Abstract
We report the molecular and functional characterization of a new α chain of laminin in Drosophila. The new laminin chain appears to be the Drosophila counterpart of both vertebrate α2 (also called merosin) and α1 chains, with a slightly higher degree of homology to α2, suggesting that this chain is an ancestral version of both α1 and α2 chains. During embryogenesis, the protein is associated with basement membranes of the digestive system and muscle attachment sites, and during larval stage it is found in a specific pattern in wing and eye discs. The gene is assigned to a locus called wing blister (wb), which is essential for embryonic viability. Embryonic phenotypes include twisted germbands and fewer pericardial cells, resulting in gaps in the presumptive heart and tracheal trunks, and myotubes detached from their target muscle attachment sites. Most phenotypes are in common with those observed in Drosophila laminin α3, 5 mutant embryos and many are in common with those observed in integrin mutations. Adult phenotypes show blisters in the wings in viable allelic combinations, similar to phenotypes observed in integrin genes. Mutation analysis in the eye demonstrates a function in rhabdomere organization. In summary, this new laminin α chain is essential for embryonic viability and is involved in processes requiring cell migration and cell adhesion.
Keywords: Drosophila, wing blister, laminin, extracellular matrix, development
Laminins are large extracellular matrix (ECM)1 molecules usually associated with basement membranes (BMs), and represent a family of molecules important for development, adhesion, and cell migration (reviewed by Timpl and Brown, 1996). Laminin was initially isolated from tumor cells as a heterotrimer composed of α1, β1, and γ1 chains (Chung et al., 1979; Timpl et al., 1979; see Fig. 2). All laminin chains are composed of a series of protein modules that occur in other ECM molecules (e.g., EGF repeats or laminin G domains; see Fig. 2 A). The size of laminin chains is usually >200 kD. Vertebrate studies have revealed the presence of at least five α chains, three β chains, and three γ chains that can assemble in a combinatorial manner to form native laminin molecules. All are classified using a recent nomenclature (Burgeson et al., 1994). Data so far show that only α, β, and γ heterotrimers are sufficiently stable to be secreted (Yurchenko et al., 1997), an issue that becomes particularly important when one of the subunits is lacking or mutated due to a genetic defect.
Figure 2.
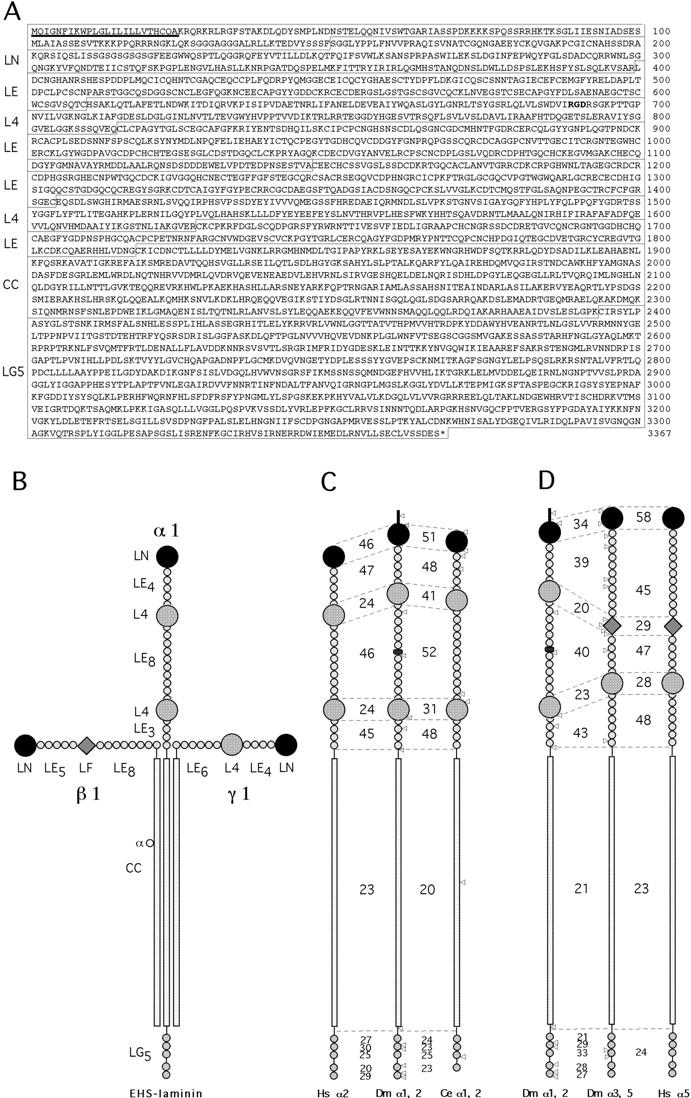
The Wb (laminin α1, 2) protein. (A) Open reading frame encoded by the wb gene. The different domains of the laminin α chain are indicated on the left as letter code defined in the SWISS-PROT data bank (Bairoch and Apweiler, 1999) and appear as boxes surrounding the sequence. A putative signal sequence is underlined. The RGD tripeptide within L4 is highlighted in bold. (B) Schematic representation of a classical laminin molecule showing EHS laminin (Timpl et al., 1979) composed of an α, β, and γ chain. (C) Schematic drawing of the domain structures and comparison of the identities between human α2, Drosophila α1, 2, and C. elegans laminin α1, 2 chain. C. elegans laminin α1, 2 sequence was available from cosmid T22A3 and H10E24, and appears compiled under accession number AF074902. Numbers indicate percentages of identity in the different domains. Triangles denote positions of introns. Note that in Drosophila laminin α1, 2, an NH2-terminal extension and an insertion between the EGF-like repeats is observed, and in C. elegans the fifth G domain is lacking. (D) Comparison of the domain structure and identities between Drosophila α1, 2, Drosophila α3, 5 (previously called lamA), and human α5 chain. Numbers indicate percentage of identities between the different domains of the chain.
Thin but extended sheets of BM require continuous molecular structures which can extend over long distances, e.g., in blood vessels. BMs are usually thought to provide sufficient mechanical stability to resist high shearing forces at the dermal–epidermal junction or to resist hydrostatic pressure in glomerular loops in the kidney. On the other hand, BM needs to be flexible, i.e., to respond to rapid changes in volume in blood capillaries. The major contribution to these properties comes from two networks formed independently from laminins and collagen IV. Laminin undergoes a thermally reversible polymerization, and electron micrographs suggest that peripheral short and long arm interactions are involved in this assembly (Yurchenco and Cheng, 1993). Additional molecules are known to interact with laminin, i.e., nidogen, which is thought to cross-link the laminin and the collagen IV network, or perlecan, a proteoglycan (reviewed by Timpl and Brown, 1996).
Different laminin isoforms are not always expressed at the same site and time. A careful examination of the occurrence in vertebrate embryonic and adult tissues of all α chains shows that laminin α chains have distinct expression patterns, with α4 and α5 showing the broadest, and α1 the most restricted expression (Miner et al., 1997). Moreover, each BM examined contains at least one α chain, but the composition of α chains within the BMs changed constantly during embryonic development, as assayed in the kidney (Miner et al., 1997).
Few data are known about the developmental function of laminins, mainly because few laminin mutations have been identified to date. However, mutations in the α2 chain of human laminin have been linked to congenital muscular dystrophy (Helbling-Leclerc et al., 1995), and the classic dy mutation in mouse could also be linked to defects in the murine α2 chain (Xu et al., 1994). In both species, the lack or partial loss of function of laminin α2 leads to variation in skeletal muscle fibers and muscle fiber necrosis. These findings demonstrate a role for the α2 chain in skeletal muscle function. Mutations in the γ2 subunit of laminin can lead to Herlitz's junctional epidermolysis bullosa (Aberdam et al., 1994; Pulkkinnen et al., 1994), characterized by blister formation within the dermal–epidermal BMs. Furthermore, mutations in the α3 and β3 laminin chain which associate with γ2 to form laminin 5 show similar phenotypes (Kivirikko et al., 1995; Cserhalmi-Friedman et al., 1998). Laminin α2 also plays a role in molecular pathogenesis of neural tropism since the bacterium Mycobacterium leprae binds to α2 on Schwann cell axon units (Rambukkana et al., 1997).
Intensive studies on the composition of the ECM in invertebrates have shown the existence of a laminin with a proposed subunit composition α3, 5; β1; γ1 (Montell and Goodman, 1988, 1989; Chi and Hui, 1989; Kusche-Gullberg et al., 1992; Henchcliffe et al., 1993). α3, 5 was previously called lamA, and this new name is proposed as a reminder that α3, 5 is the precursor of both vertebrate α3 and α5 chains. Genetic studies have shown that null mutations in the Drosophila α3, 5 chain lead to embryonic lethality, with visible defects in mesodermally derived tissues, i.e., in heart, muscles, or gut leading to dissociated cell groups in the various organs (Yarnitzki and Volk, 1995). These data suggest that laminin is used to confer structural support and adhesivity. Surprisingly, no obvious pathfinding defects in central nervous system neurons were observed during embryogenesis (Henchcliffe et al., 1993), however, at the neuromuscular junction, the extent between neuronal and muscular surfaces appeared significantly altered in α3, 5 mutants (Prokop et al., 1998).
