Abstract
Despite great interest in cancer chemoprevention, effective agents are few. Here we show that chloroquine, a drug that activates the stress-responsive Atm-p53 tumor-suppressor pathway, preferentially enhances the death of Myc oncogene–overexpressing primary mouse B cells and mouse embryonic fibroblasts (MEFs) and impairs Myc-induced lymphomagenesis in a transgenic mouse model of human Burkitt lymphoma. Chloroquine-induced cell death in primary MEFs and human colorectal cancer cells was dependent upon p53, but not upon the p53 modulators Atm or Arf. Accordingly, chloroquine impaired spontaneous lymphoma development in Atm-deficient mice, a mouse model of ataxia telangiectasia, but not in p53-deficient mice. Chloroquine treatment enhanced markers of both macroautophagy and apoptosis in MEFs but ultimately impaired lysosomal protein degradation. Interestingly, chloroquine-induced cell death was not dependent on caspase-mediated apoptosis, as neither overexpression of the antiapoptotic protein Bcl-2 nor deletion of the proapoptotic Bax and Bak affected chloroquine-induced MEF death. However, when both apoptotic and autophagic pathways were blocked simultaneously, chloroquine-induced killing of Myc-overexpressing cells was blunted. Thus chloroquine induces lysosomal stress and provokes a p53-dependent cell death that does not require caspase-mediated apoptosis. These findings specifically demonstrate that intermittent chloroquine use effectively prevents cancer in mouse models of 2 genetically distinct human cancer syndromes, Burkitt lymphoma and ataxia telangiectasia, suggesting that agents targeting lysosome-mediated degradation may be effective in cancer prevention.
Introduction
Tumorigenesis is a multi-stage process that typically involves the activation of oncogenes that provoke unchecked cell growth and division and tumor angiogenesis as well as the inactivation of a cast of tumor suppressors that harness the cell cycle and/or induce apoptosis (1). The action of oncogenes is normally held in check by induction of the Arf-p53 tumor suppressor pathway, which can trigger growth arrest or apoptosis (2, 3). Indeed, secondary events that accompany tumor progression nearly always involve inactivation of the Arf-p53 pathway (1). Because of its central role in regulating tumor development and growth, modulation of p53 pathways are an attractive target for cancer prevention and treatment.
In addition to its role as a guardian against oncogenic insults, p53 is responsive to a wide array of signals that stress the cell, including hypoxia, defects in mitotic checkpoints, nucleotide deprivation and defects in RNA synthesis, and agents that induce DNA damage (4). A key arbiter of the DNA damage response pathway is the ATM gene, which is mutated in the cancer-prone disorder ataxia telangiectasia (AT). The ATM protein kinase, which is activated by ionizing irradiation and by numerous chemotherapeutic agents that induce DNA strand breaks, phosphorylates key substrates involved in modulating cell cycle and DNA repair (5). ATM, and the related kinase ATR, are required for the efficient induction and activation of p53 in response to a variety of cellular insults (6–8).
Oncogenes such as Myc cooperate with loss of function mutations in the Atm-p53 pathway to accelerate tumorigenesis (2, 3, 9–11); therefore, we reasoned that agents targeting this pathway might influence the outcome of Myc-induced tumor formation. Here we report that intermittent treatment of Myc-overexpressing mice with the antimalarial drug chloroquine (CQ), a compound that activates the ATM kinase and induces p53 without damaging DNA (12, 13), impairs tumor formation and enhances survival. CQ preferentially killed cells overexpressing Myc, and CQ-induced cell death was dependent on p53 but was not dependent on Atm, Arf, or classical regulators of apoptosis. Accordingly, intermittent CQ treatment impaired spontaneous tumor development in mice lacking Atm but not in mice lacking p53. CQ induced hallmarks of macroautophagy, yet also disrupted lysosomal functions, and blockade of both apoptosis and autophagy was simultaneously required to reduce CQ-induced cell death. These results support a model whereby alterations in lysosomal function trigger a p53-dependent cell death response that can be exploited to remove precancerous cells and impair cancer development. Selectivity for premalignant cells, preferential killing of Myc-overexpressing cells, and the ability to overcome apoptosis resistance make this approach attractive in both chemoprevention and cancer treatment.
Results
CQ suppresses Myc-induced lymphomagenesis.
