Abstract
As for most integral membrane proteins, the intracellular transport of retroviral envelope glycoproteins depends on proper folding and oligomeric assembly in the ER. In this study, we considered the hypothesis that a panel of 22 transport-defective mutants of the human T cell leukemia virus type 1 envelope glycoprotein might be defective in ER assembly. Upon cell cotransfection with wild-type envelope, however, the vast majority of these transport-defective mutants (21 of 22) exerted a specific trans-dominant negative effect. This effect was due to random dimerization of the mutated and wild-type glycoproteins that prevented the intracellular transport of the latter. This unexpected result suggests that association of glycoprotein monomers precedes the completion of folding. The only mutation that impaired this early assembly was located at the NH2 terminus of the protein. COOH-terminally truncated, soluble forms of the glycoprotein were also trans-dominant negative provided that their NH2 terminus was intact. The leucine zipper-like domain, although involved in oligomerization of the envelope glycoproteins at the cell surface, did not contribute to their intracellular assembly. We propose that, at a step subsequent to translation, but preceding complete folding of the monomers, glycoproteins assemble via their NH2-terminal domains, which, in turn, permits their cooperative folding.
Keywords: protein processing; posttranslational; viral envelope proteins; genes, dominant; human T cell leukemia virus
Cell surface proteins are usually oligomers, and reach the plasma membrane after they undergo stepwise processes which ensure their structural integrity (Hurtley and Helenius, 1989; Einfeld and Hunter, 1991). Viral envelope glycoproteins have been extensively used as models for defining the main characteristics of the cellular “quality control” of membrane proteins because, although encoded by viruses, they follow the normal secretory pathway also used by the cellular proteins of the plasma membrane (Doms et al., 1993). Studies of the influenza hemagglutinin (HA)1 precursor HA0 and of the vesicular stomatitis virus (VSV) G protein showed that, as a rule, quality control over newly synthesized membrane proteins is exerted primarily by the ER: only membrane glycoproteins that are correctly folded and oligomerized can leave the ER and proceed to the Golgi complex and beyond, whereas proteins with incorrect tertiary or quaternary structure are retained in the ER and eventually degraded. Excess misfolded protein can, however, escape this ER control, in which case the intermediate compartment and the cis-Golgi intervene as a back-up quality control system to recycle the protein back to the ER via retrograde transport (Hammond and Helenius, 1994).
Folding of membrane proteins begins during their cotranslational translocation in the ER compartment, and continues posttranslationally. The rate and efficiency of folding vary considerably among proteins. Important differences reside in the time required and the nature of the intermediates formed before the acquisition of the mature oligomeric conformation. For VSV G, but not for HA0, the normal folding process involves the transient formation of multimolecular complexes (de Silva et al., 1993). In both cases, the bona fide trimeric assembly is a posttranslational and kinetically late step in the sequence of events leading to the transport-competent conformation. Indeed, it occurs after the monomers have reached their fully oxidized form and characteristic immunoreactivity (Copeland et al., 1988; de Silva et al., 1993; Tatu et al., 1993, 1995). This supports the contention that extensive folding must take place for monomeric subunits to recognize each other as specific assembly partners (Doms et al., 1993). In the case of the hemagglutinin-neuraminidase glycoprotein of the human parainfluenza virus type 3, however, oligomeric assembly occurs as an early event that precedes the acquisition of correct intramolecular disulfide bonds and mature immunoreactivity (Collins and Mottet, 1991).
Retroviral envelope glycoproteins also constitute a good model for studying the maturation steps required for protein transport out of the ER (Einfeld, 1996). These envelope proteins are first synthesized in the ER as a precursor membrane protein, which is then transported to the Golgi apparatus where its cleavage into two mature products occurs, mediated by a furin-like cellular protease. Transport competence is thus easily monitored by the appearance of the cleaved viral products, the surface (SU) and the transmembrane (TM) glycoproteins. Among retroviral envelopes, that of the human T cell leukemia virus type 1 (HTLV-1), an oncogenic human retrovirus, provides an interesting model for exploring the limiting steps for the acquisition of transport competence, because the quality control exerted over the structural integrity of the precursor glycoprotein is remarkably stringent (Pique et al., 1990; Delamarre et al., 1996). Indeed, most of the mutations that we have artificially introduced in the HTLV-1 glycoprotein result in a lack of precursor cleavage, which most probably indicates retention of the precursor in the ER. In the present study, we took advantage of our large series of mutants to examine whether the defect in intracellular transport exhibited by these glycoproteins might actually reflect a defect in oligomeric assembly. We designed a dominant negative assay (Herskowitz, 1987) in which the retained glycoproteins were systematically tested for their ability to interfere in trans with the transport and hence function of the wild-type (wt) glycoprotein. We found, unexpectedly, that the vast majority of the mutated glycoproteins that could not be transported into the Golgi apparatus and beyond were nevertheless capable of specific dimeric association with the wt precursor glycoprotein. That incompetence for assembly was rarely responsible for the intracellular retention of these glycoproteins suggests the occurrence of an early assembly step preceding the completion of folding.
Materials and Methods
Plasmids
The HTLV envelope expression vectors used in this study are the previously described plasmids CMV-ENV-1 (Delamarre et al., 1997) and CMV-ENV-2 (Rosenberg et al., 1998), which contain the respective HTLV-1 and HTLV-2 sequences corresponding to the env, tax, and rex genes, under the control of the simian cytomegalovirus (CMV) promoter. The CMV-ENVΔPvuII and CMV-ENVΔPmaCI constructs were used as the respective negative controls (Delamarre et al., 1997; Rosenberg et al., 1998). The mutated constructs coding for HTLV-1 glycoproteins with single amino acid substitutions were described elsewhere (Delamarre et al., 1997; Rosenberg et al., 1997). The CMV-ENV438-stop plasmid encodes a soluble form of the HTLV-1 glycoprotein under the control of the CMV promoter (Pique et al., 1993; Rosenberg et al., 1997). The human immunodeficiency virus type 1 (HIV-1) envelope expression plasmid pMA243, which is derived from an HIV-1LAI provirus and has the capacity for encoding the viral proteins Env, Tat, Rev, and Vpu, was a gift from M. Alizon (INSERM U332, ICGM, Paris, France) (Dragic et al., 1992).
Mutagenesis and Cloning
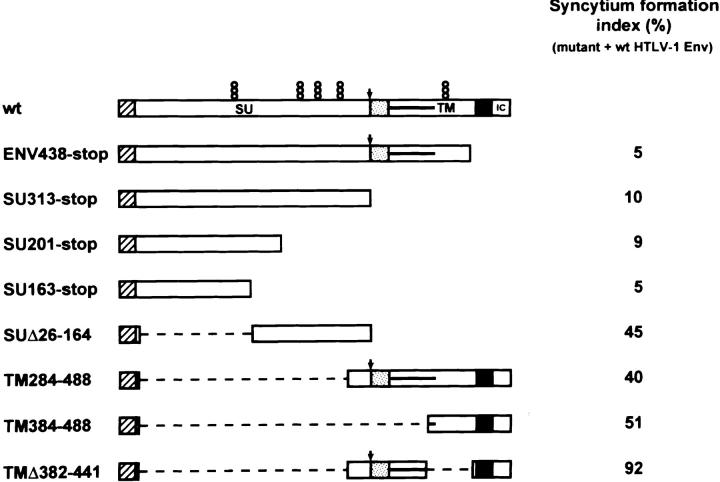
Oligonucleotide-directed mutagenesis of the sequence encoding the SU portion of the HTLV-2 envelope protein was performed as described elsewhere (Rosenberg et al., 1998). The constructs coding for HTLV-1 envelope proteins with truncations or deletions were generated using standard cloning techniques. Positions in the amino acid sequence of the HTLV-1 envelope protein are numbered from the initiator methionine. The locations of truncations and deletions relative to other features of the HTLV-1 envelope protein are depicted in Fig. 6.
Figure 6.
Effect of truncated and deleted HTLV-1 glycoprotein mutants on syncytium formation induced by wt envelope. (left) Schematic representations of wt, truncated, and deleted HTLV-1 glycoproteins. The hydrophobic stretches corresponding to the signal peptide, fusion peptide, and membrane-anchorage domain are depicted as hatched, dotted, and black boxes, repectively. The intracytoplasmic (IC) domain is indicated. The position of the cleavage site is shown with an arrow, those of the five N-glycosylation sites with piled circles. The horizontal bar marks the position of the leucine zipper-like motif within the TM ectodomain. Internal deletions are shown with broken lines that encompass the deleted regions. (Right) Effect of the truncated and deleted mutants on syncytium formation induced by wt envelope. Cells were cotransfected with the wt HTLV-1 envelope expression plasmid CMV-ENV-1 plus a threefold excess of the truncated or deleted construct. The syncytium formation index gives the percentage of fusion relative to that obtained after cell cotransfection with the same amount of CMV-ENV-1 plus a threefold excess of negative control (CMV-ENVΔPvuII).
Cell Lines
COS-1 and HeLa cells were obtained from the American Type Culture Collection. CosLTRLacZ cells, which are COS cells stably expressing the bacterial β-galactosidase gene (lacZ) under the control of the HIV-1 long terminal repeat (LTR), and HeLa-Tat cells, which are HeLa cells stably expressing the HIV-1 tat gene, were a gift from M. Alizon (Dragic et al., 1992). Also provided by M. Alizon were the HeLa-P4 cells, which are HeLa cells stably expressing both the lacZ gene under the control of the HIV-1 LTR and the human CD4 cDNA (Clavel and Charneau, 1994). All cell lines were grown in DME containing 50 μg/ml gentamicin and 5% FCS, and supplemented with 300 μg/ml hygromycin B (Calbiochem Corp.) for the CosLTRLacZ cells, or with 500 μg/ml G-418 sulphate (Geneticin, Life Technologies, Inc.) for the HeLa-P4 cells. Cell cultures were maintained at 37°C in a humidified 5% CO2 atmosphere.