Hypomorphic mutants and heteroallelic mutant combinations of α3, 5 can give rise to viable pupae and some viable adults (Henchcliffe et al., 1993). These adult escapers show abnormalities in the shape of their legs and in the organization of ommatidia in the compound eye (Henchcliffe et al., 1993). A recent report has also shown the requirement of α3, 5 in normal pathfinding by ocellar pioneer axons (Garcia-Alonso et al., 1996).
In spite of the observed pleiotropy of mutations in the α3, 5 gene, the phenotypic effects seen in mutant animals are not dramatic given the wide distribution of the protein. This predicted the existence of a second laminin α chain which can compensate for loss of α3, 5 function. Indeed, during the course of the Drosophila genome sequencing program, we noticed the presence of sequences related to laminin, and subsequent analysis of the genomic region allowed us to define a new member of the invertebrate laminin α chain family, similar to both the vertebrate α1 and α2 chain. We show that mutations from the wing blister (wb) locus are associated with lesions in this new α gene and that this second Drosophila laminin α chain is indispensable for embryonic viability and adhesiveness between cell layers.
Materials and Methods
Fly Stocks
The wb alleles, wbk05612, wbk00305, wbPZ09437, wbPZ10002, wbSF25, wbHG10, and wbCR4, were used to determine embryonic functions for the Wb protein. P element induced alleles, wbk05612, wbk00305, wbPZ09437, and wbPZ10002, were produced in the laboratories of Istvan Kiss and A. Spradling (Carnegie Institute, Baltimore, MD), and ethylmethane sulfonate (EMS) induced alleles, wbSF25 and wbHG10, were produced in the laboratory of M. Ashburner (University of Cambridge, Cambridge, UK). wbSF25, wbHG10, and wbPZ09437 have been described previously (Karpen and Spradling, 1992; Lindsley and Zimm, 1992). Df(2L)fn7 (breakpoints 34E3; 35B3-4) and Df(2L)fn36 (breakpoints 34F3-5; 35B4) were used in this study and are described in Lindsley and Zimm, (1992). Revertants of wbPZ09437 were obtained by precise excision of the P element and showed wild-type appearance and fertility. Lethal chromosomes used in this study were kept in stocks balanced over CyO (Lindsley and Zimm, 1992).
Somatic clones in the eye were produced by inducing mitotic recombination using the FLP/FRT system as described in Roote and Zusman (1996).
Videomicroscopy
Embryos from mutant lines were placed on petri perm plates (Hereus) in a drop of Voltalef 3S oil. All embryos were derived from mothers homozygous for the klarsicht (kls) mutation, which clears out yolk and makes embryonic phenotypes easily visible during filming, yet has no discernible effect on embryonic development (Wieschaus and Nüsslein-Volhard, 1986). Time lapse videomicroscopy was performed on embryos under a Zeiss Axioskop microscope with a Panasonic AG-6730 recorder and a Zeiss ZVS-47N CCD videocamera system. wb embryos were identified by their inability to hatch and the presence of a dorsal hole at the end of embryonic development.
Immunostaining and Preparation of Embryos for Whole Mounts
Embryos were collected on agar/apple juice plates and prepared for immunostaining according to the protocol described in Zusman et al. (1990) with an antibody against a pericardial protein (Mab#3; Yarnitzky and Volk, 1995) or an antibody against a tracheal protein (2A12; Samakovlis et al., 1996). Embryos stained with antibodies were dehydrated and mounted in a 3:1 solution of methyl salicylate and Canada balsam for examination under bright-field illumination.
For examination of somatic muscles, wb embryos were prepared as described by Drysdale et al. (1993) and viewed under polarized light. To confirm and examine further the wb somatic muscle phenotype, embryos derived from parents heterozygous for wb were stained with antibodies against muscle myosin (Kiehart and Feghali, 1986) using the procedures described in Young et al. (1991) and Roote and Zusman (1995).
Late stage wb or deficiency-containing embryos were identified by the dorsal hole phenotype and/or their inability to hatch. At earlier stages wb phenotypes were based on 25% of the population exhibiting defects not observed in a wild-type population, and the similarity of these defects to those observed when a dorsal hole is present. The mutant tracheal phenotype was also observed in developing wb embryos using videomicroscopy.
DNA and RNA Techniques
Southern and Northern blot analyses were performed by standard procedures (Maniatis et al., 1982). RNA was extracted by the guanidium thiocyanate/phenol/chloroform extraction method of Chomczynski and Sacchi (1987). Poly(A)+ RNA was isolated using a Pharmacia Kit (Pharmacia Biotech, Inc.). Equal specific activity of wb probes and laminin α3, 5 and γ1 probes were achieved using a standardized labeling protocol, and by using probes of similar lengths and similar GC content. Exposure times for Northern blots were 3 d. Whole mount in situ hybridizations were conducted using digoxigenin labeled wb cDNAs following the protocol of Tautz and Pfeiffle (1989).
Verification of the Sequence of Genomic DS Phages
At least three sequence errors were discovered within the published DS 03792 sequence leading to reading frame shifts. Suitable cDNAs were isolated using PCR, subcloned, and were used to correct the derived cDNA sequence. Irregularities between the domain structure of vertebrate and this new Drosophila laminin α chain were confirmed by additional isolation of suitable cDNAs by PCR and subsequent sequencing, ruling out misleading interpretations of intron–exon boundaries.
Generation of Antibodies and Staining of Embryos
Two independent fragments from either the NH2 or COOH terminus (amino acids 173–376 and amino acids 2,383–2,633, respectively) were cloned into the appropriate pMALc2 expression vectors (BioRad Laboratories). After induction and lysis of cells, fusion proteins were purified over a maltose matrix (BioRad Laboratories). Both antigens were used to generate two independent rabbit polyclonal antisera each. Polyclonal antisera were affinity purified over a corresponding GST fusion protein (Pharmacia Biotech, Inc.) column and eluted with 0.1 M glycine, pH 2.5. The specificity of the antisera was tested on Df(2L)fn36 embryos. For histochemical staining, the antifusion protein antisera were used at a concentration of 1:500.
Western Blotting
Samples of embryonic extracts and conditioned medium of Schneider S2 cells were separated under nonreducing and reducing conditions on 6% SDS-PAGE. After transfer onto nylon membranes, blots were probed with anti-wb antibodies and detected with HRP conjugated secondary antibodies, followed by ECL chemiluminescence (Nycomed Amersham, Inc.).
Results
Cloning, Sequence Analysis, and Properties of a New Drosophila Laminin Chain
In our attempt to find laminin-like sequences from Drosophila in the database, we noticed the presence of EGF-like repeats similar to laminin chains on the reverse strand of a subclone derived from the genomic phage DS 03792 (Kimmerly et al., 1996). Subsequent alignment of all subclones derived from this DS phage revealed the presence of a novel laminin chain gene in Drosophila. Analysis of the gene structure showed a genomic region spanning ∼70 kb of DNA with ≥16 exons contained within two overlapping DS phages, DS 037092 and DS 01068 (Fig. 1 B). Most intron–exon boundaries proposed by GENSCAN (Burge and Karlin, 1997) were confirmed by isolating and sequencing suitable cDNA clones spanning the region of interest (data not shown).
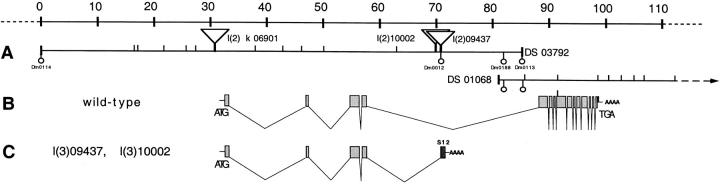
Figure 1.
Schematic representation of the cloned genomic region from 35A1. (A) The chromosomal region of 110 kb surrounding the wb gene is shown, along with three P element mutations and two P1 phages. The left end of DS 03792 was arbitrarily chosen as position 0. Underneath the DS phages, the relative position of four STSs are indicated. Vertical bars correspond to EcoRI sites. (B) Intron–exon structure of the wb gene. 15 exons are observed. (C) Intron–exon structure of the trans-spliced transcript observed in l(2) 09437 or l(2) 10002 animals (Fig. 3 B) yielding a composite transcript between the first four exons of wb and the last exon of S12.