Loss of either Atm or p53 augments Myc-induced tumorigenesis (2, 9–11) suggesting that agonists of the Atm-p53 DNA damage pathway might impair tumor development. The antimalarial drug CQ is a particularly attractive agonist of the DNA damage pathway, as it activates Atm and induces p53 without creating double-strand breaks in DNA (12). To determine whether CQ could affect spontaneous tumor development in a Myc-induced model of lymphomagenesis, weanling-age Eμ-Myc mice were either treated with a combined oral/i.p. injection CQ regimen (Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI33700DS1) or with i.p. CQ injection alone (3.5 mg/kg every 5 days) (Figure 1A). Intermittent CQ treatment markedly impaired lymphoma development in Eμ-Myc mice, doubling their overall survival (mean survival of 265 days on the oral/i.p. regimen versus 98 days for mice given PBS; P = 0.0002; Figure 1A). Importantly, CQ treatment was continuously required to suppress lymphoma development, since cessation of CQ administration led to the rapid reappearance of disease (Supplemental Figure 1).
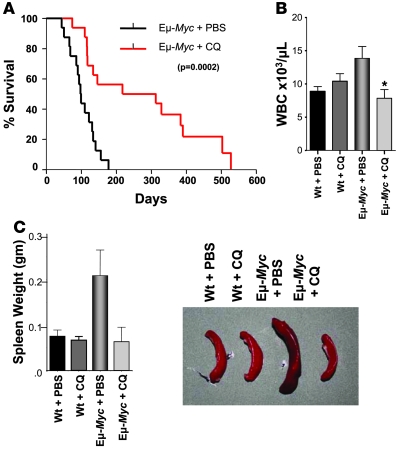
Figure 1. CQ prevents Myc-induced lymphomagenesis.
(A) Administration (i.p.) of CQ impairs lymphoma development in Eμ-Myc transgenic mice. Beginning at weaning, Eμ-Myc mice were treated with 3.5 mg/kg CQ (in PBS) i.p. every 5 days, or with PBS alone (n = 18 for each group). Median survival time was 98 days for mice treated with PBS versus 265 days for mice receiving CQ (P = 0.0002). (B) CQ treatment prevents lymphocytosis in precancerous Eμ-Myc transgenic mice. Beginning at 4 weeks, Eμ-Myc transgenic mice and their wild-type littermates (n = 5–8 for each group) were injected with 3.5 mg/kg CQ (in PBS) i.p. every 5 days, or with PBS alone. At 7 weeks, mice were then analyzed for wbc counts. CQ had no effect on wbc numbers in wild-type mice but had profound effects on wbc numbers in Eμ-Myc mice. *P < 0.05. (C) CQ treatment prevents splenomegaly in precancerous Eμ-Myc transgenic mice. Beginning at 4 weeks, Eμ-Myc transgenic mice and their wild-type littermates (n = 8 for each group) were injected with 3.5 mg/kg CQ (in PBS) i.p. every 5 days, or injected with PBS alone. At 7 weeks, mice were then analyzed for spleen weight. Pictures in inset show representative spleens from wild-type and Eμ-Myc transgenic mice injected with PBS or CQ.
Lymphoma development in Eμ-Myc transgenic mice is preceded by a precancerous phase in which there are marked increases in the numbers of B cells in the bone marrow and periphery and an accompanying splenomegaly (14, 15). To address the effects of CQ on the precancerous response, 4-week-old Eμ-Myc transgenic mice and their wild-type littermates received 3 weekly doses of PBS or CQ and were evaluated for their wbc numbers and spleen weight 15 days later. CQ treatment had no effect on wbc numbers or spleen size of wild-type mice, and, as expected, increased wbc numbers and splenomegaly were evident in PBS-injected Eμ-Myc transgenic mice (Figure 1, B and C). Strikingly, Eμ-Myc transgenic mice treated with just 3 doses of CQ had wbc numbers and spleen sizes essentially identical to those of wild-type mice (Figure 1, B and C).
CQ selectively kills Myc-expressing cells in a p53-dependent manner.
