6C females to balance the putative asl alleles. The asl mutations (asl1, asl2, and asl3) were kept over the TM6C balancer that carries the body-shape marker Tubby (Tb), allowing identification of homozygous asl larvae and pupae. All the balancers and markers used for mutagenesis and mapping are described in Lindsley and Zimm (1992). The flies were reared on standard Drosophila medium at 25 ± 1°C; dissections were performed at room temperature.
Immunofluorescence Microscopy
Cytological preparations were made with testes from third instar larvae or from young pupae. For tubulin immunostaining, KLP3A plus tubulin immunostaining, and anillin plus tubulin immunostaining, testes were fixed as described previously (Cenci et al., 1994; Williams et al., 1995). For phalloidin staining and tubulin immunostaining, testes were fixed according to Gunsalus et al., 1995. For γ tubulin plus tubulin immunostaining or centrosomin plus tubulin immunostaining, testes were dissected and frozen in liquid nitrogen as described (Cenci et al., 1994). Preparations were then fixed in cold methanol for 15 min and acetone for 30 s, and were then immersed for 10 min in PBS containing 0.1% Tween 20 and 0.1% acetic acid. Before incubation with antibodies, slides were rinsed several times in PBS containing 0.1% Tween 20.
Tubulin immunostaining and phalloidin staining plus tubulin immunostaining have been described previously (Cenci et al., 1994; Gunsalus et al., 1995). For double immunostainings, testes were first incubated overnight at 4°C with any of the following rabbit primary antibodies diluted in PBT (PBS containing 0.1% Triton X-100) containing 1% BSA: anti-γ tubulin (1:200; Callaini et al., 1997); anti-centrosomin (1:1,000; Li and Kaufman, 1996); anti-KLP3A (1:500; Williams et al., 1995); or anti-anillin raised against amino acids 1–371 (1:300; Field and Alberts, 1995). These primary antibodies were detected by 2-h incubation at room temperature with TRITC-conjugated anti-rabbit IgG (Cappel Laboratories, Malvern, PA) diluted 1:100 in PBT. Slides were then incubated with a monoclonal anti-α tubulin antibody (Pharmacia Biotech, Inc., Piscataway, NJ) diluted 1:50 in PBS, which was detected by FLUOS-conjugated sheep anti–mouse IgG (Boehringer Mannheim, Mannheim, Germany) diluted 1:10 in PBS. After these immunostainings, testis preparations were air-dried and stained with Hoechst 33258 as described (Cenci et al., 1994).
All preparations were examined with an Axioplan (Carl Zeiss, Oberkochen, Germany) microscope equipped with an HBO 50W mercury lamp for epifluorescence, and with a cooled charge-coupled device (CCD; Photometrics Inc., Woburn, MA). Hoechst 33258, FLUOS, and TRITC fluorescence were detected as described (Gunsalus et al., 1995). Gray-scale digital images were collected separately using the IP Lab Spectrum software. Images were then converted to Photoshop 2.5 format (Adobe System, Inc., Mountain View, CA), pseudocoloured, and merged.
Electron Microscopy
Testes dissected from asl1adult males were fixed in 3% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2) at room temperature for 1 h, washed four times in phosphate buffer (5 min each), and postfixed in 1% OsO4 in the same buffer for 1 h. After four washes in phosphate buffer (5 min each), testes were dehydrated with ethanol (30, 50, and 70% 3×, 5 min each at 4°C; and 95 and 100%, 3×, 10 min each at room temperature). Testes were embedded in Epon and, after sectioning, were stained with 3% uranyl acetate and lead citrate.
Results
Isolation and Characterization of asterless Mutations
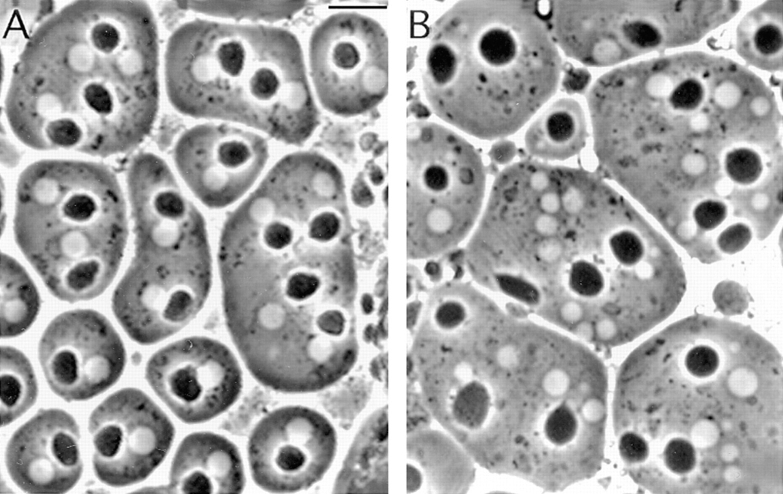
The first asl mutant allele (asl1) was isolated in the course of a cytological screen of a collection of 16 EMS-induced male sterile mutations kindly provided by Barbara Wakimoto (University of Washington, Seattle, WA). Living preparations of mutant testes were examined by phase contrast microscopy for defects in the onion stage spermatids. In wild-type, each spermatid contains one phase-light nucleus and one phase-dense mitochondrial derivative called the Nebenkern (reviewed by Fuller, 1993). At the onion stage of spermatid development, the nuclei and Nebenkern have spherical shapes and very similar sizes (Fig. 1). The regular size of both nuclei and Nebenkern depends on the correct execution of the meiotic process; abnormal-sized nuclei and Nebenkern are diagnostic of errors in chromosome segregation and in the partition of mitochondria, respectively (Gonzalez et al., 1989; Fuller, 1993). As shown in Fig. 1, asl1 spermatids are composed of nuclei and Nebenkern of very different sizes, suggesing that asl mutations disrupt both meiotic chromosome segregation and the correct distribution of mitochondria between the daughter cells.
Figure 1.
Abnormal spermatids in asl mutants. Live testis squashes were viewed by phase contrast microscopy to examine onion stage spermatids. (A) Regular spermatids from Oregon-R controls with nuclei (white structures) and nebenkern (dark structures) of similar sizes. (B) Spermatids from asl mutants showing nuclei and nebenkern of various sizes. See text for details on the origin of these aberrant spermatids. Bar, 10 μm.
asl1 is perfectly viable but sterile in both sexes. The phenotypes of male sterility, female sterility, and aberrant spermatids associated with asl1 were mapped using a ri Ki pp chromosome by examining 44 recombinants between ri and pp (these markers define an interval of 1 cM). This analysis showed that these three phenotypes comap just to the left of Kinked (Ki), from which they are separated by only one recombination event.