Conceptual translation of the 10,101-nucleotide open reading frame yields a protein of 3,367 amino acids with a deduced molecular size of ∼374 kD (Fig. 2 A). At the NH2 terminus, the predicted initiating methionine is followed by an amino acid sequence containing structural regions characteristic of a secretory signal sequence (Fig. 2 A; von Heijne, 1986). A hydropathy profile of the primary structure revealed no other long hydrophobic regions indicative of a transmembrane spanning segment (Fig. 2 A), suggesting that this laminin chain is a secreted protein.
Closer inspection of the domain structure shows that this new chain has all the domains of laminin α chains in the appropriate order (Fig. 2 C). However, the number of different modules varies in some regions. For example, the second EGF-like stretch contains 10 full and 2 half EGF repeats, while in vertebrates there are 8 full and 2 half EGF repeats (Fig. 2 C). In addition, a unique NH2-terminal extension of ∼120 amino acids is present (Fig. 2 C). Finally, the array of the second EGF repeat region is symmetrically interrupted by an insertion of 45 amino acids.
We performed domain-wise comparisons of identities to existing vertebrate α chains. The LN domain showed almost an equally high degree of identity to vertebrate α1 and α2 chains, while the LE4 domain showed a slightly higher degree of identity to vertebrate α2 than to α1. However, both L4 domains showed slightly higher scores of identity to α5, immediately followed by equally high scores to α2 and α1. The remaining two EGF-like repeats showed that the first was highly homologous to α1 but the second was homologous to α2. Finally, all five G domains showed a slightly higher similarity to α2 than to α1. In summary, the majority of the domains showed most similarity to vertebrate α2 chains, yet many were significantly similar to α1. For this reason, and to illustrate the fact this chain is a common precursor of vertebrate α2 and α1 chains, we have tentatively called this chain Drosophila laminin α1, 2 in the remainder of the text.
A special feature within the amino acid sequence should be noted: the presence of a RGD within the first L4 domain (Fig. 2). RGD tripeptides have been shown to mediate cell adhesion in Drosophila using Drosophila PS2 integrins as receptors (Bunch and Brower, 1992). In fact, a recent study based on cell culture assays demonstrated that the laminin α1, 2 subunit showed exclusive binding to one integrin isoform, αPS2m8βPS4A, while the other PS2 integrin isoforms did not show any binding (Graner et al., 1998), suggesting that α1, 2 is a ligand of a splice-specific form of the PS2 integrins.
Temporal and Spatial Distribution of Laminin α1, 2 Transcripts
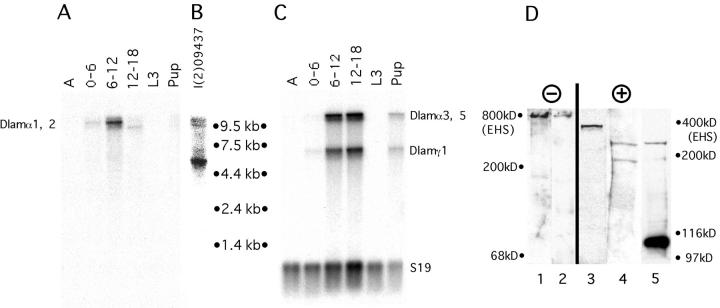
Northern analysis was performed on RNA derived from samples spanning the Drosophila life cycle using α1, 2 cDNAs as probes. An 11-kb transcript was first detected in the early stages of embryogenesis and peaked in 6–12-h embryos (Fig. 3 A). In the last part of embryogenesis (12– 18 h), a slightly smaller version of a 10.5-kb transcript was observed. We cannot exclude the possibility of an alternative spliced transcript or alternative usage of another polyadenylation site. Transcription decays in the later stages and is hardly detectable in third-instar larvae, but increases again in pupal stages. To compare the existing Drosophila laminin chains, the same Northern blot used for α1, 2 was also probed with a mixture of α3, 5 and γ1 probes (Fig. 3 C). This showed that the two laminin subunits are present at similar stages during embryogenesis. There is a marked difference, however, as these two subunits are also transcribed very strongly during the late stages of embryogenesis, in contrast to α1, 2 which fades out rapidly during this stage. Assuming that all probes in this analysis had similar specific activities (see Materials and Methods), it suggests that α1, 2 is less abundantly expressed than α3, 5, a feature already noted in vertebrate expression studies (Miner et al., 1997).
Figure 3.
Northern and Western analyses. (A) Northern blot analysis of different stages throughout the Drosophila life cycle with a wb cDNA. Each lane contains 5 μg of poly(A)+ RNA. The stage of the embryonic RNA is denoted in hours after egg laying, L3 is from the third instar larval stage, P from late pupal stages, and A from adult males and females. Two transcripts are detected which might derive from differentially polyadenylated mRNAs. (B) Trans-splicing in l(2) 09437 mutants. Northern blot analysis of 6–18-h old embryos from l(2) 09437 with a wb cDNA. Two groups of transcripts are detected, two wild-type bands ∼11 kb and a 5.6-kb band which derives from an aberrant splicing event with the last exon of ribosomal protein S12 on the mutant chromosome (Horowitz and Berg, 1995). (C) Comparison of transcriptional activity of different Drosophila laminin chains. The same Northern blot as in B was reprobed with a mixture of laminin α3, 5 and laminin γ1 cDNAs (Kusche-Gullberg et al., 1992). The amount of loaded RNA was estimated by subsequent probing with a Drosophila ribosomal protein S19 (Baumgartner et al., 1993). Transcript lengths were determined with a ladder of RNA standards. (D) Western analysis of the Wb protein. Extracts from 0–24-h embryos (lanes 1, 2, 4, and 5) and conditioned medium from Schneider S2 cells (lane 3) were fractionated on 6% SDS-PAGE under nonreducing (lanes 1 and 2), and reducing conditions (lanes 3–5), and assayed using polyclonal anti-wb antisera (anti-NH2 antibodies lanes 1 and 4; anti-COOH antibodies lanes 2, 3, and 5; see Materials and Methods). In conditioned medium, a single 360-kD band was observed (lane 3), while in extracts proteolytic cleavage occurs, resulting in a 240-kD (NH2) and a 110-kD (COOH) band (lanes 4 and 5). The 180-kD band in lane 4 might represent another cleavage or a degradation product which is not recognized by the COOH antibody. Moreover, this band did not appear under nonreducing conditions (lane 1), suggesting that it originated from laminin. Under nonreducing conditions, an 800-kD band was observed (lanes 1 and 2). Mouse EHS laminin (a gift from J. Engel) was used to determine the relative location of the 800-kD and 400-kD bands, respectively.
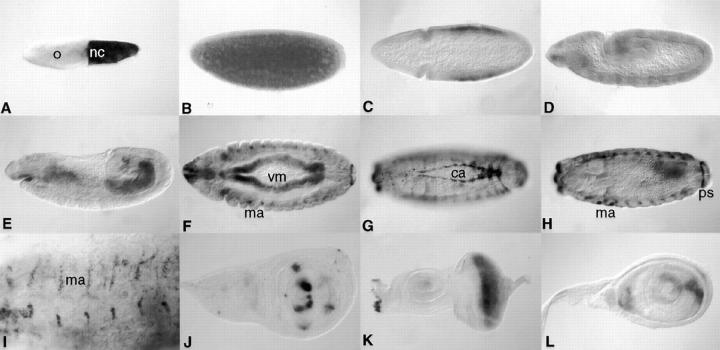
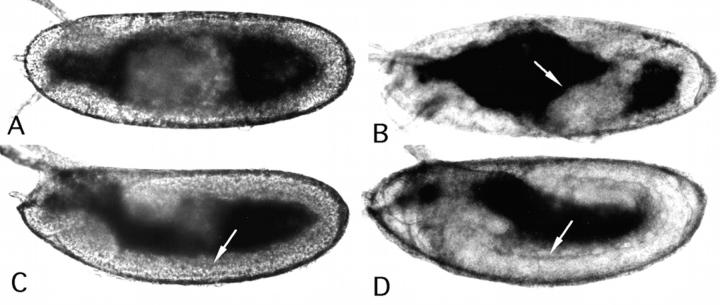
Using digoxigenin-labeled probes, the spatial expression of the α1, 2 chain was examined. Transcripts were first detected during oogenesis in nurse cells and growing oocytes (Fig. 4 A), suggesting a maternal contribution. During cleavage stage, the message is uniformly distributed in the egg (Fig. 4 B) and becomes slightly enriched in cells of the trunk region at blastoderm stage (Fig. 4 C). During germband extension (Fig. 4 D), low levels of uniform expression are observed. After germband retraction, the visceral mesoderm of the gut starts to accumulate α1, 2 transcripts (Fig. 4, E and F). At that time, cells near the presumptive muscle attachment sites show transcripts (Fig. 4 F). At stage 14, strong expression is also observed in cardiac cells (Fig. 4 G) and more prominent in cells near the muscle attachment sites (Fig. 4, H and I).
Figure 4.