Syncytium Formation Assays
The quantitative assays used to evaluate syncytium formation elicited by the HTLV-1, HTLV-2, or HIV-1 envelopes have been described elsewhere (Dragic et al., 1992; Delamarre et al., 1997; Rosenberg et al., 1998). In all these assays, the HIV-1 LTR-driven expression of β-galactosidase is transactivated by the Tat protein upon fusion of envelope-expressing cells with receptor-bearing indicator cells. To assess the dominant negative effect of the glycoprotein mutants on syncytium formation induced by the wt HTLV-1 or HTLV-2 glycoproteins, the mutated and wt envelope constructs (total amount: 3 μg DNA) were cotransfected into CosLTRLacZ cells seeded at 3 × 105 cells per 60-mm–diameter dish the previous day by a procedure using DEAE-dextran, chloroquine, and dimethyl sulfoxide (Cullen, 1987). Immunofluorescence analysis of the cells cotransfected with the constructs for the wt HTLV-1 envelope and for a truncation mutant demonstrated that more than 95% of the cells that expressed one of the envelope constructs in fact expressed both of them (data not shown). Radioimmunoprecipitation analysis also assured us that, within the range of DNA quantities used in this study, the level of protein expressed correlated with the amount of DNA transfected. 1 d (HTLV-2) or 2 d (HTLV-1) after transfection, 5 × 105 HeLa-Tat cells were added as indicator cells. After a 24-h coculture, the amount of β-galactosidase was evaluated by a chemiluminescence assay for detection of the activity of this enzyme in cell lysates (Galacto-Light; Tropix) with a luminometer (Lumat LB 9501; Berthold) (Delamarre et al., 1997; Rosenberg et al., 1998). To assess the effect of the HTLV-1 glycoprotein mutants on syncytium formation induced by the wt HIV-1 envelope, the mutated HTLV-1 envelope constructs (2.25 μg) and the pMA243 plasmid (5 ng) were cotransfected into COS-1 cells seeded at 3 × 105 cells per 60-mm–diameter dish the previous day (Cullen, 1987). 2 d after transfection, 5 × 105 HeLa-P4 cells were added as indicator cells. After a 24-h coculture, syncytia were stained in situ and counted under a light microscope as described elsewhere (Dragic et al., 1992). For each assay, the syncytium formation index gives the percentage fusion induced by the wt envelope in the presence of the glycoprotein mutant relative to that obtained in its absence.
Immunofluorescence Microscopy
The envelope constructs were transfected into COS-1 or HeLa cells by the calcium phosphate precipitation method. 1 d after transfection, the cells were seeded onto glass slides (Lab-Tek; Nunc, Inc.) at 4 × 104 cells per 80-mm2 well. The next day, cultures were rinsed in PBS and fixed with 4% paraformaldehyde for 15 min at room temperature, followed by quenching in 0.1 M glycine in PBS. Permeabilization and saturation were achieved by a 2-h incubation in PBS containing 0.05% saponin and 0.2% BSA, and all subsequent steps were performed in this buffer at room temperature. The cells were incubated for 90 min with the primary antibodies. These were mAb 1C11 (1:100; Epitope), which is a murine mAb directed to the HTLV-1 SU (Palker et al., 1989), and rabbit polyclonal antibodies directed to the α subunit of translocating chain-associating membrane protein (1:500, kindly provided by T.A. Rapoport, Department of Cell Biology, Harvard Medical School, Boston, MA) or to Rab1 (1:25, kindly provided by B. Goud, CNRS UMR 144, Institut Curie, Paris, France). Excess antibody was removed with five washes, and the secondary antibodies were allowed to bind for 60 min. These were FITC-conjugated goat anti– mouse IgG (1:400; Jackson ImmunoResearch Laboratories, Inc.) and cyanin 3–conjugated goat anti–rabbit IgG (1:300; Jackson ImmunoResearch Laboratories, Inc.). After five washes, the slides were mounted in Mowiol and observed with a confocal laser scanning microscope (MRC-1000; Bio-Rad Laboratories, with a 60× objective; Nikon Inc.). The pinhole aperture was such that optical section thickness was 0.6 μm. “Bleed-through” from the FITC to the cyanin channel was negligible. Images were processed using the Laser Sharp software. Colocalization appeared as yellow pixels after merging sections recorded at the same z level in each channel.
Radioimmunoprecipitation Assays
Immunoprecipitations of the HTLV-1 envelope glycoproteins were performed as described in our previous studies (Pique et al., 1990), using protein A–Sepharose CL-4B beads (Pharmacia Biotechnology) coated with a pool of sera from HTLV-1–infected individuals (provided by J. Coste, CRTS, Montpellier, France). For coimmunoprecipitation experiments, protein A–Sepharose beads were coated with rabbit anti–mouse Ig (Dako SA) plus purified 4D4 mAb, which is a murine mAb raised against a synthetic peptide covering the COOH-terminal domain (amino acids 287– 311) of the HTLV-1 SU (kindly donated by C. Desgranges and M.-P. Grange, INSERM U271, Lyon, France) (Grange et al., 1998). Immunoprecipitates were electrophoresed in SDS-13% polyacrylamide gels under reducing conditions (except where otherwise stated), and visualized by autoradiography.
Velocity Gradient Sedimentation
The envelope constructs were transfected into COS-1 cells (2.8 × 106) by the calcium phosphate precipitation method. 2 d after transfection, the cells were lysed in 0.8 ml of 100 mM Tris-HCl, pH 8.0, containing 100 mM NaCl, 1 mM CaCl2, and 250 mM n-octyl-β-d-glucopyranoside (Sigma Chemical Co.). The clarified lysates, together with 50 μl of size markers (kD): 66 BSA, 141 alcohol dehydrogenase, and 250 catalase, each at 9 mg/ml (Sigma Chemical Co.), were loaded onto continuous sucrose gradients (10 ml, 5–35% sucrose in 100 mM Tris-HCl, pH 8.0, containing 100 mM NaCl, 1 mM CaCl2, and 40 mM n-octyl-β-d-glucopyranoside) and centrifuged for 20 h at 4°C in an SW41 rotor at 38,000 rpm before fractionation from the bottom of the tube into 18 fractions of 600 μl. To analyze size markers, 60-μl aliquots were removed from the fractions and were separated by SDS-PAGE, followed by Coomassie blue staining. The remainders of the fractions were subjected to immunoprecipitation with a pool of sera from HTLV-1–infected individuals as described above. Immunoprecipitates were electrophoresed in SDS-10% polyacrylamide gels under reducing conditions, and proteins were transferred onto membranes (Immobilon-P; Millipore Corp.). After saturation in PBS containing 0.05% Tween-20 and 5% skim milk, the membranes were incubated with a 1:3,000 dilution of the mAb 4D4 (Grange et al., 1998) for 90 min at room temperature. Excess antibody was removed with six washes, and a 1:1,000 dilution of the second-step antibody (peroxidase-conjugated goat anti–mouse IgG; Jackson ImmunoResearch Laboratories, Inc.) was allowed to bind for 60 min. After eight washes, immunoreactive spots were detected by enhanced chemiluminescence (Amersham Buchler GmbH).
Results
Negative Dominance as a Tool to Study Determinants of Envelope Precursor Assembly
We have previously described a series of HTLV-1 envelope glycoprotein mutants that are defective in cleavage of the precursor into the mature SU and TM products (Delamarre et al., 1997; Rosenberg et al., 1997). The cleavage of the HTLV-1 glycoprotein precursor, which is required for envelope-mediated function, normally takes place in the Golgi apparatus (Pique et al., 1992). As for most integral membrane glycoproteins, transport into the Golgi apparatus is thought to be strictly dependent on proper folding and oligomeric assembly in the ER (Doms et al., 1993). We thus considered the hypothesis that our HTLV-1 glycoprotein mutants with defects in precursor cleavage might actually be defective in precursor assembly. We designed a dominant negative assay (Herskowitz, 1987) in which these cleavage-defective mutants were coexpressed with the wt HTLV-1 glycoprotein in transfected COS cells. In this approach, an assembly-competent mutant would be expected to dominantly interfere with wt envelope-mediated function by titrating the wt precursor in the formation of transport-defective heterocomplexes; in contrast, an assembly-defective mutant would spare the intracellular maturation of the wt glycoprotein and hence its function. Transfected cells expressing the wt HTLV-1 envelope at the cell surface are able to induce fusion with indicator cells expressing the receptor, leading to the formation of syncytia. Syncytium formation was thus chosen as a convenient assay to account for intracellular transport and hence function of the HTLV-1 envelope.
21 of 22 Cleavage-defective Glycoprotein Mutants Are Trans Dominant
Because several reports have suggested that the oligomerization domain of retroviral envelopes lies in the TM glycoprotein (Einfeld and Hunter, 1988, 1994; Earl et al., 1990; Rey et al., 1990; Thomas et al., 1991), we first investigated the abilities of HTLV-1 glycoproteins with mutations in the TM portion to interfere with wt envelope-mediated function. We have previously described eight single amino acid substitutions in the HTLV-1 TM that result in a lack of precursor cleavage and, consequently, in a complete loss of syncytium-forming ability (Rosenberg et al., 1997) (see Table I). The mutated envelope expression plasmids were cotransfected with the wt envelope expression plasmid (CMV-ENV-1) at a ratio of 3:1, and syncytium formation was monitored. Each of the eight cleavage-defective TM mutants exerted a marked dominant negative effect over the wt envelope, manifested by inhibition of syncytium formation (Table I).
Table I.
Effect of Cleavage-defective HTLV-1 TM Mutants on Syncytium Formation Induced by wt Envelope
| HTLV-1 glycoprotein mutant | Precursor cleavage index (%) | Syncytium formation index (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| Mutant* | Mutant + wt HTLV-1 Env‡ | Mutant + wt HIV-1 Env§ | ||||||
| Mutant* | ||||||||
| Mock‖ | <20 | <5 | 100 | 100 | ||||
| Asp351-His | <20 | <5 | 7 | 91 | ||||
| Leu368-Arg | 29 | <5 | <5 | 113 | ||||
| Ala375-Asp | 25 | <5 | 5 | 104 | ||||
| Arg379-Gly | <20 | <5 | 12 | 86 | ||||
| Asp383-Tyr | 20 | <5 | 9 | 88 | ||||
| Glu398-Val | 32 | <5 | 10 | 96 | ||||
| Ser408-Phe | 30 | 7 | 15 | 100 | ||||
| Leu419-Arg | 31 | <5 | 13 | 85 | ||||
The precursor cleavage and syncytium formation indices for the HTLV-1 glycoprotein mutants relative to the wt HTLV-1 envelope glycoprotein are from our previous studies (Rosenberg et al., 1997).
Cells were cotransfected with the mutant constructs (2.25 μg) and the wt HTLV-1 envelope construct CMV-ENV-1 (0.75 μg). Data represent the means of at least three independent transfections (standard errors were <5%).
Cells were cotransfected with the mutant constructs (2.25 μg) and the wt HIV-1 envelope construct pMA243 (5 ng).
Cells were transfected with the negative control plasmid CMV-ENVΔPvuII.