The ability of CQ to delay tumor development in Eμ-Myc transgenic mice might result from its effects on Myc-induced cell death and/or proliferation. To assess these responses, we evaluated the proliferative rates of B cells from PBS- versus CQ-treated Eμ-Myc and wild-type mice by injection with BrdU. CQ treatment did not alter the S-phase of wild-type B cells and failed to affect the increased S-phase indices of Eμ-Myc B cells (data not shown). Evaluation of cell death in vivo revealed no difference between PBS- and CQ-treated Eμ-Myc mice (Supplemental Figure 2); however, apoptotic cells are rapidly cleared by phagocytes, making detection of cells in the process of undergoing apoptosis technically challenging. Instead, using a primary B cell culture approach to tackle this issue, precancerous Eμ-Myc B cells were more sensitive to CQ-induced cell death than were B cells derived from wild-type littermates (Figure 2A). To address whether the increased sensitivity of Myc-expressing cells to CQ was evident in other cell contexts, we transduced primary early passage (p2) mouse embryonic fibroblasts (MEFs) with an MSCV retrovirus harboring the Myc-ERTAM transgene, a chimeric fusion of human c-Myc with a modified form of the estrogen-binding domain of the estrogen receptor (ER) that is selectively activated by the ER agonist 4-hydroxytamoxifen (4-HT) (16). Again, CQ treatment induced cell death, and these effects were augmented in Myc-expressing MEFs (Figure 2B).
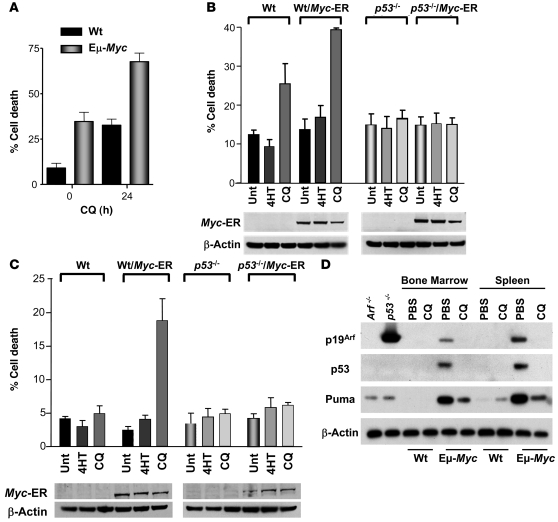
Figure 2. Myc augments p53-dependent, CQ-induced cell death.
(A) CQ augments cell death in Eμ-Myc transgenic B cells. B cells cultured from 4-week-old nontransgenic and Eμ-Myc transgenic mice were treated with 50 μM CQ for 24 h. The percentage of viable cells was determined by propidium iodide incorporation. Results shown are the mean of 3 independent B cell cultures. (B) p53 is required for sensitization of Myc-expressing MEFs to CQ. Myc-ERTAM–expressing MEFs were either left untreated (Unt) or were treated for 24 h with 4-HT alone or with 4-HT and 50 μM CQ. The percentage cell death was determined by staining cells with propidium iodide. Results shown are the mean of 3 independent experiments. Western blot analyses of the indicated cells demonstrated equal levels of expression of the Myc-ERTAM transgene. (C) Myc sensitizes HCT116 colon cancer cells to p53-dependent, CQ-induced death. Cells were treated for 24 h with 4-HT and were either left untreated or were treated with 50 μM CQ for 24 h. The percentage cell death was determined by staining cells with propidium iodide. Results shown are the mean of 3 independent experiments. (D) CQ treatment leads to clearance of Arf/p53/Puma-expressing precancerous Eμ-Myc B cells. Beginning at 4 weeks, Eμ-Myc transgenic mice and their wild-type littermates were injected with 3.5 mg/kg CQ (in PBS) i.p. every 5 days or with PBS alone. At 7 weeks, mice were sacrificed and B220+ B cells analyzed. As controls, lysates were also prepared from primary Arf–/– and p53–/– B cells. Protein extracts were prepared and evaluated for their levels of the indicated antibodies.
The administration of CQ induces p53 stabilization and activation (13). CQ treatment had no beneficial effect once the lymphomas in Eμ-Myc transgenic mice were established (data not shown). Since p53 or Arf are already mutated once these tumors are detected (2), these results suggest that the benefits of CQ might depend on p53 function. Further, the frequency of Arf and p53 mutations that arose in tumors from both PBS- and CQ-treated Eμ-Myc mice (data not shown) were the same as had been previously published using this mouse model (2). Given these results and given that Myc-induced apoptosis is at least in part p53 dependent (2, 17), we evaluated the role of p53 in CQ-induced death of early passage (p2) p53-deficient MEFs engineered to express Myc-ERTAM. Whether or not they expressed Myc-ERTAM, MEFs lacking p53 were resistant to the toxic effects of CQ (Figure 2B). To assess whether these responses to CQ were also manifest in the context of human cancer, we examined the CQ sensitivity of HCT116 human colorectal carcinoma cells. Though parental HCT116 cells were resistant to the toxic effects of CQ, activation of Myc-ERTAM rendered these cells exquisitely sensitive to this agent. However, this sensitivity was lost in Myc-ERTAM–expressing HCT116 cells lacking p53 (Figure 2C).