Spatial expression of the wb gene. Oocytes and embryos are oriented with the anterior to the left and dorsal side up unless otherwise noted. (A) Stage 10 oocyte (stages are those of King, 1970), staining is observed in nurse cells (nc) and little in growing oocytes (o). (B) Stage 2 embryo (embryonic stages are those of Campos-Ortega and Hartenstein, 1985), staining is uniform within the cleavage stage embryo. (C) Stage 6 embryo, horizontal section, staining is observed highest in the middle of the embryo. (D) Stage 10 embryo, uniform expression is observed. (E) Stage 11 embryo, diffuse staining is observed around the visceral mesoderm. (F) Stage 12 embryo, horizontal view, the visceral mesoderm (vm) and muscle attachment sites (ma) show staining. (G and H) Same embryo, different focal plane, horizontal view, cardiac cells (ca), muscle attachment sites (ma), and the posterior spiracles (ps) show staining. (I) Stage 14 embryo, high magnification on cells at muscle attachment sites. (J) Wing disc, LacZ pattern of H155 line (Fig. 1), distinct regions on the presumptive wing dorsal and ventral region show staining. (K) Eye-antennal disc of H155 line, staining is particularly strong behind the morphogenetic furrow (mf). (L) Leg disc of H155 line, a specific pattern is observed.
Transcription of laminin α1, 2 is also readily detectable in imaginal discs, as assayed by LacZ staining of imaginal discs derived from the viable P element line H155 which mimics the embryonic transcript pattern faithfully (data not shown). Particularly strong expression was found in wing discs, where certain groups of cells in the presumptive wing dorsal and ventral region show LacZ staining (Fig. 4 J). Strong staining was also observed in the eye antennal disc immediately behind the morphogenetic furrow (Fig. 4 K), and also in a specific pattern in leg discs (Fig. 4 L).
Spatial Expression of the α1, 2 Protein
To assess the nature and appearance of the α1, 2 protein, polyclonal antisera against the NH2 and COOH termini (see Materials and Methods) were produced and assayed both by Western analyses and on whole mount embryos. Western blotting of conditioned medium of Schneider S2 cells showed a single 360-kD band (Fig. 3 D, lane 3), while in embryonic extracts proteolytic cleavage was observed giving rise to a 240-kD band (lanes 4 and 5) and a 110-kD band (lane 5) which are detectable using anti-NH2 and -COOH antibodies, respectively. This suggests that proteolytic cleavage also occurs in Drosophila, as was reported for the vertebrate α2 chain (Ehrig et al., 1990). NH2 antibodies also detected a possible further degradation product of ∼180 kD (lane 4), which is not detected by COOH antibodies. Both antisera recognize a single 800-kD band under nonreducing conditions (lanes 1 and 2), suggesting that the α1, 2 protein is part of a laminin trimer. Using an immunoprecipitation assay, α1, 2 was found to be associated with the same β and γ chains, as was α3, 5 (data not shown).
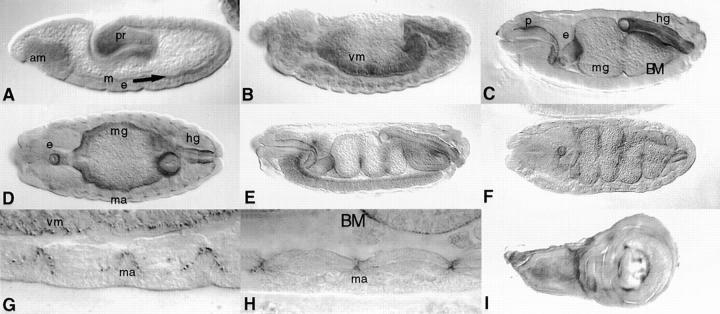
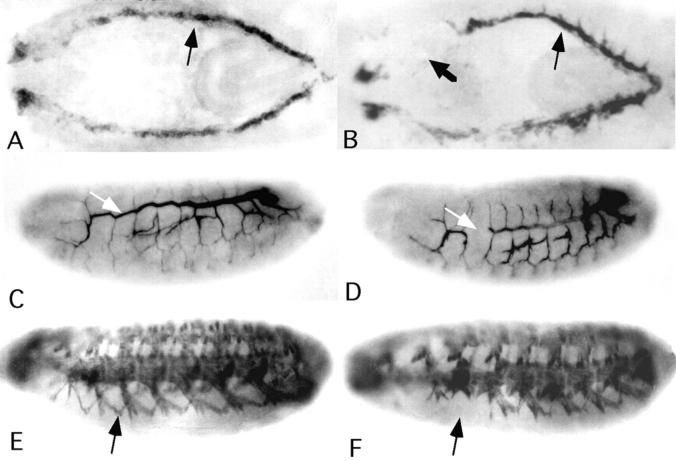
The protein is first detected at stage 10 as a weak diffusive stripe between the ectoderm and the mesoderm (Fig. 5 A). During germband retraction (Fig. 5 B) the protein is localized diffusely around areas that constitute the visceral mesoderm. At stage 14, strong staining is observed in the BMs that surround the digestive system, i.e., the gut (Fig. 5 C), or at muscle attachment sites (Fig. 5 D, G, and H). These patterns are strongly reminiscent of the expression patterns of various Drosophila integrin subunits, particularly the β subunit (Leptin et al., 1989) and the α2 subunit (Bogaert et al., 1987). Later stages include localization in dorsal structures along the ventral nerve cord (Fig. 5 E), and BMs around the digestive system (Fig. 5 F). During imaginal wing disc development, α1, 2 is localized in a specific spot pattern on the presumptive wing dorsal and ventral region (Fig. 5 I).
Figure 5.
Wb protein pattern during embryonic and larval development. Embryos are oriented with the anterior to the left and dorsal side up unless otherwise noted. (A) Stage 10 embryo (embryonic stages are those of Campos-Ortega and Hartenstein, 1985) denoting low level staining in the layer between ectoderm (e) and mesoderm (m; arrow), the anterior midgut (am), and the proctodeum (pr). (B) Stage 13 embryo showing Wb localized on visceral mesoderm of the gut. (C) Stage 14 embryo, BM surrounding the major organs of the presumptive digestive systems, pharynx (p), esophagus (e), midgut (mg), and hindgut (hg) show marked staining. (D) Same stage 14 as in C, horizontal section, muscle attachment (ma) sites are stained. (E) Stage 15 embryo, major BMs are stained. (F) Stage 16 embryo, horizontal section, BMs of the major portions of the digestive system show staining. (G and H) High magnifications of muscle attachment sites of stage 12 and stage 16 embryos, respectively. Note also the distinct staining of the BM of the midgut. (I) Wing disc, staining is particularly strong in distinct regions on the presumptive wing dorsal and ventral region.
The wb Gene Encodes Laminin α1, 2
Genomic phage DS 03792 (Fig. 1 B) was mapped to chromosomal region 35A1 (Fig. 1 A). Several P element insertion events could be detected within the genomic area of the laminin gene. Of particular interest were two fly lines conferring embryonic lethality that showed the P element inserted into the middle of the fourth intron (Fig. 1 B). Because insertions of this type showed lethality on other occasions (Horowitz and Berg, 1995) where an unusual splicing event was shown to be the cause for lethality, we wondered whether the same situation would apply here. To test whether trans-splicing between the fourth exon of laminin α1, 2 and the last exon of ribosomal protein S12, which resides on the P element construct, we performed Northern analysis on RNA derived from l(2) 09437 embryos or l(2) 10002 embryos (not shown). Two bands were visible: the doublet band ∼11 kb, already detected in the developmental Northern analysis generated by the wild-type gene from the balancer chromosome, and a smaller species of 5.6 kb, derived from the mutant chromosome whose RNA showed trans-splicing to S12, yielding a shorter transcript (Fig. 3 B). Rehybridization of the same Northern lane using a S12-specific probe confirmed the same 5.6-kb mRNA species (data not shown). We interpret the fact that the 5.6-kb mutant band is stronger than the wild-type 11-kb band as a composite result of a higher efficiency to complete the transcript, because the mutant transcript is more stable, or the transfer of larger mRNA is less efficient. The shortened mRNA codes for a protein truncated within LE8 (Fig. 2 C), and as a result no assembly of the heterotrimeric laminin molecule can occur, as only α, β, and γ heterotrimers are sufficiently stable to be secreted (Yurchenco et al., 1997). Consequently, it is likely that no functional laminin of the subunit composition α1, 2; β1; γ1 is secreted in the l(2) 09437 mutant.
A survey in the chromosomal area of 35A showed that a locus, termed wb, resulting in blisters in wings, could account for the loss of laminin function. To test this hypothesis, l(2) 09437 and l(2) 10002 flies were crossed to suitable viable and embryonic lethal wb alleles, and were tested for complementation. None of the strong ethylmethane sulfonate induced wb alleles complemented l(2) 09437 and l(2) 10002 for embryonic lethality (data not shown). This result, in combination with the mapping data, strongly argues that l(2) 09437 and l(2) 10002 are mutations in the wb locus, and that wb is indeed laminin α1, 2.