That all of the glycoproteins mutated in the TM portion were able to interfere in trans with wt envelope-mediated function prompted us to test for negative dominance of glycoproteins mutated in the SU portion. We have previously described 14 single amino acid substitutions distributed throughout the HTLV-1 SU that abolish precursor cleavage and hence envelope-mediated function (Delamarre et al., 1997) (see Table II). A marked dominant negative effect on the syncytium-forming activity of wt envelope was documented for 13 of these 14 SU mutants (Table II). Thus, the vast majority of HTLV-1 envelope mutants with defects in cleavage of the precursor glycoprotein are trans-dominant negative.
Table II.
Effect of Cleavage-defective HTLV-1 SU Mutants on Syncytium Formation Induced by wt Envelope
| HTLV-1 glycoprotein mutant | Precursor cleavage index (%) | Syncytium formation index (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| Mutant* | Mutant + wt HTLV-1 Env‡ | Mutant + wt HIV-1 Env§ | ||||||
| Mutant* | ||||||||
| Mock‖ | <20 | <5 | 100 | 100 | ||||
| Ser25-Arg | 22 | 6 | 72 | 91 | ||||
| Leu52-Arg | <20 | <5 | 7 | 86 | ||||
| Asp53-Val | <20 | <5 | 5 | 101 | ||||
| Ser58-Leu | <20 | <5 | 8 | 98 | ||||
| Ser119-Leu | <20 | <5 | <5 | 88 | ||||
| Tyr124-Asp | <20 | <5 | 9 | 85 | ||||
| Ser130-Ile | <20 | <5 | <5 | 100 | ||||
| Ser162-Phe | <20 | <5 | 7 | 96 | ||||
| Asp171-Tyr | <20 | <5 | 5 | 96 | ||||
| Ile173-Ser | <20 | <5 | 10 | 100 | ||||
| Thr178-Ala | <20 | <5 | <5 | 89 | ||||
| His238-Leu | 21 | <5 | 15 | 112 | ||||
| Ser292-Tyr | <20 | <5 | 13 | 87 | ||||
| Leu295-Arg | 20 | <5 | 13 | 92 | ||||
The precursor cleavage and syncytium formation indices for the HTLV-1 glycoprotein mutants relative to the wt HTLV-1 envelope glycoprotein are from our previous studies (Delamarre et al., 1997).
Cells were cotransfected with the mutant constructs (2.25 μg) and the wt HTLV-1 envelope construct CMV-ENV-1 (0.75 μg). Data represent the means of at least three independent transfections (standard errors were <5%).
Cells were cotransfected with the mutant constructs (2.25 μg) and the wt HIV-1 envelope construct pMA243 (5 ng).
Cells were transfected with the negative control plasmid CMV-ENVΔPvuII.
Specificity of the Trans-dominant Negative Effect
We examined whether the inhibitory effect exerted by the mutants was specific for the HTLV-1 envelope. For this purpose, we cotransfected each HTLV-1 glycoprotein mutant construct with an expression vector encoding the wt envelope glycoprotein of HIV-1 (pMA243), and we scored the number of syncytia elicited by the HIV-1 envelope. In contrast to their drastic effect on syncytium formation induced by the wt HTLV-1 envelope, the HTLV-1 glycoprotein mutants failed to interfere with syncytium formation induced by the wt HIV-1 envelope, even upon cotransfection of a 450-fold excess of the HTLV-1 plasmid (Tables I and II). As a positive control, coexpression of CD4 with the HIV-1 glycoprotein gave the expected inhibition of syncytium formation (Bour et al., 1991; data not shown). The dominant negative effect was thus specific, and could not be accounted for by some general alteration of the intracellular maturation or transport of membrane proteins.
Random Dimerization of wt and Mutated Glycoproteins
It has been shown that aggregation occurs in the ER of cells synthesizing misfolded proteins (Hurtley et al., 1989) and is not restricted to products from a single polysome (Marquardt and Helenius, 1992). It could thus be argued that the mutated glycoproteins might titrate the wt glycoprotein in the formation of large heteroaggregates rather than in a bona fide dimeric assembly process. To decide between these two hypotheses, we combined different approaches.
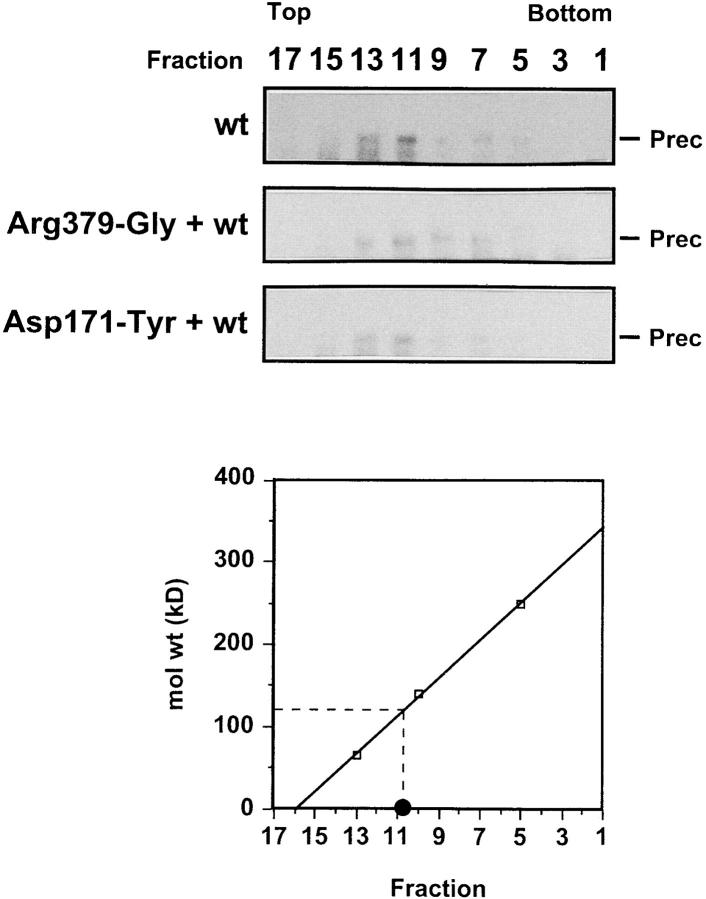
We first examined the oligomeric structure of the envelope glycoproteins from transfected cell lysates by velocity sedimentation on sucrose gradients. Consistent with previous data (Paine et al., 1994), most of the wt HTLV-1 envelope precursor glycoprotein was recovered in a peak at the position expected for the dimeric form of this 61-kD protein (Fig. 1). A small amount of faster-sedimenting material was also detected that might correspond to tetramer formation. A similar pattern of sedimentation was detected in lysates from cells coexpressing the wt glycoprotein plus a threefold excess of a cleavage-defective dominant negative mutant (see the examples of the TM mutant Arg379-Gly and of the SU mutant Asp171-Tyr in Fig. 1). Notably, the position of the envelope glycoproteins did not shift towards the bottom of the gradient, as would have been expected if aggregation had occurred. We also used nonreducing SDS-PAGE to analyze the envelope products radioimmunoprecipitated from lysates of cotransfected cells, because interchain disulfide-bonded complexes often arise in the ER of cells synthesizing misfolded proteins. No such complexes were detected (data not shown), further arguing against an aggregation bias.
Figure 1.
Velocity gradient sedimentation of the envelope glycoproteins from lysates of cells coexpressing the wt HTLV-1 envelope and a cleavage-defective mutant. COS-1 cells were transfected with the following constructs: wt HTLV-1 envelope (CMV-ENV-1), a 3:1 ratio of Arg379-Gly mutant plus wt HTLV-1 envelope, or a 3:1 ratio of Asp171-Tyr mutant plus wt HTLV-1 envelope. Cell lysates were loaded onto 5–35% sucrose gradients and, after centrifugation, the distribution of the envelope precursor glycoproteins across the gradients was determined by immunoprecipitation with sera from HTLV-1–infected individuals, followed by SDS-PAGE and Western blotting with the anti– HTLV-1 glycoprotein 4D4 mAb as the primary antibody. The gradients were calibrated with BSA (66 kD), alcohol dehydrogenase (141 kD), and catalase (250 kD), with • indicating the expected position of the HTLV-1 envelope precursor dimer (size of the monomer: 61 kD).
In addition to these biochemical approaches, we examined the extent of the trans-dominant negative interference exerted by the cleavage-defective mutants as a function of the mutant:wt ratio (Fig. 2). In a cell that coexpresses the wt glycoprotein and an assembly-competent mutant, three dimer combinations are expected: homodimers of wt precursor subunits, homodimers of mutant precursor subunits, and heterodimers consisting of both subunit types. If dimerization is random, the distribution between these three combinations can be calculated for a variety of expression ratios (Fig. 2 a). Cells were transfected with a constant amount of DNA containing various ratios of mutant to wt HTLV-1 envelope constructs (3:1, 1:1, and 1:3), and the resulting syncytium-forming activity was compared with that observed after transfection of the wt construct alone. As shown in Fig. 2 b for two TM and two SU mutants, the experimental indices of syncytium formation at each ratio approached the theoretical values for the corresponding proportions of wt homodimers expected from a random dimerization process. This corroborates our biochemical findings, since trapping of wt glycoprotein in a higher-order structure would have shifted the experimental curves to the right. Therefore, the trans-dominant negative effect most likely reflects random dimeric assembly of mutant and wt glycoproteins, with wt homodimers being the sole functional combination.
Figure 2.