Since CQ-induced cell death was p53 dependent (Figure 2, B and C), we wished to further explore the potential involvement of the Arf-p53 tumor suppressor pathway that guards against Myc-induced tumorigenesis. To test this, 4-week-old Eμ-Myc transgenic mice and their wild-type littermates were treated with 3 weekly doses of PBS or CQ and the expression of p19Arf, p53, and the p53 target gene Puma, which are increased in Eμ-Myc precancerous B cells, were evaluated (15, 18). Interestingly, CQ treatment appeared to lead to the clearance of B220+ cells that expressed these regulators (Figure 2D), suggesting that CQ treatment selectively targeted Myc-expressing B cells that had activated the Arf-p53 pathway.
CQ is a p53-dependent chemoprevention agent.
To test the potential roles of the p53 modulators Atm and Arf in the CQ-induced death response, early passage (p2) Atm- or Arf-deficient MEFs were treated with CQ and were directly compared with wild-type MEFs isolated from siblings and with p53-deficient MEFs. p53 was induced in Atm- and Arf-deficient MEFs (Supplemental Figure 4 and data not shown), and both cell types were susceptible to the toxic effects of CQ, while p53-deficient MEFs were again resistant (Figure 3A). CQ was also not toxic to M1 leukemia cells, which are null for p53 (data not shown). In addition to demonstrating that CQ is acting independently of both Atm and Arf, these results show that CQ induces a p53-dependent and Myc-enhanced cell death response in many different cell types, including MEFs, premalignant B cells, myeloid leukemia, and human colorectal cancer cells.
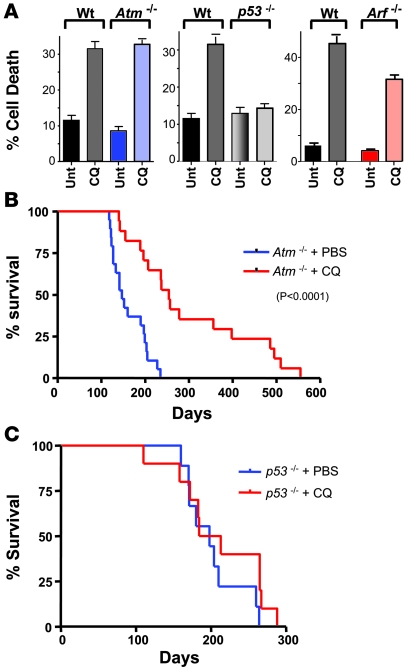
Figure 3. CQ is a p53-dependent chemoprevention agent.
(A) CQ induces cell death in wild-type, Atm- and Arf-deficient MEFs, but not in p53-deficient MEFs. Early passage (p2) MEFs paired wild-type, Atm–/–, Arf–/–, and p53–/– MEFs were left untreated or were treated with CQ (50 μM) for 24 h. The percentage cell death was determined by staining cells with propidium iodide. Results shown are the mean of 3 independent experiments. (B) Administration of CQ i.p. impairs thymoma development in Atm-deficient mice. Beginning at weaning, Atm–/– mice were treated with 3.5 mg/kg CQ (in PBS) i.p. every 5 days, or with PBS alone (n = 20 for each group). Median survival time was 147 days for mice treated with PBS versus 254 days for mice receiving CQ (P < 0.0001). (C) Administration of CQ i.p. does not affect thymoma development in p53-deficient mice. At weaning, p53–/– mice were treated with 3.5 mg/kg CQ (in PBS) i.p. every 5 days, or with PBS alone (n = 20 for each group). Median survival time was 198 days for mice in both groups.