To determine whether the asl1 phenotype is specifically elicited by this mutation or is a general characteristic of lesions in the asl locus, we isolated two additional mutant alleles. We treated 2,360 chromosomes with EMS and tested them for allelism with asl1. This screen yielded two new mutations, asl2 and asl3, that are viable over asl1 and fail to complement asl1 for male and female sterility as well as for the aberrant spermatid phenotype. However, asl2/asl2, asl2/ asl3, and asl3/asl3individuals are lethal; asl2/asl2 and asl2/ asl3larvae die at the larval pupal boundary, whereas asl3/ asl3individuals have an earlier lethal phase. In addition, recombination experiments failed to separate the late lethal phenotype from asl2 and the earlier lethal phenotype from asl3. Thus, we conclude that asl is an essential locus required for viability. At present, however, we do not know whether asl3is a null mutation. The fact that asl maps very close to Triplolethal (Tpl) prevented examination of the phenotype of asl3 over deficiency and its comparison with that of asl3/asl3individuals. In this context, it is of interest that the pattern and frequency of abnormal spermatids is very similar in all mutant combinations (asl1/ asl1, asl1/asl2, asl2/asl2, asl1/asl3, and asl2/asl3), indicating that the three mutant alleles cause similar disruptions of the asl + function during male meiosis.
asl Spermatocytes are Devoid of Asters and have Defective Centrosomes
To define the primary lesion leading to the formation of aberrant spermatids in asl mutants, we analyzed cytologically the meiotic division. Testis preparations were stained with anti-tubulin antibodies and Hoechst 33258 for simultaneous visualization of both microtubules and chromatin. Examination of male meiosis in asl1/asl1, asl1/asl2, asl2/asl2, asl1/asl3, and asl2/asl3animals revealed that all these mutant combinations cause a common cytological phenotype. Whereas wild-type spermatocytes exhibit prominent asters throughout meiotic cell division (Fig. 2; see Cenci et al. 1994 for a detailed description of male meiosis), asl spermatocytes are completely devoid of asters (Fig. 3).
Figure 3.
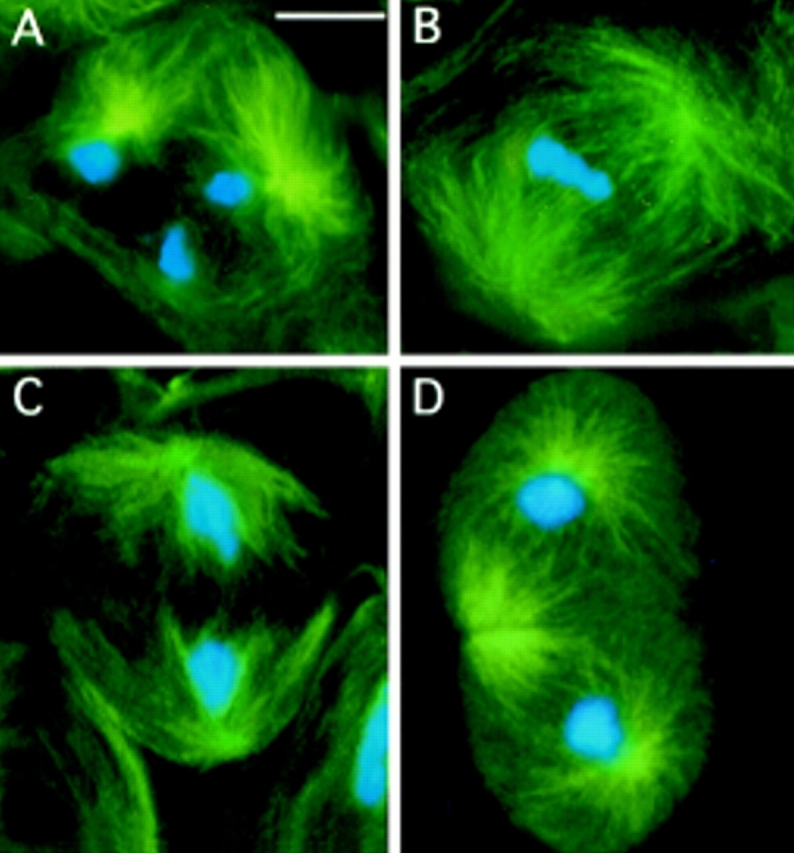
First meiotic division in asl mutant males. Cells were stained for tubulin (green) and DNA (blue). (A) A prometaphase I–like figure (at an M1-like stage as judged by the degree of chromatin condensation) with no asters. (B) Microtubule nucleation around the bivalents; this type of meiotic stage is never seen in the wild-type. (C) A metaphase I–like figure in which two large bivalents are associated with minispindles, and another large bivalent (upper right) is surrounded by microtubules that are not clearly polarized. Note that the tiny fourth chromosomes that have just begun to segregate are associated with very few microtubules. (D) An anaphase I–like stage in which three pairs of homologs (including the fourth chromosomes) have segregated, while a large bivalent (bottom left) is still unseparated. (E and F) An anaphase I–like figure showing segregation of sister chromatids; E shows only the chromosomes, while F shows both the chromosomes and the microtubules. See text for further explanation. (G) A telophase I figure showing a morphologically normal central spindle and scattered chromosomes at the poles. (H) A telophase I with a tripartite central spindle where the chromosomes have segregated only to two poles; in this cell the chromosomes are atypically congregated into discrete telophase nuclei. Bar, 10 μm.
To determine whether the absence of asters in asl mutants was the consequence of a primary defect in centrosome structure, we immunostained mutant testes with antibodies directed to either γ tubulin or centrosomin, two components of Drosophila centrosomes (Zheng et al., 1991; Li and Kaufman, 1996). In wild-type testes, anti-γ tubulin antibodies immunostain the centrosomes in premeiotic primary spermatocytes and throughout meiosis (Fig. 4). In mature primary spermatocytes, the centrosomes are located near the plasma membrane (not shown). Before the first meiotic division they migrate to the periphery of the nuclear envelope where they nucleate prominent asters that move to the opposite poles of the cell (Fig. 4 A′). In anaphase and early telophase I there is a single centrosome at each spindle pole, which in late telophase I splits into two centrosomes that start migrating to the poles of secondary spermatocytes while nucleating new asters (Fig. 4, B′ and C′). These centrosomes remain at the spindle poles throughout the second meiotic division and do not split into two separate entities in late telophase II so that each spermatid receives a single centrosome. In contrast, in asl mutants γ tubulin is not concentrated in the centrosomes during any phase of primary spermatocyte growth and meiotic cell division (Fig. 4, D–F′). Instead, it is dispersed in multiple small aggregates that do not appear to have the ability to nucleate microtubules.
Figure 4.
Failure of centrosome assembly in asl mutants. Cells were stained for α tubulin (green), γ tubulin (orange), and DNA (blue). Panels in black and white show only γ tubulin immunofluorescence; color panels show merged images. (A–C′) wild-type; (A, A′) prometaphase I; (B, B′) early anaphase I; and (C, C′) telophase I showing well-organized centrosomes that accumulate γ tubulin. In one of the telophases shown in C and C′, centrosomes have already started to separate in preparation for the second meiotic division. (D– F′) asl mutants; (D, D′) prometaphase I–like figure; (E, E′) anaphase I; and (F, F′) telophase I, showing no γ tubulin accumulations at the cell poles. Note that γ tubulin is dispersed in small aggregates that do not appear to have the ability to nucleate microtubules. Bar, 10 μm.