Defects in wb Embryos
To examine the functions of the Wb protein during embryogenesis, the development of embryos homozygous for embryonic lethal mutations in the wb gene (wbk05612, wbk00305, and wbHG10; Lindsley and Zimm, 1992; Zusman, S., unpublished results) was examined and compared with wild-type embryos and embryos homozygous for a deficiency that uncovers the wb locus (Df[2L]fn36 and Df[2L]fn7; Lindsley and Zimm, 1992).
Time lapse videomicroscopy of developing flies revealed that homozygous wbHG10, wbk05612, and Df(2L)fn36 embryos become abnormal during gastrulation. Rather than extending their germbands dorsally, mutant germbands twist and extend laterally (Fig. 6, A and B). Near the completion of germband extension, wb and Df(2L)fn36 embryos show a distinct separation between the mesodermal and ectodermal tissue layers of the germband (Fig. 6, C and D). These phenotypes are similar to that described for mys hemizygous embryos which lack the βPS subunit of integrin (Roote and Zusman, 1995), a potential receptor for laminin (reviewed by Hynes, 1992). Another phenotype in common with mys embryos (Wieschaus et al., 1984) includes a dorsal hole which often forms in the cuticle of wbk05612, and occasionally in wbk00305 embryos. Although Wb protein accumulates around the BMs of the developing embryonic gut, no defects were detected in gut morphology or midgut primordial migration.
Figure 6.
Germband phenotypes in wb embryos. Wild-type embryos (A and B) and wbk05612 embryos (C and D). (A and B) Dorsal view of living embryos at germband extension. (B) wbk05612 embryo shows the posterior midgut invagination (arrow) and germband twisted abnormally to one side and not centered on the midline as in the wild-type embryo in A. (C and D) Lateral view (anterior left) of living embryos at the onset of germband retraction. An abnormal separation is observed between the mesoderm and ectoderm layers of the germband (C versus D, arrow).
Previous studies of embryos lacking the Drosophila laminin α3, 5 chain have demonstrated functions for this molecule in the proper morphogenesis of heart, somatic muscle, and trachea (Yarnitzky and Volk, 1995; Stark et al., 1997). In laminin α3, 5 deficient embryos there is a dissociation of the pericardial cells of the heart, gaps in the dorsal trunk of the trachea, and the ventral oblique muscles fail to reach their attachment sites. Similar heart and tracheal defects are found in embryos with mutations affecting αPS3βPS integrin (Stark et al., 1997). To determine if the Wb protein is also involved in these processes, we examined the development of their heart, trachea, and somatic muscles.
The heart (dorsal vessel) forms from external pericardial cells and internal cardioblasts that migrate during dorsal closure to meet along the dorsal midline to form the heart tube (Bate, 1993). wb and wb-deficient embryos stained with antibodies that recognize pericardial cells show that homozygous wbk05612, wbk00305 (both occasionally showing dorsal holes), and Df(2L)fn36 embryos often contain fewer pericardial cells than wild-type embryos resulting in distinct gaps in the heart tube (Fig. 7, A and B). Furthermore, the pericardial cells appear to dissociate randomly and the tube often appears to curve off towards the lateral side of the embryo.
Figure 7.
Dorsal vessel, trachea, and somatic muscle defects in wb embryos. Wild-type embryos (A, C, and E) and abnormal embryos from wbk05612 producing crosses (B, D, and F) are shown with anterior at the left. (A and B) Antibody staining of stage 14 embryos shows mislocalization (arrow) and gaps (thick arrow) in the pericardial cells of the developing heart and dorsal vessel of a wb embryo. (C and D) Antibody staining of stage 16 embryos shows gaps (arrow) in the dorsal tracheal trunk of a wb embryo in D. (E and F) Ventrolateral views of stage 16 embryos stained with antimyosin antibodies to visualize somatic muscle patterning. Ventral oblique muscles (arrow) in the wb embryo of F never reach their targeted epidermal attachment sites.
The dorsal trunk of the trachea is formed by migration of the tracheal pits to form a long tracheal tube which extends the length of the embryo (reviewed in Manning and Krasnow, 1993). Antibodies were used to examine trachea formation in wb and wb deficient embryos. Embryos homozygous for wbk05612, wbHG10, and Df(2L)fn36 were observed to have significant gaps in the dorsal trunk of the trachea (Fig. 7, C and D). This was confirmed by examining the development of filmed wb embryos.
Due to the strong expression of the Wb protein in muscle attachment sites, we also examined wb and wb-deficient embryos for defects associated with the attachment of myotubes to their ectodermal attachment sites. Careful examination of somatic muscle in homozygous wbk05612, wbHG10, and Df(2L)fn36 embryos stained with antimyosin antibodies (Fig. 7, E and F), or prepared for examination under polarized light at the end of embryonic development, revealed that their somatic myotubes are often not attached to target epidermal attachment sites. This defect most commonly involves the ventral oblique muscles located in the anterior most segments of the embryo (Fig. 7 F). Random disorganization of myotubes and areas without myotubes are occasionally observed in these embryos as well.
In conclusion, several defects are observed in wb embryos, some in common with those observed in laminin α3, 5 embryos, and many in common with those observed with integrin mutations.
Defects in Adults
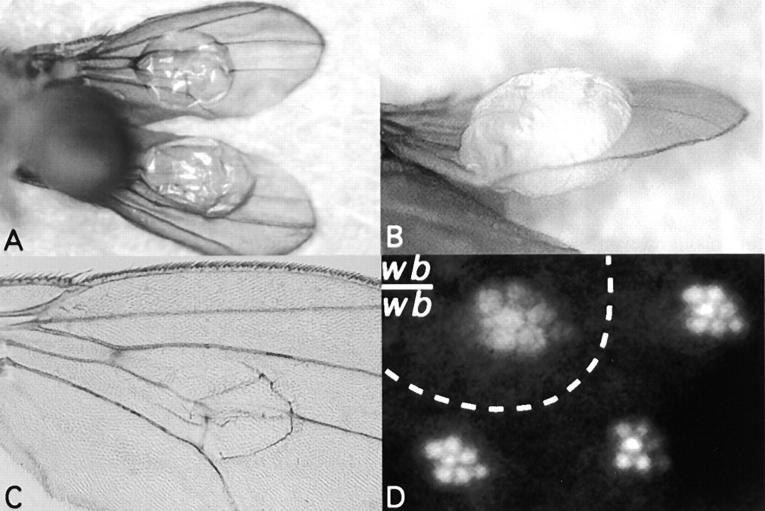
As the name implies, mutations in the wb locus can lead to blistering of the wing, in which the dorsal and ventral wing surfaces separate (Woodruff and Ashburner, 1979). As shown in Fig. 8, A and C, the blisters are located centrally within the wing, consistent with the location of laminin expression and localization in wing discs (Fig. 4 J and Fig. 5 I). The blisters vary in size, depending on the allelic combination used (data not shown). Homozygous viable alleles of wb exist that show no blistering (i.e., wbCR4), and only in combination with an embryonic lethal wb allele (i.e., l(2) 09437) or a deficiency (Df[2L]fn7 or fn36) were blisters observed, suggesting that below a certain threshold, the lack of functional laminin can lead to blistering. No haplo-insufficiency was observed in l(2) 09437 animals (data not shown). The wb phenotype strongly resembles the phenotypes associated with mutations in integrins (Brower and Jaffe, 1989; Brabant et al., 1993; Brower et al., 1995), and mutations in the Drosophila laminin α3, 5 gene can also lead to blistered wings (Henchcliffe et al., 1993).
Figure 8.
Adult wb phenotypes. (A–C) Wings from wbCR4/l(2) 09437 flies. (A) Bottom, (B) side, and (C) high magnification of the blister, suggesting weakened adhesion between dorsal and ventral wing epithelia. (D) Disorganized rhabdomeres in homozygous wbk05612 eye clones as viewed under antidromic illumination. Note that the encircled rhabdomeres (wb/wb) show the same overall organization as in the neighboring (wild-type) rhabdomeres, but appear disorganized, similar to mys eye clones (Zusman et al., 1990), or mew eye clones (Brower et al., 1995).
Due to the fact that high expression of wb was also found posterior to the morphogenetic furrow in the developing eye (Fig. 4 K), we wished to determine the function of wb during eye development. For this reason, somatic clones were induced in the eye of wbk05612 flies using the FLP technique (Golic, 1991). As evident in Fig. 8 D, the number of photoreceptor cells did not change, but they appear disorganized. Disorganized photoreceptor cells were also detected in mys and mew (PS1-encoding) mutant clones (Zusman et al., 1990; Brower et al., 1995).