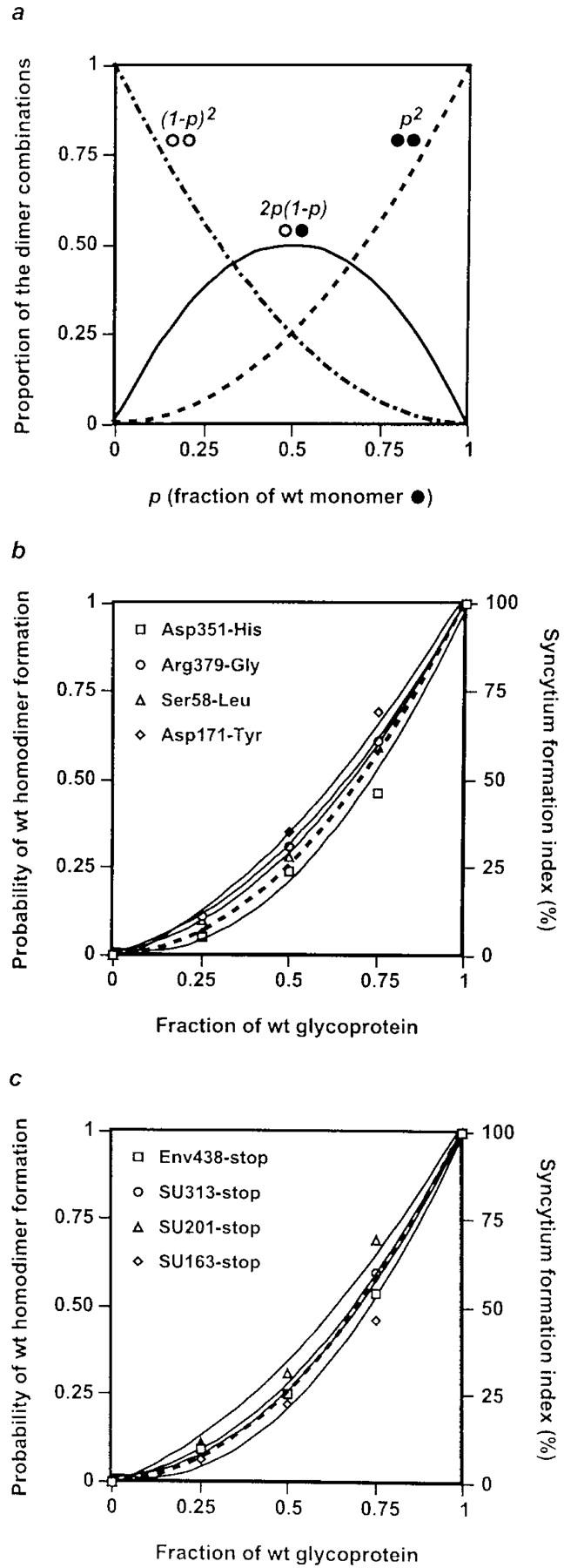
Random dimerization in cells coexpressing the wt HTLV-1 glycoprotein and an assembly-competent mutant. (a) Theoretical curves expected from a random dimerization process. In a cell that coexpresses the wt glycoprotein and an assembly-competent mutant, three dimer combinations are possible: homodimers of wt precursor subunits, homodimers of mutant precursor subunits, and heterodimers consisting of both subunit types. If dimerization is random and p is the fraction of wt monomers, then the proportion of dimers consisting solely of wt subunits is given by p 2 (••). It follows that (1 − p) represents the fraction of mutant monomers; therefore, the proportion of homodimers of mutant subunits is (1 − p)2 (○○), and the proportion of heterodimers is expressed by 2p(1 − p) (○•). (b and c) Syncytium formation as a function of the mutant:wt transfection ratio. Cells were transfected with a constant amount of DNA containing mutant:wt ratios of 3:1, 1:1, and 1:3. The theoretical curve (broken line) gives the probability of wt homodimer formation assuming that dimerization is random, as calculated in a. Superimposed are the experimental data for the indices of syncytium formation obtained at each mutant:wt ratio with (b) cleavage-defective mutants with single amino acid substitutions in the TM or SU portions of the glycoprotein and (c) transport-competent mutants with COOH-terminal truncations of the glycoprotein. The plotted curves (full lines) were fitted to the experimental points using a polynomial method.
Trans-dominant Negative Mutants Prevent the Intracellular Transport of the wt Precursor Glycoprotein
The design of the dominant negative assay was based on the assumption that a cleavage-defective mutant with competence for assembly would prevent the wt glycoprotein from being transported to, and consequently cleaved in, the Golgi apparatus. We used two complementary approaches to ascertain that this was indeed the inhibitory step.
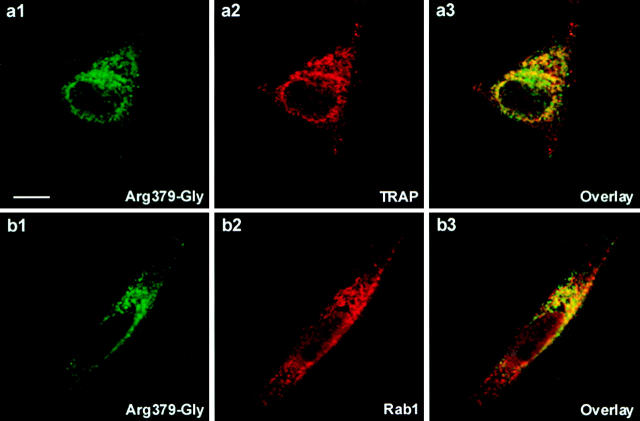
First, we performed in situ immunofluorescence analyses to examine the intracellular localization of the wt HTLV-1 glycoprotein expressed in the absence or presence of a cleavage-defective mutant (Fig. 3). When the wt envelope construct was transfected alone, most of the positive cells were committed to the formation of syncytia, and a granular staining scattered throughout the cytoplasm was detected (Fig. 3 b). In contrast, the staining was confined to the perinuclear space in cells expressing any of the cleavage-defective mutants (see the example of the Arg379-Gly mutant in Fig. 3 c). We performed double-label immunofluorescence experiments to identify the mutant-retaining organelles. As shown in Fig. 4, the staining for HTLV-1 glycoprotein mutant showed partial colocalization both with that for translocating chain-associating membrane protein (Fig. 4 a), an ER marker, and with that for Rab1 (Fig. 4 b), an intermediate compartment and cis-Golgi marker. The pattern of staining observed in cells coexpressing the wt glycoprotein and a cleavage-defective dominant negative mutant was similar to that in cells expressing the mutant alone (see Fig. 3 d). These results indicate intracellular retention of wt glycoprotein in the presence of a trans-dominant negative mutant.
Figure 3.
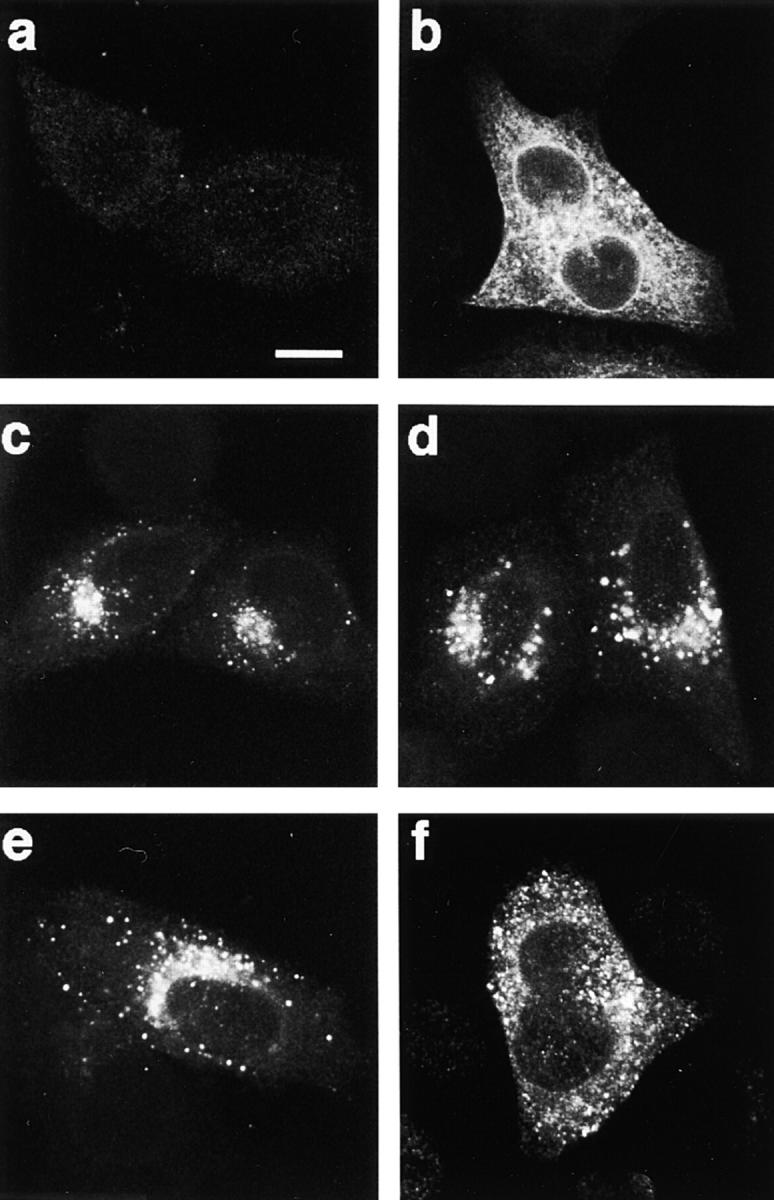
Immunofluorescence analysis of the intracellular distribution of wt and mutated HTLV-1 glycoproteins. HeLa cells were transfected with the following constructs: negative control (CMV-ENVΔPvuII) (a), wt HTLV-1 envelope (CMV-ENV-1) (b), Arg379-Gly mutant (c), a 3:1 ratio of Arg379-Gly mutant plus wt HTLV-1 envelope (d), Ser25-Arg mutant (e), or a 3:1 ratio of Ser25-Arg mutant plus wt HTLV-1 envelope (f). Transfected cells were fixed, permeabilized, and subjected to indirect immunofluorescence staining with the anti–HTLV-1 glycoprotein 1C11 mAb as the primary antibody. Shown are equatorial slices of representative fluorescent cells obtained by confocal microscopy. Bar, 10 μm.
Figure 4.
Partial colocalization of mutated HTLV-1 glycoprotein with both an ER marker and an intermediate compartment and cis-Golgi marker. HeLa cells were transfected with the expression plasmid for Arg379-Gly mutant. Transfected cells were fixed, permeabilized, and subjected to double immunofluorescence staining for detection of HTLV-1 glycoprotein (green) and marker (red), translocating chain-associating membrane protein (TRAP) (a) or Rab1 (b). Shown in each row is one single optical plane recorded in both green and red channels by confocal microscopy. Colocalization sites appeared as yellow pixels after merging the images (a3 and b3). Bar, 10 μm.