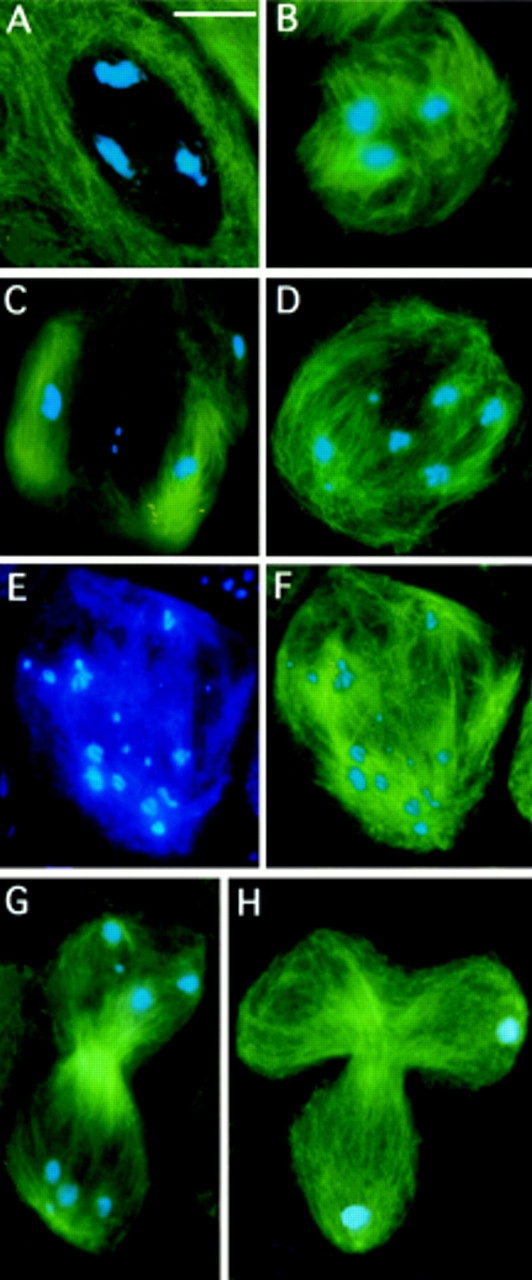
Similar but not identical results were obtained with centrosomin. In wild-type, centrosomin accumulates in the centrosomes of both premeiotic primary spermatocytes and meiotic cells, just as γ tubulin (Fig. 5). In asl mutants, antibodies directed to centrosomin fail to detect discrete centrosomal entities in most mature primary spermatocytes at the S5 stage (Fig. 5 F). However, in late prophase/ prometaphase primary spermatocytes at the M1 stage, anti-centrosomin antibodies immunostain two structures located near the nuclear envelope. These structures are consistently paired, as are the wild-type centrosomes before their migration to the cell poles, but are much less fluorescent than regular centrosomes and fail to nucleate astral microtubules (Fig. 5, G and G′). During ana–telophase I, the centrosomin-enriched bodies are always detected at only one of the cell poles, whereas the other pole is consistently devoid of them (Fig. 5, H–I′). In addition, although they usually appear as a pair of fluorescent spots (Fig. 5 H), they are occasionally resolved into four entities (Fig. 5 I). At telophase I, the centrosomin-positive bodies are transmitted to only one of the daughter cells, and are therefore inherited by only one half of the secondary spermatocytes. These bodies tend to remain associated either as doublets or quartets during the second meiotic division, and are usually transmitted together to one-fourth of the spermatids (Fig. 5 J). These observations strongly suggest that each element of the fluorescent doublets corresponds to a pair of centrioles, and that each element of the quartets consists of a single centriole. However, neither the doublets nor the quartets have nucleating ability, as they are never associated with astral arrays of microtubules.
Figure 5.
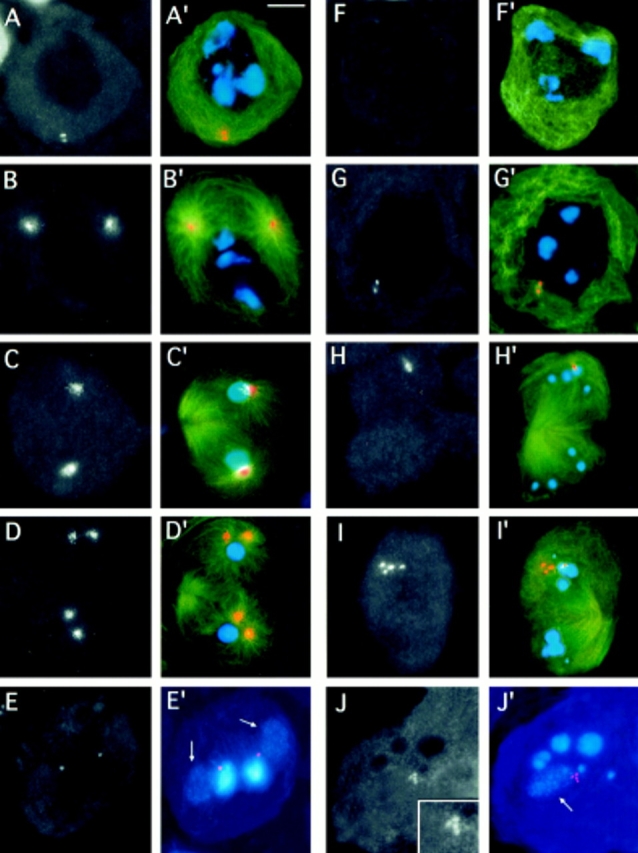
Centrosomin immunostaining of asl testes. Cells were stained for α tubulin (green), centrosomin (orange), and DNA (blue). Panels in black and white show only centrosomin immunofluorescence. Color panels show merged images; tubulin immunofluorescence was not merged in the color images shown in E′ and J′. (A– E′) wild-type; (A, A′) mature primary spermatocyte at the S5 stage showing a pair of centrosomes just under the plasma membrane; (B, B′) prometaphase I; (C, C′) telophase I; and (D, D′) late telophase I showing prominent centrosomin-decorated centrosomes. Note that in the telophase shown in D and D′, the centrosomes have already started to separate. (E and E′) Two spermatids, each consisting of a nucleus and a Nebenkern. The weak fluorescence of the Nebenkern (arrows) is due to the mitochondrial DNA they con-tain. Note that the anticentrosomin antibodies detect the basal body located between the nucleus and the Nebenkern. (F–J′) asl mutants; (F and F′) mature primary spermatocyte at the S5 stage showing no centro– somin accumulations; (G and G′) prometaphase I showing a doublet of centrosomin- enriched bodies near the nuclear envelope; (H and H′) telophase I with a pair of centrosomin-enriched bodies at one of the poles; (I and I′) telophase I showing a quartet of centrosomin- enriched bodies at one of its poles. (J and J′) Highly irregular spermatid associated with four centrosomin-containing entities (enlarged in the insert of J; the arrow points at the Nebenkern). Bar, 10 μm.
To ascertain the presence of centrioles in asl spermatocytes and spermatids, we examined thin sections of testes by EM. This analysis revealed that asl spermatocytes have morphologically normal centrioles (Fig. 6). However, centriole separation is abnormal in that we observed that in some spermatids, Nebenkern are associated with two centrioles instead of a single one, as occurs in the wild-type (Fig. 6). Moreover, the two centrioles of the spermatid shown in Fig. 6, C and D, are lying parallel to each other instead of at a right angle, as do the parent and its daughter centriole in the wild-type. This spermatid may therefore contain four centrioles, with only two of them in the plane of the section.
Figure 6.