Both Atm- and p53-deficient mice spontaneously develop T cell lymphomas (19, 20). The resistance of p53-deficient, but not Atm-deficient, cells to the toxic effects of CQ in vitro suggested that CQ might display selective efficacy in vivo in preventing lymphomagenesis. To test this notion, CQ was administered once every 5 days to weanling-age Atm- and p53-deficient mice, and effects on spontaneous thymoma development and survival were determined. Strikingly, CQ administration more than doubled the lifespan of Atm-deficient mice (Figure 3B; P < 0.0001). In contrast, CQ was ineffective as a chemoprevention agent in p53-deficient mice (Figure 3C). Collectively these data suggest that CQ prevents lymphoma development by inducing cell death in an Atm-independent yet p53-dependent manner.
CQ can provoke cell death independent of apoptosis.
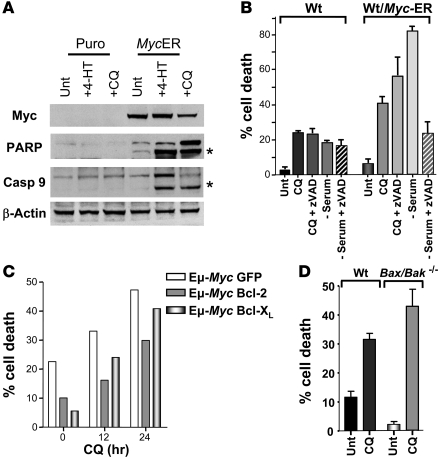
Previous reports suggested that CQ treatment might affect apoptosis (21, 22). Consistent with these data, we observed cleavage of caspase-9 and the caspase-3 substrate poly-ADP-ribose polymerase (PARP; Figure 4A) as well as increases in Annexin V staining (Supplemental Figure 3). Since CQ treatment induced obvious signs of apoptotic cell death in Myc-expressing MEFs, we tested whether cell death induced by CQ was indeed dependent on apoptosis. Interestingly, pretreatment with the broad-spectrum caspase inhibitors zVAD-fmk or qVD-fmk failed to affect the CQ-induced death of either wild-type or Myc-overexpressing MEFs (Figure 4B and data not shown). In contrast, as expected (23), caspase inhibition did block the death of Myc-expressing MEFs deprived of serum, which stimulates Myc-induced apoptosis (24, 25). Furthermore, CQ-induced death of Eμ-Myc B cells was not impaired by the overexpression of the antiapoptotic proteins Bcl-2 or Bcl-XL (Figure 4C), which potently inhibit Myc-induced apoptosis (26, 27). Apoptosis requires the concerted functions of the multidomain proapoptotic Bcl-2 family members Bax and Bak (28). However, Bax/Bak-deficient MEFs were also sensitive to CQ-induced cell death (Figure 4D). Therefore, though CQ induces a p53-dependent cell death, these results demonstrate that CQ can provoke cell death independent of classical apoptosis.
Figure 4. CQ induces hallmarks of apoptosis, yet can induce cell death independent of apoptosis.
(A) CQ induces hallmarks of apoptosis in Myc-expressing MEFs. Early passage MEFs were treated for 24 h with 4-HT to activate Myc-ERTAM and then treated with or without CQ (50 μM) for 24 h. Western blot analyses of the indicated cells demonstrated cleavage of caspase-9 and the caspase substrate PARP; asterisks indicate the specific cleavage products. (B) CQ-induced cell death is not inhibited by the broad-spectrum caspase inhibitor zVAD-fmk. Early passage MEFs were pretreated with zVAD-fmk for 1 h prior to being treated with CQ (50 μM) for 24 h. Myc-overexpressing MEFs were also pretreated with zVAD-fmk for 1 h and either serum starved (0.1%) or treated with CQ (50 μM) for a further 24 h. Viability was determined by propidium iodide staining. Results shown are the mean of 2 independent experiments. (C) CQ-induced cell death is not inhibited by Bcl-2 or Bcl-XL. Primary bone marrow B cell cultures derived from Eμ-Myc transgenic mice were infected with MSCV-IRES-GFP, MSCV–Bcl-2–IRES-GFP or MSCV–Bcl-XL–IRES–GFP retroviruses. GFP+ cells were isolated and expanded in culture and then were treated with CQ (50 μM) for 24 h. The percentage cell death was determined by staining cells with propidium iodide. By 24 h, CQ induced a 2.1-, 3.1-, or 7-fold increase in the death of GFP-, Bcl-2–, or Bcl-XL–expressing Eμ-Myc B cells, respectively. (D) CQ induces cell death in Bax/Bak–/– MEFs. Early passage MEFs were treated with or without CQ (50 μM) for 24 h. The percentage cell death was determined by propidium iodide incorporation. Results shown are the mean of 3 independent experiments.