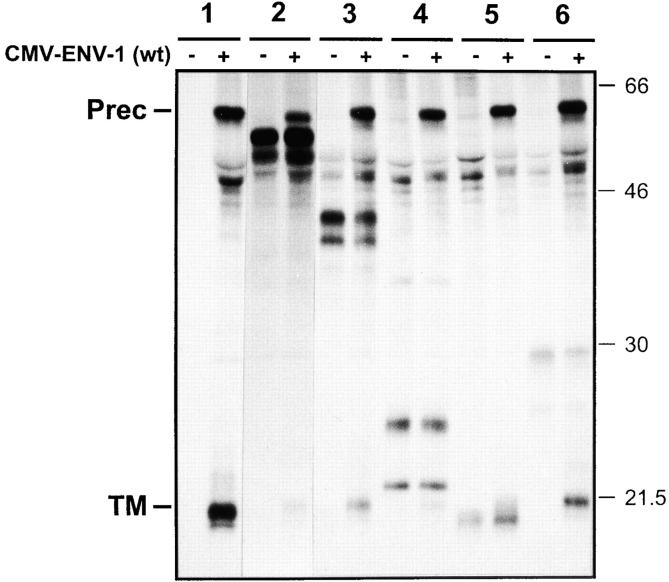
We next verified that the mutants that by themselves were defective in precursor cleavage also impaired in trans the cleavage of the wt envelope precursor (Fig. 5). Indeed, when expressed in the absence of mutant, the wt precursor gave rise to its mature products as appreciated by detection of the 20-kD band corresponding to the TM glycoprotein (Fig. 5, a, lane 2, and b, lane 2), whereas in the presence of any of the cleavage-defective dominant negative mutants, the TM-gp20 was barely or not detected (Fig. 5, a, lanes 3–10, and b, lanes 4–16).
Figure 5.
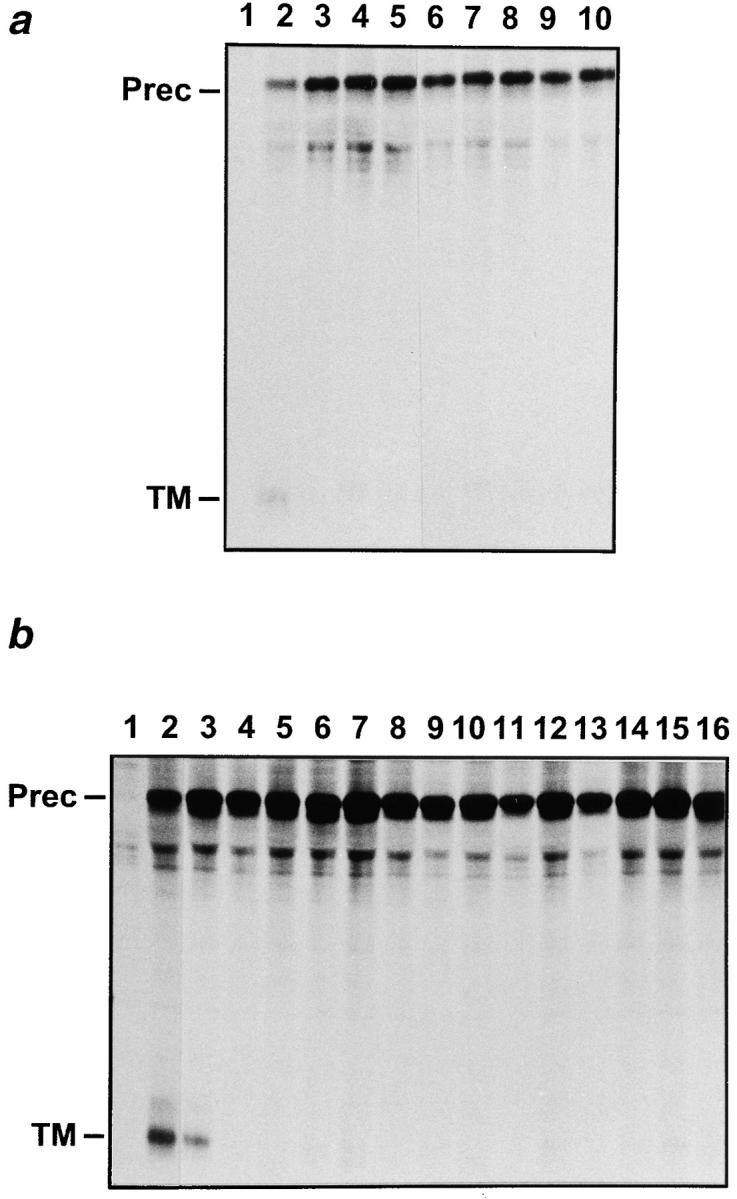
Radioimmunoprecipitation of the envelope products from lysates of cells coexpressing the wt HTLV-1 glycoprotein and a cleavage-defective mutant. Transfected COS-1 cells were metabolically labeled for 16 h, and the lectin-purified glycoproteins from the cell lysates were subjected to immunoprecipitation with sera from HTLV-1–infected individuals. Positions of the bands corresponding to the precursor (Prec) and TM glycoproteins are indicated on the left. (a) HTLV-1 glycoprotein mutants with single amino acid substitutions in the TM portion. Cells were transfected with the negative control plasmid (CMV-ENVΔPvuII) (lane 1) or with the wt HTLV-1 envelope expression plasmid CMV-ENV-1 plus a threefold excess of the following constructs: (lane 2) negative control (CMV-ENVΔPvuII), (lane 3) Asp351-His, (lane 4) Leu368-Arg, (lane 5) Ala375-Asp, (lane 6) Arg379-Gly, (lane 7) Asp383-Tyr, (lane 8) Glu398-Val, (lane 9) Ser408-Phe, (lane 10) Leu419-Arg. (b) HTLV-1 glycoprotein mutants with single amino acid substitutions in the SU portion. Cells were transfected with the negative control plasmid (CMV-ENVΔPvuII) (lane 1), or with the wt HTLV-1 envelope expression plasmid CMV-ENV-1 plus a threefold excess of the following constructs: (lane 2) negative control (CMV-ENVΔ- PvuII), (lane 3) Ser25-Arg, (lane 4) Leu52-Arg, (lane 5) Asp53-Val, (lane 6) Ser58-Leu, (lane 7) Ser119-Leu, (lane 8) Tyr124-Asp, (lane 9) Ser130-Ile, (lane 10) Ser162-Phe, (lane 11) Asp171-Tyr, (lane 12) Ile173-Ser, (lane 13) Thr178-Ala, (lane 14) His238-Leu, (lane 15) Ser292-Tyr, (lane 16) Leu295-Arg.
Taken together, these data confirm our hypothesis that the dominant negative effect exerted by cleavage-defective mutants results from their ability to prevent it from being transported to and cleaved in the Golgi apparatus.
The Only Mutation that Impairs Precursor Assembly Is Located at the NH2 Terminus of the Glycoprotein
Among the 22 cleavage-defective HTLV-1 glycoprotein mutants, the SU mutant Ser25-Arg represented a notable exception: it spared 72% of the syncytium formation elicited by the wt envelope (Table II). Immunofluorescence microscopy showed that the Ser25-Arg mutant, like the other cleavage-defective mutants, was defective in intracellular transport (Fig. 3 e). Upon its coexpression with wt glycoprotein, however, giant multinucleated cells with a granular staining scattered throughout the cytoplasm were detected (Fig. 3 f). The Ser25-Arg mutant thus failed to abolish in trans the intracellular transport of the wt glycoprotein, in sharp contrast with the other cleavage-defective HTLV-1 glycoprotein mutants. Furthermore, we confirmed that the cleavage of the wt glycoprotein was only partially inhibited by coexpression of a threefold excess of the Ser25-Arg mutant (Fig. 5 b, lane 3). We also verified that the phenotype of the Ser25-Arg mutant was not due to a greater instability of this glycoprotein compared with the dominant negative mutants (data not shown). Thus, assembly of the HTLV-1 envelope precursor is impaired by a single amino acid substitution located at the NH2 terminus of the glycoprotein (the serine at position 25 is the fifth amino acid of the protein after removal of the signal peptide; Lee et al., 1984).
The HTLV-1 and HTLV-2 Glycoproteins Have a Similar Determinant of Precursor Assembly
We asked whether HTLV-2, a human retrovirus phylogenetically related to HTLV-1, has comparable requirements for envelope precursor assembly. We mutated the serine residue at position 21 of the HTLV-2 envelope protein, equivalent to the serine at position 25 in HTLV-1 (Sodroski et al., 1984; Rosenberg et al., 1998). Like the Ser25-Arg HTLV-1 mutant, the Ser21-Arg HTLV-2 mutant by itself exhibited a cleavage-defective phenotype (Table III). It also failed to abolish wt envelope-mediated function when expressed in a threefold excess with the wt HTLV-2 glycoprotein, sparing 85% of the syncytium-forming activity (Table III). This shows that, in both HTLVs, the substitution of one serine at the NH2 terminus of the glycoprotein is sufficient to impair precursor assembly.
Table III.
Effect of Cleavage-defective HTLV-2 SU Mutants on Syncytium Formation Induced by wt Envelope
| HTLV-2 glycoprotein mutant | Precursor cleavage index (%) | Syncytium formation index (%) | ||||
|---|---|---|---|---|---|---|
| Mutant* | Mutant* | Mutant + wt HTLV-2 Env‡ | ||||
| Mock§ | <20 | <5 | 100 | |||
| Ser21-Arg | 33 | 11 | 85 | |||
| Ser70-Phe | <20 | <5 | 7 | |||
The indices for the HTLV-2 glycoprotein mutants relative to the wt HTLV-2 envelope glycoprotein were calculated as described elsewhere (Rosenberg et al., 1998). Data represent the means of two independent transfections.
Cells were cotransfected with the mutant constructs (2.25 μg) and the wt HTLV-2 envelope construct CMV-ENV-2 (0.75 μg). Data represent the means of two independent transfections.
Cells were transfected with the negative control plasmid CMV-ENVΔPmaCI.
A COOH-Terminally Truncated, Soluble Form of the Glycoprotein Is Trans-dominant Negative
We generated mutants with truncations or deletions to map the HTLV-1 glycoprotein domains involved in precursor assembly (Fig. 6). We verified that none of them elicited syncytium formation when expressed alone (data not shown), and we tested them for negative dominance.
A truncation mutant lacking the membrane-anchorage and intracytoplasmic domains (ENV438-stop) abolished the syncytium-forming activity of the wt HTLV-1 envelope (Fig. 6). As for point mutants, the trans-dominant negative effect was specific, because the truncated HTLV-1 glycoprotein mutant did not interfere with the syncytium formation elicited by the wt HIV-1 envelope (data not shown). It also followed the titration curve expected from a random dimeric association of the two glycoproteins (Fig. 2 c). The cleavage of the wt envelope precursor was greatly impaired in cells coexpressing the ENV438-stop mutant (Fig. 7, compare lane 2 + with 1 +). Conversely, secretion of the soluble products (SU of normal size and truncated TM) of the ENV438-stop mutant was reduced in the supernatant of these cells (data not shown). We concluded that (a) the ectodomain of the HTLV-1 envelope protein contains structural information that is sufficient for precursor subunit association, and (b) the heterodimers formed by the full-length wt glycoprotein and a COOH-terminally truncated, soluble mutant are defective in intracellular transport even though both subunits by themselves are competent for this process (Pique et al., 1993; Rosenberg et al., 1997).