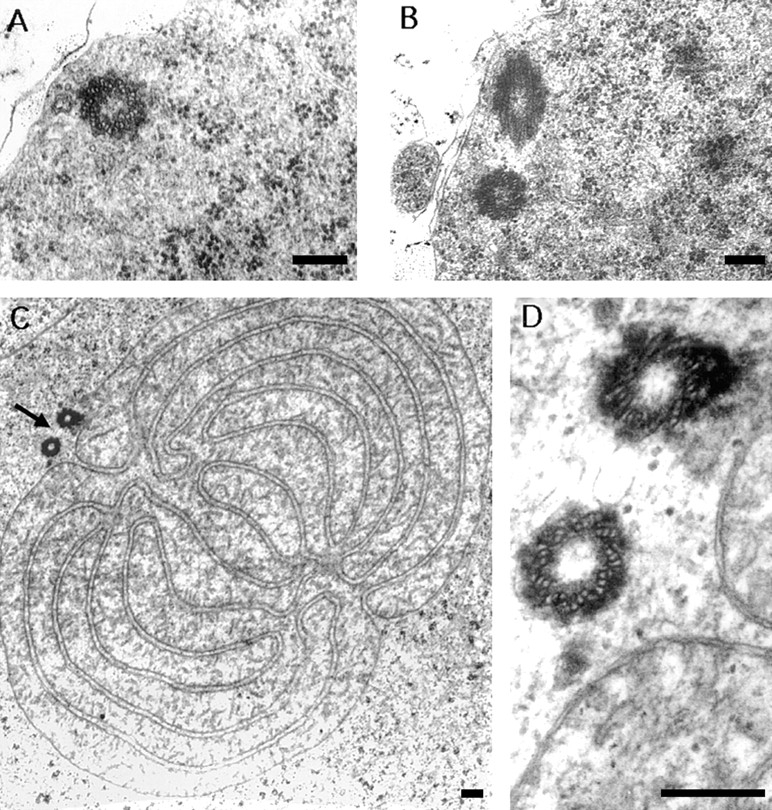
Presence of morphologically normal centrioles in asl1mutants. (A) cross-section through the proximal part of a centriole in a mature primary spermatocyte. (B) A pair of centrioles lying at approximately right angles, located at the periphery of a primary spermatocyte. (C) Cross-section through a Nebenkern of an onion-stage spermatid, irregularly associated with two basal bodies (arrow). (D) Higher magnification of the basal bodies in C, consisting of nine peripheral triplets of tubules. (A) 28,000×; (B) 22,000×; (C) 13,000×; (D) 60,000×. Bars, 0.2 μm.
asl Mutants Organize Anastral Spindles that Mediate Chromosome Segregation
Despite the absence of asters, asl primary spermatocytes develop a peculiar anastral spindle. After the breakdown of the nuclear envelope, microtubules grow from multiple sites near the chromosomes, and form radial arrays extending from each bivalent (Fig. 3 B). These microtubules then organize into bipolar bundles, creating minispindles associated with individual bivalents (Fig. 3 C). However, in many asl spermatocytes, not all the bivalents within the same cell develop clear minispindles; some bivalents remain associated with a nonpolarized or poorly polarized network of microtubules (Fig. 3 C). In addition, the bundles of microtubules associated with the bivalents are often oriented in different directions (Fig. 3, C–F; Fig. 4 E′). As a consequence, the bivalents never congregate into a metaphase I plate during male meiosis in asl mutants.
Interestingly, the network of microtubules associated with the tiny fourth chromosomes is always much smaller than that associated with the larger bivalents, indicating that microtubule growth around the chromosomes is promoted by the whole chromatin, and not by the kinetochores alone. It is worth noting that in most metaphase-like figures the fourth chromosomes exhibit precocious segregation (Fig. 3 C), as occurs in wild-type metaphase I female meiosis (McKim and Hawley, 1995).
Despite these problems in congression, asl meiotic chromosomes progress into a highly irregular anaphase A (Fig. 3, D–F; Fig. 4 E′). The homologs manage to segregate, but their separation is often asynchronous (Fig. 3 D; Fig. 4 E′). Moreover, in ∼35% of anaphase I–like figures, the sister chromatids of one or more half bivalents split and separate from each other (Fig. 3, E and F). These peculiar ana– phases are genuine anaphase I figures, and are not cells undergoing anaphase II. This conclusion is suggested by the finding that in telophase I figures we never observed abnormal segregations with all the chromosomes migrating to a single pole (see below). Thus, most if not all the dividing cells with a 2N complement are likely to be primary spermatocytes undergoing meiosis I, and not diploid secondary spermatocytes in meiosis II. The phenomenon of precocious sister chromatid separation observed in asl anaphase I figures is probably due to the structure of the kinetochore of their half bivalents. During wild-type prometaphase, each half bivalent has a single hemispherical kinetochore that differentiates into two planar kinetochores between late prometaphase I and early anaphase I (Goldstein, 1981). In asl mutants where spindle formation is likely to be delayed with respect to the wild-type (see below), kinetochore duplication may occur before the onset of anaphase I. As a consequence, some half bivalents may become connected to both poles through their duplicated kinetochores, leading to separation of their component sister chromatids.
Regardless of the type of segregation they exhibit, anaphase I chromosomes of asl mutants are never organized into two discrete sets, but are instead scattered throughout the cell (compare Fig. 2 C with Fig. 3, D–F and Fig. 4 E′). Most likely this irregular anaphase chromosome arrangement reflects both the poor polarization of the spindles and the asynchrony in chromosome segregation.
After anaphase A, asl primary spermatocytes undergo anaphase B. Despite the aberrant configuration of anaphase A, ∼85% of these cells develop a central spindle, which is indistinguishable from its wild-type counterpart (compare Figs. 2 D and 4 C′ with Figs. 3 G and 4 F′; Table I). The remaining 15% of ana-telophases form tripartite or multipartite central spindles (Fig. 3 H; Table I). Moreover, central spindles elongate normally and are pinched in the middle during cytokinesis (Figs 3 G, 4 F′, and 5 H′, I′; see below). However, in about 80% of the ana-telophases with morphologically normal central spindles, these structures are asymmetrically located with respect to the cell poles, so that cytokinesis would produce two daughter cells of different size (Fig. 7; Table I). In addition, in most cells (60%) chromosomes also remain scattered during anaphase B and do not congregate into two daughter nuclei as in wild type (compare Figs. 2 D and 4 C′ with Figs. 3 G, 4 F′ and 5 H′, I′; Table I).
Table I.
Types of Ana–telophases Observed in asl1 Mutants
| Meiotic division | Total ana– telophases scored | Multipartitecentral spindle |
Bipartite central spindle | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symmetric | Asymmetric | |||||||||||||||||||
| a | b | c | d | a | b | c | d | |||||||||||||
| I | 190 | 26 | 9 | 3 | 11 | 11 | 16 | 9 | 36 | 69 | ||||||||||
| II | 99 | 7 | 14 | 0 | 14 | 12 | 7 | 1 | 16 | 28 | ||||||||||
a, Regular chromosome segregation; congregated chromosomes at the poles. b, Regular chromosome segregation; scattered chromosomes at the poles. c, Unequal chromosome segregation; congregated chromosomes at the poles. d, Unequal chromosome segregation; scattered chromosomes at the poles.
Figure 7.