CQ inhibits lysosomal protein degradation to induce cell death.
Autophagy is an evolutionarily ancient program that normally directs the turnover of long-lived proteins and typically enhances cell survival in times of starvation or other forms of stress (29, 30). It has been suggested that CQ can affect p53-dependent cell death by inhibiting autophagy (22, 31, 32). While some reports have suggested that CQ stimulates cell death by blocking the fusion of autophagosomes with lysosomes (21, 33, 34), other studies have suggested that CQ inhibits a later stage of autophagy by blocking degradation of cargo delivered to the lysosome (22).
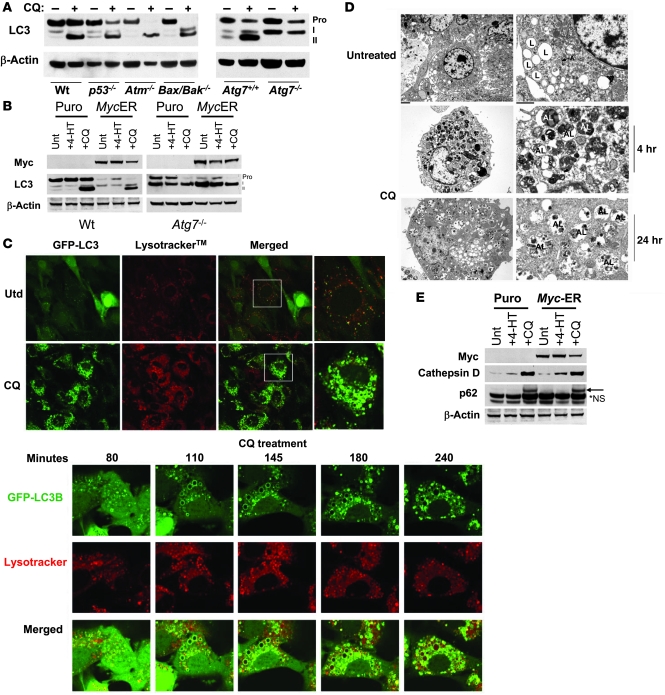
To investigate the effects of CQ on autophagy and whether autophagy was the mechanism of p53-dependent cell death induced by CQ in our model systems, we initially evaluated the effects of CQ on LC3, a conserved protein in the macroautophagy pathway that is necessary for the formation of autophagosomes. LC3 becomes modified in an ubiquitin-like reaction with phosphatidylethanolamine (PE) that utilizes the E1-like enzyme Atg7, which is essential for this modification and for autophagosome formation (35). PE-modified LC3 (LC3-II) can be distinguished from the pro-form of LC3 and from cleaved but unmodified LC3 (LC3-I) by gel electrophoresis, since LC3-II migrates most rapidly in denaturing polyacrylamide gels (36). Levels of LC3-II have thus been used to determine the abundance of autophagosomes (36, 37). Western blot analyses demonstrated that CQ induced a marked and rapid accumulation of LC3-II (within 2–4 hours; Supplemental Figure 4) in both primary wild-type MEFs and in p53-, Atm-, and Bax/Bak-deficient MEFs. Therefore, although CQ-induced death is p53 dependent, its ability to provoke this early hallmark of autophagy is p53 independent. In contrast, as expected, CQ failed to induce the formation of LC3-II in Atg7-deficient MEFs (Figure 5A). Furthermore, LC3-II was also detected in Myc-expressing wild-type MEFs treated with CQ, and again this modification failed to occur in Atg7-deficient MEFs engineered to overexpress Myc (Figure 5B).
Figure 5. CQ provokes markers of macroautophagy yet inhibits lysosomal functions.