Discussion
We have demonstrated the existence of a second laminin α chain in Drosophila, and sequence analysis shows that it is homologous to the α2 and α1 chain in vertebrates. Most likely, this chain represents one of the ancestral versions of a vertebrate α chain of laminin, as some marked changes are observed in comparison to α1, 2. The protein is slightly larger than vertebrate α1 or α2, mainly due to the addition of a NH2-terminal extension, an insertion in the first EGF-like region, and by acquisition of two additional EGF-like modules (Fig. 2 C). Other discrepancies have been observed in the Caenorhabditis elegans α1, 2 where one G module is deleted (Fig. 2 C). Laminins have also been isolated in lower organisms such as Hydra vulgaris (Sarras et al., 1994) where they are expressed in the subepithelial zone involved in attachment of mesoderm to the ectoderm. Sequence comparisons suggest that the α chain associated with this laminin corresponds to an ancestral version of the α3 and α5 chain (Sarras, M., personal communication).
Virtually no exon boundaries match the gene structure observed in human laminin α2 or C. elegans laminin α1, 2, nor is the number of exons similar (16 versus 64 and 10, respectively; Zhang et al., 1996; Fig. 2), suggesting that α chains in higher animals have become more complex by splitting coding sequences through uptake of new noncoding sequences. In addition, no exon boundaries of Drosophila α1, 2 fit those of Drosophila α3, 5 (Fig. 2 D) or even of C. elegans α1, 2 (Fig. 2 C), suggesting that the two α chains diverged much earlier. Based on the sequenced C. elegans genome, which discovered only two α chains, it is plausible to assume that invertebrate genomes such as Drosophila or C. elegans probably possess only two α chains, one β and one γ chain, respectively, which may limit the number of possible assemblies into functional laminin trimers to two.
A comparison between expression patterns of α1, 2 and vertebrate laminins reveals that the expression of vertebrate α2 fits better to Drosophila α1, 2, as α1 shows a highly restricted expression in kidney, as compared with α2 whose expression was reported to be widespread in mesenchymal cells (Miner et al., 1997). In accordance with vertebrate expression studies (Miner et al., 1997) where α5 was shown to be the most widely expressed α chain, Drosophila α3, 5 is more widely expressed than α1, 2.
Interestingly, Wb harbors a RGD sequence located on the L4 domain (Fig. 2 C) which makes it a likely ligand for integrins. Biochemical studies on integrin-mediated adhesion using Drosophila cell lines identified Wb as a distinct ligand for αPS2m8βPS4A integrin (Graner et al., 1998), one of four splice forms of the αPS2βPS integrins (Brown et al., 1989; Zusman et al., 1990). The αPS2 isoform is also the predominant splice form present at developmental stages during which Wb is expressed (Brown et al., 1989). No data have been reported to date on the isoform distribution of βPS integrin. In contrast, other RGD containing proteins such as tiggrin (Fogerty et al., 1994), or ten-m (Baumgartner et al., 1994) show no absolute requirement for a specific splice isoform of βPS: both proteins need only exon 8 of αPS2 to be present. Using a similar approach, Drosophila laminin containing α3, 5 was shown to be a specific ligand for αPS1βPS integrin (Gotwals et al., 1994). This suggests that Drosophila laminins (subunit composition α1, 2; β1; γ1, and α3, 5; β1; γ1) can serve as PS2 and PS1 integrin ligands, respectively. Moreover, the model for embryonic muscle and pupal wing attachment proposed by Gotwals et al. (1994) holds true, by juxtaposing another partner to tiggrin facing the PS2 binding site. Interestingly, the region harboring the RGD in L4 of Wb is highly related to the RGD-containing site of vertebrate laminin α5 (Graner et al., 1998), which could indicate that vertebrate α5 has taken up this motif during evolution, in contrast to the existing Drosophila α3, 5 which does not harbor an RGD site. Genetic data further support an association of wb with integrins, since weak mys mutations increase the size and frequency of blisters in wb flies (Khare, N., and S. Baumgartner, manuscript in preparation). No conclusive genetic interaction data were reported to occur between α3, 5 and mys (Henchcliffe et al., 1993).
Several embryonic wb phenotypes (Fig. 6) were shown to be remarkably similar to those of single integrin mutations, i.e., the separation of mesoderm and ectoderm, and the twisted germband common to mys (Fig. 6, B and D; Roote and Zusman, 1995) or to scb (Stark et al., 1997). Notably, separated mesoderm/ectoderm and twisted germband were not observed in mutations in the α3, 5 chain (Yarnitzki and Volk, 1995). The α3, 5 chain was only found to be required for later stages of patterning of mesodermally derived cells, suggesting that α1, 2 is exclusively used to confer early adhesion between mesoderm and ectoderm. In contrast, common phenotypes between α1, 2 and α3, 5 were detected in late stages of embryogenesis where the formation of the ventral oblique muscles is disturbed, particularly in the anterior segments (Fig. 7 F; Yarnitzki and Volk, 1995; Prokop et al., 1998). Finally, the formation of the heart was reported to be disturbed in mutations of both genes (Fig. 7 B; Yarnitzki and Volk, 1995).
No phenotype reminiscent of the muscular dystrophy-like phenotype in vertebrates was observed in our mutants. Although we did not observe wb expression in muscles, we cannot rule out marginal expression levels below the sensitivity of our detection method. However, certain myotubes do appear disorganized in wb mutant embryos. This cannot be considered an analogous situation to dy/dy mice (Xu et al., 1994), because the defects observed are most likely due to the inability of muscle cells to migrate properly and a failure in attaching to muscle attachment sites. Similar phenotypes were also observed in laminin α3, 5 mutants (Prokop et al., 1998).
Previous studies have shown that integrin-mediated adhesivity between the two epithelial cell layers of the wing is particularly sensitive to mutations involving either integrin ligands (this paper) or upstream factors of integrins, i.e., the blistered (bs) gene encoding a Drosophila serum response factor (SRE; Montagne et al., 1996). bs and integrins interact genetically (Fristrom et al., 1994) and mys expression appears to be greatly reduced in hypomorphic bs mutants (Montagne et al., 1996), suggesting a scenario where bs might directly control integrin gene expression on the transcriptional level. It is plausible to assume that bs might also directly control wb expression, as the transcript pattern of both show striking coexpression (Fig. 4 J; Montagne et al., 1996), and a corresponding SRE has been located 260 bp upstream of the putative TATA box of the wb gene (data not shown).
Specific screens have been performed for mutations affecting adhesion between wing surfaces (Prout et al., 1997; Walsh and Brown, 1998). To our surprise, none of the loci described correspond to wb, suggesting that the formation of blisters in the wing depends on subtle changes of wb activity. This is further suggested by the fact that only suitable wb allelic combinations show blisters. For example, blisters were only detected in transheterozygous allelic combinations of a weak (homozygous viable) allele, wbCR4, and Df(2L)fn7 or l(2) 09437 which behaves as a null allele. In other words, only the range of wb activity slightly below 50% of wild-type activity is capable of forming blisters, while a level of ≥50% does not affect wing blistering, as no haplo-insufficiency is observed in l(2) 09437 flies.
In parallel to the wing, wb clones induced in the eye cause similar phenotypes to clones induced in integrin mutations, i.e., αPS1 (mew) mutants (Roote and Zusman, 1995) or βPS (mys) mutants (Zusman et al., 1990; Brower et al., 1995), but not in αPS2 (if) mutants (Brower et al., 1995) which result in virtually wild-type eyes. Similar phenotypes were also observed in laminin α3, 5 mutant combinations, however, the degree of severity of disorganization is higher than in wb or integrin mutant clones (Henchcliffe et al., 1993).
Acknowledgments
We wish to thank John Roote and Michael Ashburner for their generous contribution of wb alleles, and Denise Montell for providing the H155 line. We also wish to thank Talila Volk, Mark Krasnow, and Dan Kiehart for providing antibodies, and Jürgen Engel for providing mouse EHS laminin. We would like to thank the Berkeley Drosophila Genome Project and particularly Suzanne Lewis for providing sequence information before publication. The help of Herbert Angliker in resequencing portions of the gene is acknowledged. In addition, we appreciate the efforts of Kassandra Gorham, Christopher Wright, Liam Casey, and Mark Hickery on this project.
This work was supported by National Science Foundation grant 9404055 to S. Zusman and by the Schybergs Stiftelse and a Swedish Naturvetenskapliga Forskningradet Grant 409005501-5 to S. Baumgartner.
Abbreviations used in this paper
- BM
basement membrane
- ECM
extracellular matrix
- wb
wing blister
Footnotes
Sequence data reported in this paper appears in GenBank/EMBL/DDBJ under the accession number AF 135118.