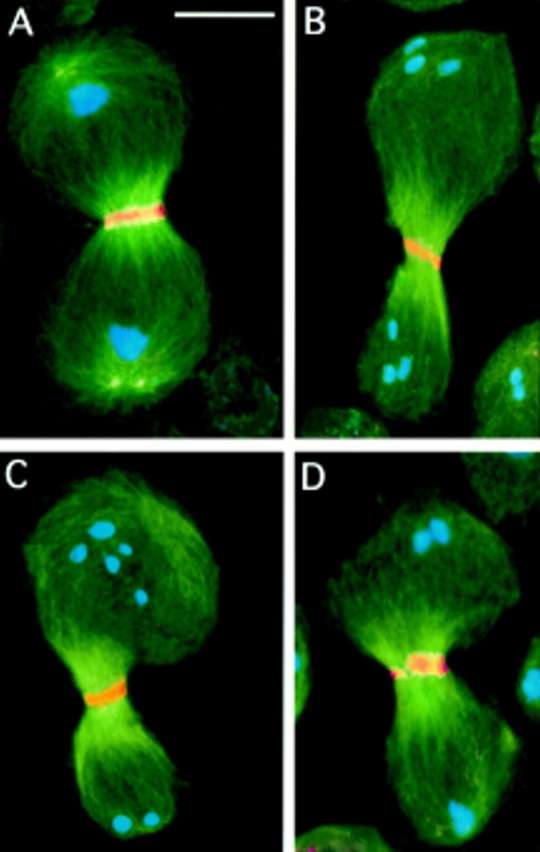
Central spindle formation and cytokinesis in asl mutants. Cells were immunostained for tubulin (green); DNA (blue); and either actin (orange; A and B), anillin (orange; C), or KLP3A (orange; D). (A) A wild-type telophase I and (B) an asl telophase I showing similar actin bands in the middle of their central spindles. (C and D) asl telophase I figures showing normal anillin (C) and KLP3A (D) accumulations in the spindle midzone. Note that in the cells in B and C, the central spindle is asymmetrically located with respect to the cell poles. Bar, 10 μm.
About 70% of telophase I figures exhibit unequal chromosome segregation (Fig. 4 F′; Fig. 7 C; Table I). In cells showing both an asymmetrically located central spindle and unequal chromosome segregation, there is no correlation between the size of the daughter cells and their chromosomal content, indicating that central spindle positioning and chromosome segregation are independent events.
As a consequence of the abnormal first meiotic division, asl secondary spermatocytes receive variable numbers of chromosomes. These cells undergo an anastral second meiotic division that has the same features reported above for mutant first meiotic divisions. Mutant secondary spermatocytes form an irregular apolar spindle that mediates chromosome segregation, and eventually assemble an apparently normal central spindle (data not shown).
To obtain insight into the dynamics and timing of the meiotic process in asl mutants, we determined the frequencies of the various meiotic figures in asl testes, and compared them with those observed in wild-type controls (Table II). An inspection of Table II reveals that the frequencies of late prophase/early prometaphase I and anaphase/telophase I figures found in asl testes are only slightly higher than those observed in controls. In contrast, the frequencies of prometaphase/metaphase I and early anaphase I figures are much higher in asl than in controls. Because the frequency of each meiotic stage should be proportional to its duration in vivo, these findings suggest that the duration of asl prophase/prometaphase I is only slightly increased with respect to the control. However, prometaphase I, metaphase I, and especially early anaphase I appear to last much longer in asl mutants than in the wild-type. A likely explanation of this observation is that the process of spindle organization in asl mutants lasts longer than it does in wild-type because of the absence of astral microtubules nucleated by the centrosomes. The fact that asl mutants and wild-type controls exhibit similar frequencies of ana–telophases with a well-formed central spindle strongly suggests that most if not all the asl cells that enter meiosis I progress until ana–telophase I.
Table II.
Frequencies of Meiotic Figures Observed in asl1 and Control (Oregon R) Testes
| Genotype | Total cells scored | Phases and stages of meiosis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L. Prophase I E. Prometa. I (M1a, b) | Prometa. I Metaphase I (M2; M3) | E. Anaphase I (M4a) | Anaphase I Telophase I (M4b,c; M5) | Interphase II Prometa. II Metaphase II E. Anaphase II (M6; M7; M8; M9; M10a) | Anaphase II Telophase II (M10b,c; M11) | |||||||||
| % | % | % | % | % | % | |||||||||
| asl1/asl 1 | 820 | 21.6 | 19.0 | 13.0 | 23.2 | 11.1 | 12.1 | |||||||
| Oregon R | 1,058 | 14.8 | 5.8 | 0.9 | 19.5 | 31.8 | 27.2 | |||||||
| Ratios | 1.5 | 3.3 | 14.4 | 1.2 | 0.3 | 0.4 | ||||||||
The stages of wild-type meiosis are described in detail by Cenci et al. (1994; see also Figs. 2, 4, and 5). Some of the equivalent stages observed in asl1 mutants are shown in Figs. 3, 4, 5, and 7. For example, the meiotic figures in Fig. 3, A, B, C, D–F, and G correspond to the M1, M2, M3, M4a, and M5 stages of the wild type, respectively. In asl mutants, early anaphase II is difficult to distinguish from metaphase II because in both types of cells some chromosomes exhibit sister chromatid separation. Therefore, these stages have been grouped together. Ratios are between the frequencies of meiotic figures observed in asl and in control. The numbers of meiotic figures have been determined by examining 11 Oregon R testes and 10 asl1 testes. E, early; L, late; Prometa, prometaphase.
The frequencies of meiosis II figures are substantially lower in asl than in the control, but the ratios between interphase-early anaphase II cells and anaphase/telophase II figures are similar in both mutants and control. Because there is not reason to postulate that the second meiotic division is more rapid in asl than in the wild-type, the most straightforward explanation for these results is that only a fraction of asl secondary spermatocytes has the ability to organize an anastral spindle. However, once this anastral spindle is assembled, the cells can progress to ana−telophase and complete the second meiotic division.
The Central Spindle has the Ability to Stimulate Cytokinesis
An open question about cell cleavage in animal cells is the source of signals that stimulate contractile ring formation and cytokinesis. At present it is unclear whether these signals emanate from the asters or from the central spindle (reviewed by Fishkind and Wang, 1995; Glotzer, 1997; Goldberg et al., 1997). The fact that asl mutants form a central spindle in the absence of asters provided us with a unique opportunity to discriminate between these alternatives. We stained asl testes with rhodamine-phalloidin, which detects the actomyosin contractile ring during male meiotic cytokinesis (Gunsalus et al., 1995). In addition, we immunostained asl testes for KLP3A (Williams et al., 1995) and anillin (Field and Alberts, 1995), two proteins that concentrate in the cleavage furrow during wild-type meiotic cytokinesis (Williams et al., 1995; Hime et al., 1996). As shown in Fig. 7, both symmetrically and asymmetrically located central spindles exhibit a regular actin-based contractile ring and normal accumulations of both KLP3A and anillin. Regardless, the positioning of the central spindle within the cell, actin, anillin, and KLP3A are always localized in the middle of this structure, as occurs in the wild-type. In addition, in correspondence with the localization of these proteins, the central spindle is pinched, suggesting regular execution of cytokinesis.
Discussion
asl Mutants are Defective in Centrosome Assembly
We have identified a gene we call asterless (asl), that specifies a function necessary for aster formation during Drosophila male meiosis. In interphase primary spermatocytes and meiotic cells of wild-type males, centrosomes are enriched in γ tubulin. In contrast, in the same cell types of asl mutants this protein does not accumulate in the centrosomes but remains dispersed in multiple cytoplasmic aggregates that do not have microtubule-nucleating ability. Most likely, this primary defect in centrosome assembly prevents aster formation throughout meiotic cell division in asl mutants.
A similar but not identical situation has been observed in the acentriolar Drosophila cell line 1182–4, established from aploid embryos produced by the female sterile mutant mh 1182 (Gans et al., 1975; Debec, 1978; Debec et al., 1995). In control embryonic cell lines, γ tubulin accumulates in both interphase and mitotic centrosomes. In 1182-4 acentriolar cells γ tubulin fails to associate with the interphase centrosomes, but it concentrates in the spindle poles where it exhibits different patterns of accumulation (Debec et al., 1995). However, the γ tubulin polar spots seen in the acentriolar cells are not true centrosomes in that they readily disappear upon microtubule disassembly with either cold or colchicine treatment (Debec et al., 1995). Based on these results, Debec et al. (1995) suggested that centrioles play an important role in the assembly of centrosomal material.