(A) CQ induces the accumulation of PE-modified LC3. Early passage (p2) MEFs were treated with 50 μM CQ for 24 h. Expression and modification of LC3 (pro-form, LC3-I, and PE-modified LC3-II) was analyzed by western blot. (B) Indicated MEFs were treated for 24 h with 4-HT to activate Myc-ERTAM and with or without 50 μM CQ for 24 h. Expression and modification of LC3 were monitored by western blot analyses. (C) Top: GFP-LC3B–expressing MEFs were incubated with 100 nM Lysotracker for 30 min with or without CQ (50 μM) for 6 h. Cells were then imaged using a Nikon inverted confocal fluorescent microscope. Bottom: Time course analyses of CQ-induced changes in GFP-LC3B–expressing MEFs. Cells were incubated with 100 nM Lysotracker for 30 min, then treated with CQ (50 μM), followed by real-time video microscopy (see Supplemental Videos 1 and 2). Images were taken at the indicated times using a Nikon inverted confocal fluorescent microscope. Original magnification, ×63. (D) Wild-type early passage (p2) MEFs were treated with or without CQ (50 μM) for 4 h or 24 h. Cells were fixed with 2% (vol/vol) glutaraldehyde, and 1-μM sections were analyzed by transmission electron microscopy. Magnification, ×5,000. Scale bars: 1 μM. L, lysosome; A, autophagosome; AL, autophagolysosome. (E) CQ induces the accumulation of p62 and cathepsin D. MEFs were treated for 24 h with 4-HT to activate Myc-ERTAM and then treated with or without CQ for 24 h. Expression of p62 and cathepsin D was analyzed by western blot. *NS, nonspecific.
LC3 localizes to the membranes of autophagosomes following its modification with PE, and this can be monitored using a functional GFP-LC3B fusion protein (36). To further explore the effects of CQ on autophagy, MEFs were transduced with MSCV retrovirus expressing GFP-LC3B, GFP+ cells were expanded in culture, and the effects of CQ on LC3 localization was followed by time-lapse video microscopy. To monitor lysosomes, cells were also loaded with Lysotracker, a cell-permeable pH-dependent dye that becomes fluorescent in acidic functional lysosomes. Untreated cells demonstrated a mostly diffuse cytoplasmic staining of GFP-LC3 with very few punctuate autophagosomes and lysosomes (Figure 5C). In contrast, treatment of MEFs with CQ resulted in a rapid (2–4 hours) and massive induction of GFP-LC3–positive autophagosomes (Figure 5C). Further, CQ-treated cells displayed a marked increase in the number and size of lysosomes, which retained Lysotracker fluorescence, indicating they did not lose their acidic nature (Figure 5C).
We used real-time video microscopy to follow the fate of GFP-LC3–bound vesicles and observed that CQ induced the accumulation of large autophagosomes that then accumulated Lysotracker fluorescence. Although at 2–4 hours GFP-LC3–positive vesicles readily lost their GFP staining after the accumulation of Lysotracker fluorescence (indicating that GFP-LC3 was degraded), by 24 hours there were many vesicles that retained GFP staining and Lysotracker fluorescence, indicating that these lysosomes were only partially functional (Figure 5C and Supplemental Videos 1 and 2). Importantly, kinetic analyses of CQ-induced LC3-II generation (data not shown) and real-time video microscopy of GFP-LC3B (Supplemental Videos 1 and 2) suggested that CQ treatment initially induces aspects of the autophagy pathway, but that chronic exposure to CQ causes defects in lysosomal degradation, the final step in the pathway, which can also contribute to LC3-II accumulation. Finally, all of these effects of CQ were also independent of p53 (data not shown). Therefore, the p53 dependence of CQ-induced death is not due to overt effects of p53 on the ability of CQ to induce early hallmarks of macroautophagy, nor upon the ability of p53 to modify lysosomal functions. These results are also consistent with the more rapid kinetics of LC3 modification compared with p53 induction during CQ treatment (Supplemental Figure 4). Taken together, these observations suggest that p53 induction is a consequence, not a cause, of CQ-induced macroautophagy.
Electron microscopy confirmed that CQ treatment led to a massive accumulation of autophagosomes and the rapid delivery of cytosolic material to the lysosome (Figure 5D). At 4 hours, CQ induced the formation of numerous double-membrane autophagosomes and autophagolysosomes, consistent with the active fusion and delivery of cargo of autophagosomes with lysosomes (Figure 5D). CQ did not block the fusion of autophagosomes with lysosomes, as by 24 hours virtually all of vesicles in the cytosol were lysosomes, yet many of these still contained significant amounts of undegraded cytosolic material, indicating that lysosomal function was impaired by CQ. Similar findings were also evident in human diploid fibroblasts, Myc-expressing human fibroblasts, and HeLa cells, in which CQ treatment induced a marked accumulation of vesicles that included autophagosomes, autophagolysosomes, and lysosomes that contained partially degraded material (Supplemental Figures 5 and 6).