Figure 7.
Radioimmunoprecipitation of the envelope products from lysates of cells coexpressing the wt HTLV-1 glycoprotein and a truncation mutant. COS-1 cells were transfected with (+) or without (−) the wt HTLV-1 envelope expression plasmid CMV-ENV-1 plus a threefold excess of the following constructs: negative control (CMV-ENVΔPvuII) (1), CMV-ENV438-stop (2), CMV-SU313-stop (3), CMV-SU201-stop (4), CMV-SU163-stop (5), or CMV-SUΔ26-164 (6). Transfected cells were metabolically labeled for 16 h, and the lectin-purified glycoproteins from the cell lysates were subjected to immunoprecipitation with sera from HTLV-1–infected individuals. Positions of molecular weight markers (kilodaltons) are indicated on the right, positions of the bands corresponding to the wt precursor (Prec) and TM glycoproteins are indicated on the left.
The NH2 Terminus of the SU Portion Is Critical for Precursor Assembly
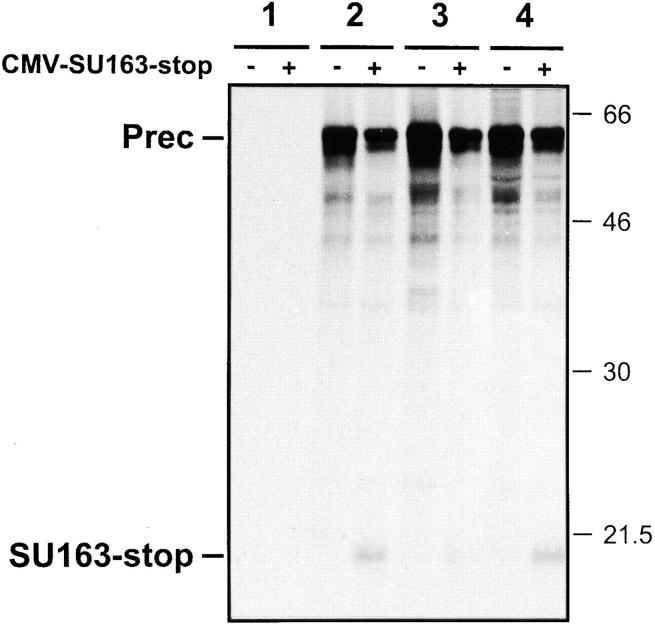
We further sought to determine which regions within the HTLV-1 glycoprotein ectodomain are critical for intracellular assembly. A major role could be attributed to the SU portion, because a truncated mutant lacking all the TM sequences (SU313-stop) was sufficient to exert a strong dominant negative effect (Fig. 6). The phenotype remained unchanged after further truncations of the SU that removed its COOH-terminal third (SU201-stop) or even its COOH-terminal half (SU163-stop). Again, titration of wt glycoprotein followed the theoretical curve expected from a random dimerization process (Fig. 2 c). The cleavage of the wt envelope precursor was also greatly impaired in cells coexpressing any of these three truncated proteins (Fig. 7, lanes 3+, 4+, and 5+). In contrast, an SU glycoprotein deleted of its NH2-terminal half (SUΔ26-164) was less effective in trans-inhibition of wt function, sparing 45% of the syncytium formation (Fig. 6). It was also less effective in trans-inhibition of wt glycoprotein cleavage (see Fig. 7, lane 6+; the labeling of the mutated protein was fainter than that of the other mutants because the deletion removed 10 of the 14 cysteine residues of the SU glycoprotein). Therefore, the integrity of the NH2-terminal domain of the SU was both necessary and sufficient for full trans-dominant inhibition of the intracellular transport and hence the function of the wt envelope. These findings underscore the importance of the NH2-terminal domain in the intracellular assembly of the glycoprotein.
The TM Portion Contributes to Precursor Assembly, but Not via the Leucine Zipper-like Domain
We reasoned that removal of the SU might unmask a potential contribution of the TM portion in precursor assembly. Indeed, a deletion mutant lacking most of the SU sequences (TM284-488) exerted a partial dominant negative effect on wt function (coexpression in a 3:1 ratio resulted in 40% syncytium-forming activity; Fig. 6). The effect seemed mostly due to the COOH-terminal region of the ectodomain, and not to the leucine zipper-like domain, because an NH2-terminally deleted protein lacking the leucine zipper-like motif (TM384-488) still exerted a dominant negative effect, whereas a TM protein deleted of the COOH-terminal half of the ectodomain (TMΔ382-441) was ineffective in trans-inhibition (Fig. 6). Thus, in addition to the contribution of the NH2-terminal domain of the SU, a role in intracellular assembly could be attributed to the COOH-terminal region of the TM ectodomain.
Physical Interaction between HTLV-1 Envelope Glycoproteins Having an Intact NH2 Terminus
Finally, we took advantage of truncation mutants to provide direct demonstration of the physical interaction between HTLV-1 envelope proteins having an intact NH2-terminal domain. We used the 4D4 mAb, which is directed to the COOH-terminal domain (amino acids 287–311) of the HTLV-1 SU (Grange et al., 1998), to perform coimmunoprecipitation experiments (Fig. 8). The 4D4 mAb was able to immunoprecipitate similar amounts of precursor glycoproteins for the wt envelope, the Ser25-Arg mutant, or a trans-dominant mutant, Arg379-Gly (Fig. 8, lanes 2–4), but did not allow immunoprecipitation of the COOH-terminally truncated envelope protein SU163-stop, which does not contain the 4D4 epitope (Fig. 8, lane 1+). However, the 4D4 mAb did coimmunoprecipitate the SU163-stop protein together with the wt precursor glycoprotein in cells coexpressing the two proteins (Fig. 8, lane 2+). Therefore, the dominant negative effect displayed by the SU163-stop mutant could indeed be accounted for by the physical association of the truncated protein with the wt precursor glycoprotein in the coexpressing cell. A comparable level of SU163-stop protein was also coimmunoprecipitated together with a trans-dominant glycoprotein, Arg379-Gly, confirming that competence for assembly resulted in coimmunoprecipitation (Fig. 8, lane 4+). In contrast, a smaller amount of SU163-stop protein was brought down from cells coexpressing the Ser25-Arg mutated glycoprotein (Fig. 8, lane 3+). Similar results were obtained when the SU201-stop mutant was tested instead of the SU163-stop mutant (data not shown). These data provide direct evidence that competence for assembly is impaired by a single amino acid substitution in the NH2-terminal domain of the glycoprotein.
Figure 8.
Coimmunoprecipitation of HTLV-1 envelope glycoproteins when the NH2 terminus is intact. COS-1 cells were transfected with (+) or without (−) the CMV-SU163-stop construct plus the following constructs: negative control (CMV-ENVΔPvuII) (1), wt HTLV-1 envelope (CMV-ENV-1) (2), Ser25-Arg mutant (3), or Arg379-Gly mutant (4). Transfected cells were metabolically labeled for 16 h, and the lectin-purified glycoproteins from the cell lysates were subjected to immunoprecipitation with the 4D4 mAb directed to the COOH-terminal domain of the HTLV-1 SU. Positions of molecular weight markers (kilodaltons) are indicated on the right, positions of the bands corresponding to the wt or mutated precursor glycoproteins (Prec) and to the SU163-stop glycoprotein are indicated on the left.
Discussion
In this study, we designed a dominant negative assay to explore the basis for the transport defect of a series of mutants of the HTLV-1 envelope, a glycoprotein which is subject to a very tight quality control (Pique et al., 1990; Delamarre et al., 1996). To our surprise, we found that, provided the NH2 terminus was intact, all mutated forms of the glycoprotein were capable of interfering in trans with the wt. Incompetence for assembly thus cannot explain the incompetence for transport of a large series of retained glycoproteins. It should be noted here that the mutated HTLV-1 glycoproteins were not retained in the ER alone, since we also observed partial colocalization with an intermediate compartment and cis-Golgi marker. This is reminiscent of the retention of a VSV G mutant, which has been shown to involve cycling between the ER, intermediate compartment, and cis-Golgi (Hammond and Helenius, 1994).
It could be argued that, rather than resulting from an assembly process, the trans-dominant interference exerted by the HTLV-1 glycoprotein mutants might be due to a nonspecific effect, such as the titration of a factor required for the intracellular transport of membrane proteins. This is very unlikely, however, because the HTLV-1 glycoprotein mutants had no effect on another retroviral glycoprotein, that of HIV-1. Moreover, coimmunoprecipitation experiments provided direct demonstration of a physical interaction between mutated glycoproteins exerting a dominant negative effect and the wt HTLV-1 precursor glycoprotein. Another caveat with the dominant negative approach is that it could reflect the constitution of aggregates rather than a true competence for oligomeric assembly. Although this hypothesis seemed unlikely because of the specificity of the trans inhibition, we addressed it directly by showing that the HTLV-1 glycoproteins from cotransfected cells were not recovered as aggregates in sucrose gradients. Moreover, for all mutants tested, the experimental titration curves for the dominant negative effect followed the theoretical curve expected from a dimeric assembly of the wt and mutated glycoproteins. We infer from these observations that our dominant negative assay reveals a specific dimeric assembly process taking place between HTLV-1 envelope precursor glycoproteins in the living cell.