We have shown that in wild-type testes, antibodies directed to centrosomin immunostain the centrosomes in mature primary spermatoytes and throughout meiosis. In asl mutants these antibodies detect either doublets or quartets of discrete structures that are present in all late prophase/prometaphase primary spermatocytes, but are transmitted to only one half of the secondary spermatocytes and to one fourth of the spermatids. The behavior of these centrosomin-enriched bodies seen in asl mutants can be easily explained if one assumes that they correspond to the centrioles.
In wild-type, each mature primary spermatocyte contains two pairs of duplicated centrioles, with the daughter centriole lying at a right angle with respect to its parent. In preparation of meiosis I, both pairs of centrioles migrate together from the plasma membrane to the nuclear envelope, become associated with centrosomal material, and move to the cell poles while nucleating astral microtubules. Thus, during meiosis I each centrosome contains a pair of duplicated centrioles. However, there is not centriole duplication before the second meiotic division; in secondary spermatocytes each pair of centrioles splits into two single centrioles that migrate to the opposite cell poles. Therefore, each spermatid inherits a single centriole that becomes the basal body of the elongating axoneme (reviewed by Fuller, 1993).
Based on centriole behavior in the wild-type, we propose that the centrosomin-enriched doublets seen in asl primary spermatocytes correspond to the centrioles. The fact that these doublets are occasionally resolved into four entities further suggests that each element of the doublets does in fact consist of a pair of centrioles. In addition, we propose that the two pairs of centrioles, due to the absence of astral microtubules (Waters and Salmon, 1997), fail to separate and migrate to the cell poles during both meiotic divisions of asl mutants. Thus, during each meiotic division they are transmitted together to only one of the two daughter cells. This model for centriole behavior in asl mutants is supported by the results obtained by EM. EM analysis has shown that asl cells contain morphologically normal centrioles that in several cases fail to separate properly. We have observed several Nebenkern associated with two instead of a single centriole. Moreover, in some asl spermatids, these two centrioles are lying parallel to each other instead of at a right angle, as do the parent and its daughter centriole in wild-type. This parallel centriole arrangement is consistent with the possibility that the two centrioles in the plane of the section belong to different pairs of centrioles that have been transmitted together to the sectioned spermatid.
Centrosomin immunostaining and EM analysis clearly indicate that asl meiotic cells contain centrioles of regular morphology that duplicate normally. Thus, the asl1function does not appear to be required for either centriole fine structure or duplication. However, the observation that asl centrioles are never associated with γ tubulin and accumulate much less centrosomin than their wild-type counterparts, strongly suggests that asl specifies a function required for the assembly of centrosomal material around the centrioles. The identification of such a function must await the molecular analysis of asl, which, however, may turn out to be particularly difficult. We have not succeeded in isolating asl alleles by P-mutagenesis, and molecular cloning of asl by chromosome walking is hampered by its vicinity to the Tpl locus.
Spindle Assembly in asl Mutants
We have shown that despite the absence of asters, asl mutants assemble a peculiar anastral spindle. Meiotic chromosomes appear to play an important role in this process, acting as microtubule-organizing centers and promoting formation of bipolar minispindles. This finding was anticipated by micromanipulation experiments showing that Drosophila male bivalents detached from the spindle can trigger the formation of minispindles (Church et al., 1986).
The aberrant meiosis observed in asl males has many similarities with naturally occurring anastral divisions, such as those accompanying female meiosis in mice, Caenorhabditis, Xenopus, and Drosophila (reviewed in McKim and Hawley, 1995). The asl spindle formation pathway is also reminiscent of the in vitro spindle assembly induced by DNA-coated beads in Xenopus egg extracts (Heald et al., 1996; Heald et al., 1997). In all these systems, chromatin can induce microtubule nucleation and stabilization. These microtubules are initially randomly oriented; their minus-ends then focus at the spindle poles through the action of minus-end-directed motors and their associated proteins (Hatsumi and Endow, 1992; Heald et al., 1996; Matthies et al., 1996; Merdes et al., 1996; Heald et al., 1997). However, the minispindles associated with the asl bivalents are not always clearly organized into a bipolar array. Moreover, when they do exhibit a bipolar configuration, the poles are broad and are never as focused as those observed in Drosophila female meiosis or in the Xenopus in vitro systems. This result suggests that Drosophila spermatocytes do not have sufficient minus-end motor activity to complete spindle polarization in the absence of centrosomes.
Our results on asl mutants indicate that cells in which spindle assembly is normally driven by centrosomes nonetheless have the ability to form anastral spindles. Similar findings have been obtained with crane fly spermatocytes (Dietz, 1966; Steffen et al., 1986), but not with grasshopper spermatocytes where both the chromosomes and the centrosomes are essential for spindle formation (Zhang and Nicklas, 1995). In addition, a series of studies has clearly shown that spindle assembly during mitotic division of a variety of vertebrate cell types invariably requires the presence of functional centrosomes (reviewed in Rieder et al., 1993). Together, these findings raise the question of why the ability to form anastral spindles in cells that normally contain centrosomes is restricted to a few meiotic systems. It is possible that this property reflects different types of interaction between chromosomes and microtubules. In vertebrate mitotic cells and in grasshopper spermatocytes, the chromosomes can only capture and stabilize the microtubules nucleated by the centrosomes, and do not appear to have the ability to stimulate microtubule growth (Rieder et al., 1993; Zhang and Nicklas, 1995). In contrast, in Drosophila male meiosis and most likely also in Pales meiosis, the chromosomes act as microtubule- organizing centers, even in the absence of centrosomes (Fig. 3 B; see also Church et al., 1986). Thus, we suggest that anastral spindles are assembled only in those centrosome-containing systems where the chromosomes can induce formation of a sufficient number of microtubules. In systems where the chromosomes are unable to promote substantial microtubule growth, there would not be enough microtubules to form a bipolar spindle.
asl Mutants Form a Normal Central Spindle that is Fully Able to Induce Cytokinesis
One of the most remarkable features of asl male meiosis is the formation of a morphologically normal central spindle in most ana–telophases. This finding challenges the classical view of central spindle assembly through interaction of antiparallel polar microtubules. Our results argue for a self-organization of the central spindle using either preexisting or newly formed microtubules (Masuda and Cande, 1987). Most likely, central spindle formation during male meiosis is mediated by microtubule cross-linking, plus-end–directed kinesin-like motors (reviewed in Sawin and Endow, 1993; Ault and Rieder, 1994; Hoyt, 1994). This hypothesis is supported by the finding that mutations in KLP3A, a Drosophila gene encoding a kinesin-like protein that concentrates in the central spindle midzone during male meiosis, disrupts central spindle formation and cytokinesis (Williams et al., 1995; Giansanti et al., 1998).
An open question about cell cleavage in animal systems is the source of signals that stimulates contractile ring formation and cytokinesis (reviewed by Fishkind and Wang, 1995; Glotzer, 1997; Goldberg et al., 1997). It has been suggested that these signals may be provided either by the metaphase chromosomes (Earnshaw et al., 1991) or the asters (Rappaport, 1961; Hiramoto, 1971; Rappaport, 1986) or the central spindle (Rappaport and Rappaport, 1974; Cao and Wang, 1996; Fishkind et al., 1996). Our results clearly show that the asters are not needed for the cytokinetic signal. Moreover, the fact that asl chromosomes are scattered within the cell and never congress into a metaphase plate strongly suggests that chromosomes cannot dictate the positioning of the cleavage furrow. This conclusion agrees very well with the results of recent micromanipulation experiments showing that cytokinesis can occur in the absence of chromosomes in grasshopper spermatocytes (Zhang and Nicklas, 1996). Thus, of the three components of the anaphase spindle—the asters, the chromosomes, and the central spindle—only the latter appears to be required for signaling cytokinesis. In this respect, we would like to point out that our findings rule out the possibility of the central spindle merely accumulating cytokinetic signals originating from the asters.