D. Martin and S. Zusman contributed equally to this work.
S. Zusman and X. Li's present address is Department of Functional Genomics, Novartis Pharmaceuticals, Life Sciences Building, 556 Moris Avenue, Summit, NJ 07901-1398. E.L. Williams' present address is Department of Biochemistry and Cell Biology, Rice University, Houston, TX 77096.
References
- Aberdam D, Galliano MF, Vailly J, Pulkkinen L, Bonifas J, Christiano AM, Tryggvason K, Uitto J, Epstein EH, Ortonne JP, et al. Herlitz's junctional epidermolysis bullosa is linked to mutations in the gene (LAMC2) for the gamma 2 subunit of nicein/kalinin (LAMININ-5) Nat Genet. 1994;6:299–304. doi: 10.1038/ng0394-299. [DOI] [PubMed] [Google Scholar]
- Bairoch A, Apweiler R. The SWISS-Prot protein sequence data bank and its supplement in 1999. Nucleic Acids Res. 1999;27:49–54. doi: 10.1093/nar/27.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate, M. 1990. The mesoderm and its derivatives. In The Development of Drosophila melanogaster. M. Bate, and A. Martinez-Arias, editors. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 1013–1090.
- Baumgartner S, Martin D, Chiquet-Ehrismann R. Drosophilaribosomal protein S19 sequence. Nucl Acids Res. 1993;21:3897. doi: 10.1093/nar/21.16.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner S, Martin D, Hagios C, Chiquet-Ehrismann R. ten-m, a Drosophilagene related to tenascin, is a new pair-rule gene. EMBO (Eur Mol Biol Organ) J. 1994;13:3728–3740. doi: 10.1002/j.1460-2075.1994.tb06682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaert T, Brown N, Wilcox M. The DrosophilaPS2 antigen is an invertebrate integrin that, like the fibronectin receptor, becomes localized to muscle attachments. Cell. 1987;51:929–940. doi: 10.1016/0092-8674(87)90580-0. [DOI] [PubMed] [Google Scholar]
- Brabant MC, Brower DL. PS2 integrin requirements in Drosophilaembryos and wing morphogenesis. Dev Biol. 1993;157:49–59. doi: 10.1006/dbio.1993.1111. [DOI] [PubMed] [Google Scholar]
- Brower DL, Jaffe SM. Requirement for integrin during Drosophilawing development. Nature. 1989;342:285–287. doi: 10.1038/342285a0. [DOI] [PubMed] [Google Scholar]
- Brower DL, Bunch TA, Mukai L, Adamson TE, Wehrli M, Lam S, Friedlander E, Roote CE, Zusman S. Non-equivalent requirements for PS1 and PS2 integrin at cell attachments in Drosophila; genetic analysis of the αPS1 integrin subunit. Development. 1995;121:1311–1320. doi: 10.1242/dev.121.5.1311. [DOI] [PubMed] [Google Scholar]
- Brown NH, King DL, Wilcox M, Kafatos FC. Developmentally regulated alternative splicing of Drosophilaintegrin PS2α transcripts. Cell. 1989;59:185–195. doi: 10.1016/0092-8674(89)90880-5. [DOI] [PubMed] [Google Scholar]
- Bunch TA, Brower DL. DrosophilaPS2 integrin mediates RGD-dependent cell–matrix interactions. Development. 1992;116:239–247. doi: 10.1242/dev.116.1.239. [DOI] [PubMed] [Google Scholar]
- Burge C, Karlin S. Prediction of complete gene structures in human genomic DNA. J Mol Biol. 1997;268:78–94. doi: 10.1006/jmbi.1997.0951. [DOI] [PubMed] [Google Scholar]
- Burgeson R, Chiquet M, Deutzmann R, Ekblom P, Engel J, Kleinman H, Martin GR, Meneguzzi G, Paulsson M, Sanes J, et al. A new nomenclature for the laminins. Matrix Biol. 1994;14:209–211. doi: 10.1016/0945-053x(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega, J.A., and V. Hartenstein. 1985. The Embryonic Development of Drosophila melanogaster. Springer-Verlag, Berlin. 9–84.
- Chi H-C, Hui C-F. Primary structure of the Drosophila laminin B2 chain and comparison with human, mouse and DrosophilaB1 and B2 chains. J Biol Chem. 1989;264:1543–1550. [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chung AE, Jaffe R, Freeman JP, Vergnes JP, Braginsk JE, Carlin B. Properties of a basement membrane related glycoprotein synthesized by a mouse embryonal carcinoma-derived cell line. Cell. 1979;16:277–287. doi: 10.1016/0092-8674(79)90005-9. [DOI] [PubMed] [Google Scholar]
- Cserhalmi-Friedman PB, Baden H, Burgeson RE, Christiano AM. Molecular basis of non-lethal junctional epidermolysis bullosa: identification of a 38 basepair insertion and a splice site mutation in exon 14 of the LAMB3 gene. Exp Dermatol. 1998;7:105–111. doi: 10.1111/j.1600-0625.1998.tb00309.x. [DOI] [PubMed] [Google Scholar]
- Drysdale R, Rushton E, Bate M. Genes required for embryonic muscle development in Drosophila melanogaster. . Roux's Arch Dev Biol. 1993;202:276–295. doi: 10.1007/BF00363217. [DOI] [PubMed] [Google Scholar]
- Ehrig K, Leivo I, Argaves WS, Ruoslahti E, Engvall E. Merosin, a tissue-specific basement membrane protein, is a laminin-like protein. Proc Natl Acad Sci USA. 1990;87:3264–3268. doi: 10.1073/pnas.87.9.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogerty FJ, Fessler LI, Bunch TA, Yaron Y, Parker CG, Nelson RE, Brower DL, Gullberg D, Fessler JH. Tiggrin, a novel Drosophila extracellular matrix protein that functions as a ligand for DrosophilaαPS2βPS integrins. Development. 1994;120:1747–1758. doi: 10.1242/dev.120.7.1747. [DOI] [PubMed] [Google Scholar]
- Fristrom D, Gotwals P, Eaton S, Kornberg TB, Sturtevant M, Bier E, Fristrom JW. blistered: a gene required for vein/intervein formation in wings of Drosophila. . Development. 1994;120:2661–2671. doi: 10.1242/dev.120.9.2661. [DOI] [PubMed] [Google Scholar]
- Garcia-Alonso L, Fetter RD, Goodman CS. Genetic analysis of laminin A in Drosophila: extracellular matrix containing laminin A is required for ocellar axon pathfinding. Development. 1996;22:2611–2621. doi: 10.1242/dev.122.9.2611. [DOI] [PubMed] [Google Scholar]
- Golic KG. Site-specific recombination between homologous chromosomes in Drosophila. . Science. 1991;252:958–961. doi: 10.1126/science.2035025. [DOI] [PubMed] [Google Scholar]
- Gotwals PJ, Paine-Saunders SE, Stark KA, Hynes RO. Drosophilaintegrins and their ligands. Curr Opin Cell Biol. 1994;6:734–739. doi: 10.1016/0955-0674(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Graner MW, Bunch TA, Baumgartner S, Kerschen A, Brower DL. Splice variants of the DrosophilaPS2 integrins differentially interact with the extracellular ligands Tiggrin, D-Laminin α2 and Ten-m. J Biol Chem. 1998;273:18235–18241. doi: 10.1074/jbc.273.29.18235. [DOI] [PubMed] [Google Scholar]
- Helbling-Leclerc A, Zhang X, Topaloglu H, Cruaud C, Tesson F, Weissenbach J, Tome FM, Schwartz K, Fardeau M, Tryggvason K, et al. Mutations in the laminin alpha2-chain cause merosin-deficient congenital muscular dystrophy. Nat Genet. 1995;11:216–218. doi: 10.1038/ng1095-216. [DOI] [PubMed] [Google Scholar]
- Henchcliffe C, Garcia-Alonso L, Tang J, Goodman CS. Genetic analysis of laminin A reveals diverse functions during morphogenesis in Drosophila. . Development. 1993;118:325–337. doi: 10.1242/dev.118.2.325. [DOI] [PubMed] [Google Scholar]
- Horowitz H, Berg CA. Aberrant splicing and transcription termination caused by P element insertion into the intron of a Drosophilagene. Genetics. 1995;139:327–335. doi: 10.1093/genetics/139.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Karpen GH, Spradling AC. Analysis of subtelomeric heterochromatin in the Drosophila minichromsome Dp1187by single P element insertional mutagenesis. Genetics. 1992;132:737–753. doi: 10.1093/genetics/132.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehart DP, Feghali R. Cytoplasmic myosin from Drosophila melanogaster. . J Cell Biol. 1986;103:1517–1525. doi: 10.1083/jcb.103.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmerly W, Stultz K, Lewis S, Lewis K, Lustre V, Romero R, Benke J, Sun D, Shirley G, Martin C, Palazzolo M. A P1-based physical map of the Drosophilaeuchromatic genome. Genome Res. 1996;6:414–430. doi: 10.1101/gr.6.5.414. [DOI] [PubMed] [Google Scholar]
- King, R.C. 1970. Ovarian development in Drosophila melanogaster. Academic Press Inc., New York.