For most membrane-anchored glycoproteins studied so far, assembly in the ER is a late step which takes place after folding of the monomeric subunits (Doms et al., 1993). We therefore expected that the majority of the transport-defective HTLV-1 glycoprotein mutants would be non– trans dominant, due either to a defect in the assembly step per se or to a defect in a previous folding step necessary for assembly. We found, on the contrary, that incompetence for assembly was rarely responsible for the intracellular retention of the mutated glycoproteins: 21 of the 22 glycoproteins incapable of transport were nevertheless capable of dimeric association with the wt precursor. These data suggest that the assembly process unraveled here is unlikely to occur as a late event in the acquisition of transport competence. The apparent discrepancy between our results and the previous studies reviewed in Doms et al. (1993) can be resolved if one considers the methodology employed in each case. In the kinetic analyses that suggested that the essentially complete folding of monomers is a prerequisite to oligomerization, velocity gradient sedimentation was generally used as the assembly assay. Such a technique can only pick those oligomers that are stable enough to withstand detergent solubilization and centrifugation (Einfeld and Hunter, 1988; Doms, 1990). Alternatively, monoclonal antibodies have been employed, but they too may detect only the mature, stable oligomer. An assembly assay based on negative dominance is complementary to these biochemical methods, because it evaluates the functional consequences of an interaction having occurred between subunits within the cell, but which would not necessarily have withstood the experimental manipulations involved in protein isolation and may not necessarily correspond to the ultimate oligomeric conformation. With this in mind, our results suggest the occurrence of an early and transient assembly step of the glycoprotein, preceding the appearance of the stable oligomer having the quaternary structure required for transport. Studies with HA (Copeland et al., 1986) have also suggested that the intracellular assembly of membrane-anchored proteins is in fact a multistep process, although it is detected as a discrete event when studied by a single method.
Even though the association of HTLV-1 envelope precursor subunits is presumed here to occur early in the maturation process, it is a posttranslational event. Indeed, our titration curves were consistent with a random association of monomers having arisen from different polysomes. This feature is shared by the HA glycoprotein (Boulay et al., 1988), and is likely to be a general rule of the assembly of membrane proteins, because their confinement to the ER compartment and their oriented state in the plane of the membrane facilitate spatial proximity between subunits (Grasberger et al., 1986). By contrast, a posttranslational mechanism is unlikely to be adopted by cytosolic oligomeric proteins (Gilmore et al., 1996).
Our work allowed us to map the structural domain involved in the ER association of HTLV-1 envelope precursor subunits to the NH2 terminus of the glycoprotein. This conclusion was drawn from a number of concordant observations. First, of the 22 point mutants tested, the only one that spared the intracellular transport of the wt precursor had a single amino acid substitution at the NH2 terminus of the glycoprotein. Second, this mutant did not allow coimmunoprecipitation of an assembly-competent glycoprotein, whereas a trans-dominant mutant did. Third, the use of truncation and deletion mutants showed that the NH2-terminal half of the SU was both necessary and sufficient for full trans-dominant inhibition. Finally, we also showed that HTLV-2 has a similar NH2-terminal determinant of envelope precursor assembly. To our knowledge, this study is the first to demonstrate the involvement of the SU portion of retroviral glycoproteins in ER assembly of the precursor. This feature was probably obscured by the fact that mature TM glycoproteins of retroviruses have always been found as stable oligomers (Einfeld and Hunter, 1988, 1994; Pinter et al., 1989; Schawaller et al., 1989; Earl et al., 1990; Rey et al., 1990; McGuire et al., 1992), whereas mature SU glycoproteins have usually been detected as monomers (Einfeld and Hunter, 1988, 1994; Earl et al., 1990; Thomas et al., 1991). It should be noted, however, that oligomers of mature SU glycoproteins were observed in some studies (Owens and Compans, 1990; Weiss et al., 1990; Tucker et al., 1991). Whatever the contribution of SU to the oligomerization of the mature envelope glycoproteins may be, our study brings to light an SU requirement for the ER assembly of the HTLV-1 envelope precursor, and underscores that domains involved in the ER assembly of precursor glycoproteins should not be directly inferred from those defined for the mature oligomers.
The difficulties in interpreting structural data for proteins that adopt different conformations in their lifetimes are further exemplified by the study of the leucine zipper-like motif present in the TM. Retroviral envelopes undergo at least two successive oligomerization events: the first is the assembly of the precursor glycoprotein in the ER, while the second is the formation of the fusion-competent oligomer triggered by receptor recognition at the cell surface. Although it was first thought that the zipper motif might fold into a coiled coil in the context of the precursor molecule, the leading hypothesis now is that it drives only the second oligomerization event (Dubay et al., 1992; Chen et al., 1993; Wild et al., 1994). Using an in vivo experimental strategy, our study with the HTLV-1 envelope glycoprotein further corroborates the idea that the leucine zipper-like domain is not required for ER assembly, since glycoproteins with deletions encompassing the corresponding region were still capable of intracellular association with the wt precursor.
Collectively, our data indicate that the NH2-terminal domain of the HTLV-1 envelope glycoprotein determines its ER assembly at a step that is not the last one in the acquisition of transport competence. The phenotype exhibited by most of our HTLV-1 envelope mutants (i.e., transport deficiency despite competence for oligomeric assembly) was previously observed with mutants of HA0 (Gething et al., 1986), VSV G (Doms et al., 1988), and the envelope of the Moloney murine leukemia virus (Kamps et al., 1991). Thus, oligomerization per se is not sufficient to meet the quality control of the ER. The possibility of additional events in the maturation process was also suggested by the observation of a significant time lag between the ER dimerization of the HIV-1 envelope precursor and its Golgi cleavage (Earl et al., 1991). What is the exact nature of these postoligomerization events? It is possible that significant folding of the subunits proceeds within the framework of the oligomer. Consistent with this hypothesis, the HIV-1 envelope precursor has been found to acquire reactivity to stringent conformational antibodies only after its oligomeric assembly (Otteken et al., 1996). Studies on the reovirus cell attachment protein σ1, a cytosolic protein, have revealed a schema of protein maturation that may also apply to membrane proteins in the ER. The maturation of this protein involves an initial trimerization of the NH2 terminus that, in turn, permits the trimerization of the COOH terminus and completion of folding; the trans-dominant negative effects exerted by COOH-terminally truncated mutants led to the proposal that the folding of the NH2-terminally assembled subunits is a cooperative process requiring the integrity of all subunits (Leone et al., 1992). A similar mechanism is likely to account for the trans-dominant negative effects observed in our study of the HTLV-1 glycoprotein. It is indeed noteworthy that not only the heterodimers formed by the wt and any of the transport-defective mutants but also those formed by the wt and any of the COOH-terminally truncated, transport-competent mutants were defective in transport. Together with previous findings, our study thus emphasizes that the condition imposed on membrane proteins by the cellular quality control is not simply the acquisition of an oligomeric status as such, but rather the attainment of the correct quaternary structure. We propose that this process involves the cooperative folding of “preassembled” subunits.
Acknowledgments
We are grateful to L.L. Pritchard and M. Bomsel for critical reading of the manuscript and helpful suggestions, to I. Bouchaert for excellent assistance with confocal microscopy, and to C. Boivin and C. Raguénès-Nicol for help in editing the images. We thank C. Desgranges and M.-P. Grange for their gift of the 4D4 mAb, J. Coste for repeatedly providing us with sera from infected individuals, and M. Alizon for the HIV-1 plasmid and the cell lines used in the syncytium formation assays.
This work was supported by grants from the Association Nationale pour la Recherche sur le SIDA (ANRS, Paris, France) and from the Association pour la Recherche sur le Cancer (ARC, Villejuif, France), as well as by equipment grants from the Fondation pour la Recherche Médicale (FRM, Paris, France).
Abbreviations used in this paper
- CMV
cytomegalovirus
- HA
influenza virus hemagglutinin
- HTLV
human T cell leukemia virus
- SU
surface glycoprotein
- TM
transmembrane glycoprotein
- VSV
vesicular stomatitis virus
- wt
wild-type
References
- Boulay F, Doms RW, Webster RG, Helenius A. Posttranslational oligomerization and cooperative acid activation of mixed influenza hemagglutinin trimers. J Cell Biol. 1988;106:629–639. doi: 10.1083/jcb.106.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bour S, Boulerice F, Wainberg MA. Inhibition of gp160 and CD4 maturation in U937 cells after both defective and productive infections by human immunodeficiency virus type 1. J Virol. 1991;65:6387–6396. doi: 10.1128/jvi.65.12.6387-6396.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SS-L, Lee C-N, Lee W-R, McIntosh K, Lee T-H. Mutational analysis of the leucine zipper-like motif of the human immunodeficiency virus type 1 envelope transmembrane glycoprotein. J Virol. 1993;67:3615–3619. doi: 10.1128/jvi.67.6.3615-3619.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel F, Charneau P. Fusion from without directed by human immunodeficiency virus particles. J Virol. 1994;68:1179–1185. doi: 10.1128/jvi.68.2.1179-1185.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PL, Mottet G. Homooligomerization of the hemagglutinin-neuraminidase glycoprotein of human parainfluenza virus type 3 occurs before the acquisition of correct intramolecular disulfide bonds and mature immunoreactivity. J Virol. 1991;65:2362–2371. doi: 10.1128/jvi.65.5.2362-2371.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland CS, Doms RW, Bolzau EM, Webster RG, Helenius A. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J Cell Biol. 1986;103:1179–1191. doi: 10.1083/jcb.103.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland CS, Zimmer K-P, Wagner KR, Healey GA, Mellman I, Helenius A. Folding, trimerization, and transport are sequential events in the biogenesis of influenza virus hemagglutinin. Cell. 1988;53:197–209. doi: 10.1016/0092-8674(88)90381-9. [DOI] [PubMed] [Google Scholar]