We have recently shown that during Drosophila male meiosis, there is a cooperative interaction between the central spindle and the contractile ring; when one of these structures is disrupted the other one is also affected (Giansanti et al., 1998). Thus, the central spindle appears to play an essential role during cytokinesis. The asters, however, may be important for symmetrical positioning of the central spindle between the two daughter cells.
Acknowledgments
We thank B. Wakimoto for EMS-induced male sterile mutants; C. Field, W.G. Whitfield, T.C. Kaufman, and B.C. Williams for anti-anillin, anti-γ tubulin, anti-centrosomin, and anti-KLP3A antibodies, respectively; C. Goday, L. Lascari, and F. Pasquetti for advice and help with EM; and M.L. Goldberg for comments on the manuscript.
This work was supported in part by a grant from Progetto Strategico del CNR Cell Cycle and Apoptosis.
Footnotes
Address all correspondence to Silvia Bonaccorsi, Dipartimento di Genetica e Biologia Molecolare, Universita' di Roma La Sapienza, P.le Aldo Moro 5, 00185 Rome, Italy. Tel.: 39-6-49912593; Fax: 39-6-4456866; E-mail: bonaccorsi@axcasp.caspur.it
1. Abbreviation used in this paper: EMS, ethyl methane sulfonate.
References
- Albertson DG, Thomson JN. Segregation of holocentric chromosomes at meiosis in the nematode Caenorhabditis elegans. . Chromosome Res. 1993;1:15–26. doi: 10.1007/BF00710603. [DOI] [PubMed] [Google Scholar]
- Ault JG, Rieder CL. Centrosome and kinetochore movement during mitosis. Curr Opin Cell Biol. 1994;6:41–49. doi: 10.1016/0955-0674(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Callaini G, Whitfield WG, Riparbelli MG. Centriole and centrosome dynamics during the embryonic cell cycles that follow the formation of the cellular blastoderm in Drosophila. . Exp Cell Res. 1997;234:183–190. doi: 10.1006/excr.1997.3618. [DOI] [PubMed] [Google Scholar]
- Cao L-G, Wang Y-L. Signals from the spindle midzone are required for the stimulation of cytokinesis in cultured epithelial cells. Mol Biol Cell. 1996;7:225–232. doi: 10.1091/mbc.7.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci G, Bonaccorsi S, Pisano C, Verni F, Gatti M. Chromatin and microtubule organization during premeiotic, meiotic, and early postmeiotic stages of Drosophila melanogasterspermatogenesis. J Cell Sci. 1994;107:3521–3534. doi: 10.1242/jcs.107.12.3521. [DOI] [PubMed] [Google Scholar]
- Church K, Nicklas RB, Lin H-PP. Micromanipulated bivalents can trigger mini-spindle formation in Drosophila melanogasterspermatocyte cytoplasm. J Cell Biol. 1986;103:2765–2773. doi: 10.1083/jcb.103.6.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debec A. Aploid cell cultures of Drosophila melanogaster. . Nature. 1978;374:255–256. doi: 10.1038/274255a0. [DOI] [PubMed] [Google Scholar]
- Debec A, Detraves C, Montmory C, Geraud G, Wright M. Polar organization of γ tubulin in acentriolar mitotic spindles of Drosophila melanogastercells. J Cell Sci. 1995;108:2645–2653. doi: 10.1242/jcs.108.7.2645. [DOI] [PubMed] [Google Scholar]
- Dietz R. The dispensability of the centrioles in the spermatocyte divisions of Pales ferruginea (Nematocera). . Heredity. 1966;19(Suppl.):161–166. [Google Scholar]
- Earnshaw WC, Bernat RL, Cooke CA, Rothfield NF. Role of the centromere/kinetochore in cell cycle control. Cold Spring Harbor Symp Quant Biol. 1991;56:675–685. doi: 10.1101/sqb.1991.056.01.076. [DOI] [PubMed] [Google Scholar]
- Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endow SA, Komma DJ. Spindle dynamics during meiosis in Drosophilaoocytes. J Cell Biol. 1997;137:1321–1336. doi: 10.1083/jcb.137.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field C, Alberts BM. Anillin, a contractile ring protein that cycles from the nucleus to the cell cortex. J Cell Biol. 1995;131:165–178. doi: 10.1083/jcb.131.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishkind DJ, Silverman JD, Wang Y-L. Function of spindle microtubules in directing cortical movements and actin filaments organization in dividing cultured cells. J Cell Sci. 1996;109:2041–2051. doi: 10.1242/jcs.109.8.2041. [DOI] [PubMed] [Google Scholar]
- Fishkind DJ, Wang Y-L. New horizons for cytokinesis. Curr Opin Cell Biol. 1995;7:23–31. doi: 10.1016/0955-0674(95)80041-7. [DOI] [PubMed] [Google Scholar]
- Fuller, M.T. 1993. Spermatogenesis. In The Development of Drosophila melanogaster. Vol. I. M. Bate and A.M. Arias, editors. Cold Spring Harbor Laboratory Press, Plainview, NY. 71–147.
- Gaglio T, Dionne MA, Compton DA. Mitotic spindle poles are organized by structural and motor proteins in addition to centrosomes. J Cell Biol. 1997;138:1055–1066. doi: 10.1083/jcb.138.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio T, Saredi A, Compton DA. NuMA is required for the organization of microtubules into aster-like mitotic arrays. J Cell Biol. 1995;131:693–708. doi: 10.1083/jcb.131.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gans M, Audit C, Masson M. Isolation and characterization of sex-linked female sterile mutants in Drosophila melanogaster. . Genetics. 1975;81:683–704. doi: 10.1093/genetics/81.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DL. Microtubule organization during maturation of Xenopusoocytes: assembly and rotation of the meiotic spindles. Dev Biol. 1992;151:516–530. doi: 10.1016/0012-1606(92)90190-r. [DOI] [PubMed] [Google Scholar]
- Giansanti MG, Bonaccorsi S, Williams B, Williams EV, Santolamazza C, Goldberg ML, Gatti M. Cooperative interaction between the central spindle and the contractile ring during Drosophilacytokinesis. Genes Dev. 1998;12:396–410. doi: 10.1101/gad.12.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M. The mechanism and control of cytokinesis. Curr Opin Cell Biol. 1997;9:815–823. doi: 10.1016/s0955-0674(97)80082-8. [DOI] [PubMed] [Google Scholar]
- Goldberg, M.L., K. Gunsalus, R.E. Karess, and F. Chang. 1998. Cytokinesis, or breaking up is hard to do. In Dynamics of Cell Division. S. Endow and D. Glover, editors. Oxford University Press, London. In press.