- Kivirikko S, McGrath JA, Baudoin C, Aberdam D, Ciatti S, Dunnill MG, McMillan JR, Eady RA, Ortonne JP, Meneguzzi G, et al. A homozygous nonsense mutation in the alpha 3 chain gene of laminin 5 (LAMA3) in lethal (Herlitz) junctional epidermolysis bullosa. Hum Mol Genet. 1995;4:959–962. doi: 10.1093/hmg/4.5.959. [DOI] [PubMed] [Google Scholar]
- Kusche-Gullberg M, Garrison K, MacKrell AJ, Fessler LI, Fessler JH. Laminin A chain: expression during Drosophiladevelopment and genomic sequence. EMBO (Eur Mol Biol Organ) J. 1992;11:4519–4527. doi: 10.1002/j.1460-2075.1992.tb05553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leptin M, Bogaert T, Lehmann R, Wilcox M. The function of PS integrins during Drosophilaembryogenesis. Cell. 1989;56:401–408. doi: 10.1016/0092-8674(89)90243-2. [DOI] [PubMed] [Google Scholar]
- Lindsley, D.L., and G. Zimm. 1992. The genome of Drosophila melanogaster. Academic Press Inc., London. p. 777.
- Maniatis, T., E.F. Fritsch, and J. Sambrook. 1982. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Manning, G., and M.A. Krasnow. 1993. Development of the Drosophila tracheal system. In The Development of Drosophila melanogaster. M. Bate and A. Martinez-Arias, editors. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 609–685.
- Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR. The laminin α chains: expression, developmental transitions, and chromosomal locations of α1-5, identification of heterotrimeric laminins 8–11, and cloning of a novel α3 isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne J, Groppe J, Guillemin K, Krasnow MA, Gehring WJ, Affolter M. The Drosophila serum response factor gene is required for the formation of intervein tissue of the wing and is allelic to blistered. . Development. 1996;122:2589–2597. doi: 10.1242/dev.122.9.2589. [DOI] [PubMed] [Google Scholar]
- Montell DJ, Goodman CS. Drosophilasubstrate adhesion molecule: sequence of laminin B1 chain reveals domains of homology with mouse. Cell. 1988;53:463–473. doi: 10.1016/0092-8674(88)90166-3. [DOI] [PubMed] [Google Scholar]
- Montell DJ, Goodman CS. Drosophilalaminin: sequence of B2 subunit and expression of all three subunits during embryogenesis. J Cell Biol. 1989;109:2441–2453. doi: 10.1083/jcb.109.5.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A, Martin-Bermudo MD, Bate M, Brown N. Absence of PS integrins or laminin A affects extracellular adhesion, but not intracellular assembly, of hemiadherens and neuromuscular junctions in Drosophilaembryos. Dev Biol. 1998;196:58–76. doi: 10.1006/dbio.1997.8830. [DOI] [PubMed] [Google Scholar]
- Prout M, Damania Z, Soong J, Fristrom D, Fristrom JW. Autosomal mutations affecting adhesion between wing surfaces in Drosophila melanogaster. . Genetics. 1997;46:275–285. doi: 10.1093/genetics/146.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulkkinen L, Christiano AM, Airenne T, Haakana H, Tryggvason K, Uitto J. Mutations in the gamma 2 chain gene (LAMC2) of kalinin/laminin 5 in the junctional forms of epidermolysis bullosa. Nat Genet. 1994;6:293–297. doi: 10.1038/ng0394-293. [DOI] [PubMed] [Google Scholar]
- Rambukkana A, Salzer JL, Yurchenco PD, Tuomanen EI. Neural targeting of Mycobacterium lepraemediated by the G domain of the laminin-alpha2 chain. Cell. 1997;88:811–821. doi: 10.1016/s0092-8674(00)81927-3. [DOI] [PubMed] [Google Scholar]
- Roote CE, Zusman S. Functions for PS integrins in tissue adhesion, migration and shape changes during early embryonic development in Drosophila. . Dev Biol. 1995;169:322–336. doi: 10.1006/dbio.1995.1147. [DOI] [PubMed] [Google Scholar]
- Roote CE, Zusman S. Alternatively spliced forms of the DrosophilaαPS2 subunit of integrin are sufficient for viability and can replace the function of the αPS1 subunit of integrin in the retina. Development. 1996;122:1985–1994. doi: 10.1242/dev.122.6.1985. [DOI] [PubMed] [Google Scholar]
- Samakovlis C, Hacohen N, Manning G, Sutherland DC, Guillemin K, Krasnow MA. Development of the Drosophilatracheal system occurs by a series of morphologically distinct, but genetically coupled branching events. Development. 1996;122:1395–1407. doi: 10.1242/dev.122.5.1395. [DOI] [PubMed] [Google Scholar]
- Sarras MP, Yan L, Grens A, Zhang X, Agbas A, Huff JK, St John PL, Abrahamson DR. Cloning and biological function of laminin in Hydra vulgaris. . Dev Biol. 1994;164:312–324. doi: 10.1006/dbio.1994.1201. [DOI] [PubMed] [Google Scholar]
- Stark KA, Yee GH, Roote CE, Williams EL, Zusman S, Hynes RO. A novel alpha integrin subunit associates with βPS and functions in tissue morphogenesis and movement during Drosophiladevelopment. Development. 1997;124:4583–4594. doi: 10.1242/dev.124.22.4583. [DOI] [PubMed] [Google Scholar]
- Tautz D, Pfeiffle C. A nonradioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals a translational control of the segmentation gene hunchback. . Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Timpl R, Brown JC. Supramolecular assembly of basement membranes. BioEssays. 1996;18:123–132. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin-a glycoprotein from basement membranes. J Biol Chem. 1979;254:993–997. [PubMed] [Google Scholar]
- von Heijne, G. 1986. A new method for predicting signal sequence cleavage sites. Nucl. Acids Res 14:4683–4690. [DOI] [PMC free article] [PubMed]
- Walsh EP, Brown NH. A screen to identify Drosophilagenes required for integrin-mediated adhesion. Genetics. 1998;150:791–805. doi: 10.1093/genetics/150.2.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieschaus E, Nüsslein-Volhard C, Jürgens G. Mutations affecting the pattern of the larval cuticle in D. melanogaster. III. Zygotic loci on the X chromosome. Roux's Arch Dev Biol. 1984;193:296–307. doi: 10.1007/BF00848158. [DOI] [PubMed] [Google Scholar]
- Wieschaus, E., and C. Nüsslein-Volhard. 1986. Looking at embryos. In Drosophila: a Practical Approach. D.B. Roberts, editor. IRL Press, Washington D.C. pp. 199–227.
- Woodruff R, Ashburner M. The genetics of a small autosomal region of Drosophila melanogastercontaining the structural gene for alcohol dehydrogenase. II. Lethal mutations in the region. Genetics. 1979;92:133–149. doi: 10.1093/genetics/92.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wu X-R, Wewer UM, Engvall E. Murine muscular dystrophy caused by a mutation in laminin α2 (lama2) gene. Nat Genet. 1994;8:297–302. doi: 10.1038/ng1194-297. [DOI] [PubMed] [Google Scholar]
- Yarnitzky T, Volk T. Laminin is required for heart, somatic muscles and gut development in the Drosophilaembryo. Dev Biol. 1995;169:609–618. doi: 10.1006/dbio.1995.1173. [DOI] [PubMed] [Google Scholar]
- Young PE, Kiehart DP. Nonmuscle myosin is required throughout Drosophiladevelopment. J Cell Biol. 1991;115:184–193. [Google Scholar]
- Yurchenco PD, Cheng YS. Self-assembly and calcium-binding sites in laminin. A three-arm interaction model. J Biol Chem. 1993;26:7286–7299. [PubMed] [Google Scholar]
- Yurchenco PD, Quan Y, Colognato H, Mathus T, Harrison D, Yamada Y, O'Rear JJ. The alpha chain of laminin-1 is independently secreted and drives secretion of its beta- and gamma-chain partners. Proc Natl Acad Sci USA. 1997;94:10189–10194. doi: 10.1073/pnas.94.19.10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Vuolteenaho R, Tryggvason K. Structure of the human laminin α2-chain gene (LAMA2), which is affected in congenital muscular dystrophy. J Biol Chem. 1996;271:27664–27669. doi: 10.1074/jbc.271.44.27664. [DOI] [PubMed] [Google Scholar]
- Zusman S, Patel KR, Ffrench-Constant C, Hynes RO. Requirements for integrins during Drosophiladevelopment. Development. 1990;108:391–402. doi: 10.1242/dev.108.3.391. [DOI] [PubMed] [Google Scholar]