- Cullen BR. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- de Silva A, Braakman I, Helenius A. Posttranslational folding of vesicular stomatitis virus G protein in the ER: involvement of noncovalent and covalent complexes. J Cell Biol. 1993;120:647–655. doi: 10.1083/jcb.120.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamarre L, Rosenberg AR, Pique C, Pham D, Callebaut I, Dokhélar M-C. The HTLV-I envelope glycoproteins: structure and functions. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:S85–S91. doi: 10.1097/00042560-199600001-00015. [DOI] [PubMed] [Google Scholar]
- Delamarre L, Rosenberg AR, Pique C, Pham D, Dokhélar M-C. A novel human T-leukemia virus type 1 cell-to-cell transmission assay permits definition of SU glycoprotein amino acids important for infectivity. J Virol. 1997;71:259–266. doi: 10.1128/jvi.71.1.259-266.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms RW. Oligomerization and protein transport. Methods Enzymol. 1990;191:841–854. doi: 10.1016/0076-6879(90)91051-7. [DOI] [PubMed] [Google Scholar]
- Doms RW, Lamb RA, Rose JK, Helenius A. Folding and assembly of viral membrane proteins. Virology. 1993;193:545–562. doi: 10.1006/viro.1993.1164. [DOI] [PubMed] [Google Scholar]
- Doms RW, Ruusala A, Machamer C, Helenius J, Helenius A, Rose JK. Differential effects of mutations in three domains on folding, quaternary structure, and intracellular transport of vesicular stomatitis virus G protein. J Cell Biol. 1988;107:89–99. doi: 10.1083/jcb.107.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragic T, Charneau P, Clavel F, Alizon M. Complementation of murine cells for human immunodeficiency virus envelope/CD4-mediated fusion in human/murine heterokaryons. J Virol. 1992;66:4794–4802. doi: 10.1128/jvi.66.8.4794-4802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubay JW, Roberts SJ, Brody B, Hunter E. Mutations in the leucine zipper of the human immunodeficiency virus type 1 transmembrane glycoprotein affect fusion and infectivity. J Virol. 1992;66:4748–4756. doi: 10.1128/jvi.66.8.4748-4756.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl PL, Doms RW, Moss B. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci USA. 1990;87:648–652. doi: 10.1073/pnas.87.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl PL, Moss B, Doms RW. Folding, interaction with GRP78-BiP, assembly, and transport of the human immunodeficiency virus type 1 envelope protein. J Virol. 1991;65:2047–2055. doi: 10.1128/jvi.65.4.2047-2055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einfeld D. Maturation and assembly of retroviral glycoproteins. Curr Top Microbiol Immunol. 1996;214:133–176. doi: 10.1007/978-3-642-80145-7_5. [DOI] [PubMed] [Google Scholar]
- Einfeld D, Hunter E. Oligomeric structure of a prototype retrovirus glycoprotein. Proc Natl Acad Sci USA. 1988;85:8688–8692. doi: 10.1073/pnas.85.22.8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einfeld D, Hunter E. Transport of membrane proteins to the cell surface. Curr Top Microbiol Immunol. 1991;170:107–139. doi: 10.1007/978-3-642-76389-2_4. [DOI] [PubMed] [Google Scholar]
- Einfeld DA, Hunter E. Expression of the TM protein of Rous sarcoma virus in the absence of SU shows that this domain is capable of oligomerization and intracellular transport. J Virol. 1994;68:2513–2520. doi: 10.1128/jvi.68.4.2513-2520.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M-J, McCammon K, Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986;46:939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Gilmore R, Coffey MC, Leone G, McLure K, Lee PWK. Co-translational trimerization of the reovirus cell attachment protein. EMBO (Eur Mol Biol Organ) J. 1996;15:2651–2658. [PMC free article] [PubMed] [Google Scholar]
- Grange MP, Rosenberg AR, Horal P, Desgranges C. Identification of exposed epitopes on the envelope glycoproteins of human T-cell lymphotropic virus type I (HTLV-I) Int J Cancer. 1998;75:804–813. doi: 10.1002/(sici)1097-0215(19980302)75:5<804::aid-ijc22>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Grasberger B, Minton AP, DeLisi C, Metzger H. Interaction between proteins localized in membranes. Proc Natl Acad Sci USA. 1986;83:6258–6262. doi: 10.1073/pnas.83.17.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Helenius A. Quality control in the secretory pathway: retention of a misfolded viral membrane glycoprotein involves cycling between the ER, intermediate compartment, and Golgi apparatus. J Cell Biol. 1994;126:41–52. doi: 10.1083/jcb.126.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987;329:219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- Hurtley SM, Bole DG, Hoover-Litty H, Helenius A, Copeland CS. Interactions of misfolded influenza virus hemagglutinin with binding protein (BiP) J Cell Biol. 1989;108:2117–2126. doi: 10.1083/jcb.108.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley SM, Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Kamps CA, Lin Y-C, Wong PKY. Oligomerization and transport of the envelope protein of Moloney murine leukemia virus-TB and of ts1, a neurovirulent temperature-sensitive mutant of MoMuLV-TB. Virology. 1991;184:687–694. doi: 10.1016/0042-6822(91)90438-h. [DOI] [PubMed] [Google Scholar]
- Lee TH, Coligan JE, Homma T, McLane MF, Tachibana N, Essex M. Human T-cell leukemia virus-associated membrane antigens: identity of the major antigens recognized after virus infection. Proc Natl Acad Sci USA. 1984;81:3856–3860. doi: 10.1073/pnas.81.12.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone G, Maybaum L, Lee PWK. The reovirus cell attachment protein possesses two independently active trimerization domains: basis of dominant negative effects. Cell. 1992;71:479–488. doi: 10.1016/0092-8674(92)90516-f. [DOI] [PubMed] [Google Scholar]
- Marquardt T, Helenius A. Misfolding and aggregation of newly synthesized proteins in the endoplasmic reticulum. J Cell Biol. 1992;117:505–513. doi: 10.1083/jcb.117.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire TC, Knowles DP, Jr, Davis WC, Brassfield AL, Stem TA, Cheevers WP. Transmembrane protein oligomers of caprine arthritis-encephalitis lentivirus are immunodominant in goats with progressive arthritis. J Virol. 1992;66:3247–3250. doi: 10.1128/jvi.66.5.3247-3250.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otteken A, Earl PL, Moss B. Folding, assembly, and intracellular trafficking of the human immunodeficiency virus type 1 envelope glycoprotein analyzed with monoclonal antibodies recognizing maturational intermediates. J Virol. 1996;70:3407–3415. doi: 10.1128/jvi.70.6.3407-3415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens RJ, Compans RW. The human immunodeficiency virus type 1 envelope glycoprotein precursor acquires aberrant intermolecular disulfide bonds that may prevent normal proteolytic processing. Virology. 1990;179:827–833. doi: 10.1016/0042-6822(90)90151-g. [DOI] [PubMed] [Google Scholar]
- Paine E, Gu R, Ratner L. Structure and expression of the human T-cell leukemia virus type 1 envelope protein. Virology. 1994;199:331–338. doi: 10.1006/viro.1994.1131. [DOI] [PubMed] [Google Scholar]
- Palker TJ, Tanner ME, Scearce RM, Streilein RD, Clark ME, Haynes BF. Mapping of immunogenic regions of human T cell leukemia virus type I (HTLV-I) gp46 and gp21 envelope glycoproteins with env-encoded synthetic peptides and a monoclonal antibody to gp46. J Immunol. 1989;142:971–978. [PubMed] [Google Scholar]
- Pinter A, Honnen WJ, Tilley SA, Bona C, Zaghouani H, Gorny MK, Zolla-Pazner S. Oligomeric structure of gp41, the transmembrane protein of human immunodeficiency virus type 1. J Virol. 1989;63:2674–2679. doi: 10.1128/jvi.63.6.2674-2679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pique C, Pham D, Tursz T, Dokhélar M-C. Human T-cell leukemia virus type I envelope protein maturation process: requirements for syncytium formation. J Virol. 1992;66:906–913. doi: 10.1128/jvi.66.2.906-913.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pique C, Pham D, Tursz T, Dokhélar M-C. The cytoplasmic domain of the human T-cell leukemia virus type I envelope can modulate envelope functions in a cell type–dependent manner. J Virol. 1993;67:557–561. doi: 10.1128/jvi.67.1.557-561.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pique C, Tursz T, Dokhelar M-C. Mutations introduced along the HTLV-I envelope gene result in a non-functional protein: a basis for envelope conservation? . EMBO (Eur Mol Biol Organ) J. 1990;9:4243–4248. doi: 10.1002/j.1460-2075.1990.tb07872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey M-A, Laurent AG, McClure J, Krust B, Montagnier L, Hovanessian AG. Transmembrane envelope glycoproteins of human immunodeficiency virus type 2 and simian immunodeficiency virus SIV-mac exist as homodimers. J Virol. 1990;64:922–926. doi: 10.1128/jvi.64.2.922-926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg AR, Delamarre L, Pique C, Pham D, Dokhélar M-C. The ectodomain of the human T-cell leukemia virus type 1 TM glycoprotein is involved in postfusion events. J Virol. 1997;71:7180–7186. doi: 10.1128/jvi.71.10.7180-7186.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg AR, Delamarre L, Preira A, Dokhélar M-C. Analysis of functional conservation in the surface and transmembrane glycoprotein subunits of human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2. J Virol. 1998;72:7609–7614. doi: 10.1128/jvi.72.9.7609-7614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schawaller M, Smith GE, Skehel JJ, Wiley DC. Studies with crosslinking reagents on the oligomeric structure of the env glycoprotein of HIV. Virology. 1989;172:367–369. doi: 10.1016/0042-6822(89)90142-6. [DOI] [PubMed] [Google Scholar]
- Sodroski J, Patarca R, Perkins D, Briggs D, Lee T-H, Essex M, Coligan J, Wong-Staal F, Gallo RC, Haseltine WA. Sequence of the envelope glycoprotein gene of type II human T lymphotropic virus. Science. 1984;225:421–424. doi: 10.1126/science.6204380. [DOI] [PubMed] [Google Scholar]
- Tatu U, Braakman I, Helenius A. Membrane glycoprotein folding, oligomerization and intracellular transport: effects of dithiothreitol in living cells. EMBO (Eur Mol Biol Organ) J. 1993;12:2151–2157. doi: 10.1002/j.1460-2075.1993.tb05863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatu U, Hammond C, Helenius A. Folding and oligomerization of influenza hemagglutinin in the ER and the intermediate compartment. EMBO (Eur Mol Biol Organ) J. 1995;14:1340–1348. doi: 10.1002/j.1460-2075.1995.tb07120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DJ, Wall JS, Hainfeld JF, Kaczorek M, Booy FP, Trus BL, Eiserling FA, Steven AC. gp160, the envelope glycoprotein of human immunodeficiency virus type 1, is a dimer of 125-kilodalton subunits stabilized through interactions between their gp41 domains. J Virol. 1991;65:3797–3803. doi: 10.1128/jvi.65.7.3797-3803.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker SP, Srinivas RV, Compans RW. Molecular domains involved in oligomerization of the Friend murine leukemia virus envelope glycoprotein. Virology. 1991;185:710–720. doi: 10.1016/0042-6822(91)90542-j. [DOI] [PubMed] [Google Scholar]
- Weiss CD, Levy JA, White JM. Oligomeric organization of gp120 on infectious human immunodeficiency virus type 1 particles. J Virol. 1990;64:5674–5677. doi: 10.1128/jvi.64.11.5674-5677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild C, Dubay JW, Greenwell T, Baird T, Jr, Oas TG, McDanal C, Hunter E, Matthews T. Propensity for a leucine zipper-like domain of human immunodeficiency virus type 1 gp41 to form oligomers correlates with a role in virus-induced fusion rather than assembly of the glycoprotein complex. Proc Natl Acad Sci USA. 1994;91:12676–12680. doi: 10.1073/pnas.91.26.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]