- Goldstein LSB. Kinetochore structure and its role in chromosome orientation during the first meiotic division in male D. melanogaster. . Cell. 1981;25:591–602. doi: 10.1016/0092-8674(81)90167-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Casal J, Ripoll P. Relationship between chromosome content and nuclear diameter in early spermatids of Drosophila melanogaster. . Genet Res. 1989;54:205–212. doi: 10.1017/s0016672300028664. [DOI] [PubMed] [Google Scholar]
- Gunsalus KC, Bonaccorsi S, Williams E, Verni F, Gatti M, Goldberg ML. Mutations in twinstar, a Drosophilagene encoding a cofilin/ADF homologue, result in defects in centrosome migration and cytokinesis. J Cell Biol. 1995;131:1–17. doi: 10.1083/jcb.131.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsumi M, Endow SA. Mutants of the microtubule motor protein, nonclaret disjunctional, affect spindle structure and chromosome movement in meiosis and mitosis. J Cell Sci. 1992;101:547–559. doi: 10.1242/jcs.101.3.547. [DOI] [PubMed] [Google Scholar]
- Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, Karsenti E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopusegg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- Heald R, Tournebize R, Habermann A, Karsenti E, Hyman A. Spindle assembly in Xenopusegg extracts: respective roles of centrosomes and microtubule self-organization. J Cell Biol. 1997;138:615–628. doi: 10.1083/jcb.138.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hime GR, Brill JA, Fuller MT. Assembly of ring canals in the male germ line from structural components of the contractile ring. J Cell Sci. 1996;109:2779–2788. doi: 10.1242/jcs.109.12.2779. [DOI] [PubMed] [Google Scholar]
- Hiramoto Y. Analysis of cleavage stimulus by means of micromanipulation of sea urchin eggs. Exp Cell Res. 1971;8:291–298. doi: 10.1016/0014-4827(71)90153-4. [DOI] [PubMed] [Google Scholar]
- Hoyt MA. Cellular roles of kinesin and related proteins. Curr Opin Cell Biol. 1994;6:63–68. doi: 10.1016/0955-0674(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Kirschner M, Mitchison TJ. Beyond self-assembly: from microtubule to morphogenesis. Cell. 1986;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Lewis EB, Bacher F. Method for feeding ethyl methane sulphonate (E.M.S.) to Drosophilamales. Drosophila Inform Serv. 1968;43:193. [Google Scholar]
- Li K, Kaufman TC. The homeotic target gene centrosomin encodes an essential centrosomal component. Cell. 1996;85:585–596. doi: 10.1016/s0092-8674(00)81258-1. [DOI] [PubMed] [Google Scholar]
- Lindsley, D.L., and G.G. Zimm. 1992. The Genome of Drosophila melanogaster. Academic Press, San Diego. CA.
- Mastronarde DN, McDonald KL, Ding R, McIntosh JR. Interpolar spindle microtubules in PtK cells . J Cell Biol. 1993;123:1475–1489. doi: 10.1083/jcb.123.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H, Cande WZ. The role of tubulin polymerization during spindle elongation in vitro. Cell. 1987;49:193–202. doi: 10.1016/0092-8674(87)90560-5. [DOI] [PubMed] [Google Scholar]
- Matthies HJG, McDonald HB, Goldstein LSB, Theurkauf WE. Anastral meiotic spindle morphogenesis: role of the non-claret disjunctional kinesin-like protein. J Cell Biol. 1996;134:455–464. doi: 10.1083/jcb.134.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim KS, Hawley RS. Chromosomal control of meiotic cell division. Science. 1995;270:1595–1601. doi: 10.1126/science.270.5242.1595. [DOI] [PubMed] [Google Scholar]
- Merdes A, Cleveland DW. Pathways of spindle pole formation: different mechanisms; conserved components. J Cell Biol. 1997;138:953–956. doi: 10.1083/jcb.138.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A, Ramyar K, Vechio JD, Cleveland DW. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell. 1996;87:447–458. doi: 10.1016/s0092-8674(00)81365-3. [DOI] [PubMed] [Google Scholar]
- Rappaport R. Experiments concerning the cleavage stimulus in sand dollar eggs. J Exp Zool. 1961;148:81–89. doi: 10.1002/jez.1401480107. [DOI] [PubMed] [Google Scholar]
- Rappaport R. Establishment of the mechanism of cytokinesis in animal cells. Int Rev Cytol. 1986;105:245–281. doi: 10.1016/s0074-7696(08)61065-7. [DOI] [PubMed] [Google Scholar]
- Rappaport R, Rappaport BN. Establishment of cleavage furrows by the mitotic spindle. J Exp Zool. 1974;189:189–196. doi: 10.1002/jez.1401890206. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Alexander SP. Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J Cell Biol. 1990;110:81–95. doi: 10.1083/jcb.110.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder, C.L., J.G. Ault, U. Eichenlaub-Ritter, and G. Sluder. 1993. Morphogenesis of the mitotic and the meiotic spindle: conclusions obtained from one system are not necessarily applicable to the other. In Chromosome Segregation and Aneuploidy. NATO ASI Series. Vol. H72. B.K. Vig, editor. Springer-Verlag, Berlin. 183–197.
- Sawin KE, Endow SA. Meiosis, mitosis and microtubule motors. BioEssays. 1993;15:399–407. doi: 10.1002/bies.950150606. [DOI] [PubMed] [Google Scholar]
- Sluder G, Miller FJ, Rieder CL. The reproduction of centrosomes: nuclear versus cytoplasmic controls. J Cell Biol. 1986;103:1873–1881. doi: 10.1083/jcb.103.5.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G, Rieder CL. Experimental separation of pronuclei in fertilized sea urchin eggs: chromosomes do not organize a spindle in the absence of centrosomes. J Cell Biol. 1985;100:897–903. doi: 10.1083/jcb.100.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova EA, Bajer AS. Spindle poles in higher plant mitosis. Cell Motil Cytoskelet. 1992;23:1–7. doi: 10.1002/cm.970230102. [DOI] [PubMed] [Google Scholar]
- Steffen W, Fuge H, Dietz R, Bastmeyer M, Muller G. Aster-free spindle poles in insect spermatocytes: evidence for chromosome-induced spindle formation. J Cell Biol. 1986;102:1679–1687. doi: 10.1083/jcb.102.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf WE, Hawley RS. Meiotic spindle assembly in Drosophilafemales: behavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein. J Cell Biol. 1992;116:1167–1180. doi: 10.1083/jcb.116.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters JC, Mitchison TJ, Rieder CL, Salmon ED. The kinetochore microtubule minus-ends disassembly associated with poleward flux produces a force that can do work. Mol Biol Cell. 1996;7:1547–1558. doi: 10.1091/mbc.7.10.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters JC, Salmon ED. Pathways of spindle assembly. Curr Opin Cell Biol. 1997;9:37–43. doi: 10.1016/s0955-0674(97)80149-4. [DOI] [PubMed] [Google Scholar]
- Williams BC, Riedy MF, Williams EV, Gatti M, Goldberg ML. The Drosophilakinesin-like protein KLP3A is a midbody component required for central spindle assembly and initiation of cytokinesis. J Cell Biol. 1995;129:709–723. doi: 10.1083/jcb.129.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Nicklas B. The impact of chromosomes and centrosomes on spindle assembly as observed in living cells. J Cell Biol. 1995;129:1287–1300. doi: 10.1083/jcb.129.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Nicklas B. Anaphase and cytokinesis in the absence of chromosomes. Nature. 1996;382:466–468. doi: 10.1038/382466a0. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Jung K, Oakley BR. γ-tubulin is present in Drosophila melanogaster and homo sapiensand is associated with the centrosome. Cell. 1991;65:817–823. doi: 10.1016/0092-8674(91)90389-g. [DOI] [PubMed] [Google Scholar]