To assess the effects of CQ on protein degradation and lysosome function, we examined the effects of CQ on p62/SQSTM1, a protein recently shown to be uniquely degraded by autophagy pathway (38). Indeed, CQ treatment of MEFs and Myc-expressing MEFs led to a marked increase in the levels of p62 (Figure 5E), an effect that has been attributed to the inhibition of autophagy and protein degradation (38, 39). CQ is a weak base and has been reported to induce lysosomal membrane permeabilization and to provoke the release of neutral protease cathepsin D from lysosomes and the accumulation of cathepsin D in the cytosol (32, 40). Indeed, CQ induced marked increases in cathepsin D in both MEFs and Myc-expressing MEFs, an event that would impair lysosomal degradation of its cargo (Figure 5E).
Blockade of both apoptosis and autophagy impairs CQ-induced cell death.
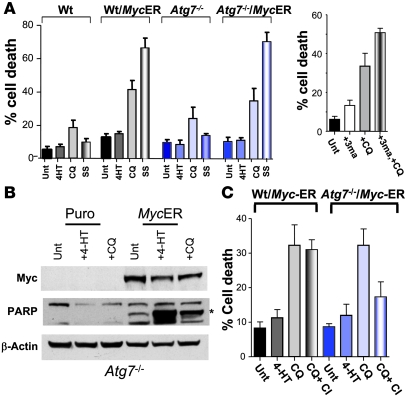
Collectively the effects of CQ indicated that it impaired the terminal steps of autophagic degradation. Since Atg7-deficient cells are defective in macroautophagy and these cells failed to display LC3-II modification following CQ treatment, we explored whether Atg7 loss spared Myc-expressing cells from CQ-induced death. Early passage wild-type or Atg7-deficient MEFs were infected with either control MSCV-puro– or MSCV–Myc-ERTAM–puro–expressing retroviruses. CQ treatment induced cell death in Myc-overexpressing Atg7-deficient cells as efficiently as that manifest in wild-type MEFs (Figure 6A). Similar results were also seen with the use of 3-methyladenine (3-MA; Figure 6A), a drug known to inhibit class III PI3K and a widely used inhibitor of autophagy (41, 42).
Figure 6. CQ-induced death is impaired by blockade of both apoptosis and autophagy.
(A) CQ can induce cell death in the absence of autophagy. Left: Early passage (p2) wild-type and Atg7-deficient MEFs expressing MSCV-IRES-puro or MSCV–IRES–Myc-ERTAM–puro retroviruses were treated for 24 h with 4-HT to activate Myc-ERTAM and were then left untreated or were treated with 50 μM CQ for 24 h or serum starved (SS; 0.1% FBS) for 24 h. The percentage cell death was determined by staining cells with propidium iodide. Results shown are the mean of 3 independent experiments. Right: Wild-type MEFs were treated with either 3-MA alone (2 mM) or in combination with CQ (50 μM) for 24 h. The percentage cell death was determined by staining cells with propidium iodide. Results shown are the mean of 3 independent experiments. (B) Blockade of autophagy does not prevent CQ from inducing hallmarks of apoptosis. Atg7-deficient MEFs expressing MSCV-IRES-puro or MSCV–IRES–Myc-ERTAM–puro retroviruses were treated for 24 h with 4-HT to activate Myc-ERTAM and were then left untreated or were treated with 50 μM CQ for 24 h. Cleavage of the caspase-3 substrate PARP was detected by western blot analysis in Myc-expressing Atg7-deficient MEFs. The asterisk indicates the specific cleavage product. (C) CQ-induced cell death is prevented by the combined blockade in autophagy and apoptosis. Early passage (p2) wild-type and Atg7-deficient MEFs expressing the MSCV–IRES–Myc-ERTAM–puro retrovirus were pretreated with qVD-fmk (CI; 20 μM) for 1 h prior to being treated with CQ (50 μM) for 24 h. Viability was determined by propidium iodide staining. Results shown are the mean of 3 independent experiments.
The effects of CQ in MEFs (Figure 4A), as well as the recent studies of others (21, 22), established that CQ treatment can induce hallmarks of apoptosis. To test whether the effects of CQ on autophagy were required for the apoptotic response, Atg7-deficient MEFs and Myc-expressing Atg7–/– MEFs were treated with CQ and assessed for markers of apoptosis. Loss of Atg7 had no effect on the cleavage of PARP in CQ-treated cells (Figure 6B and data not shown) nor upon increases in Annexin V staining in CQ-treated wild-type versus Atg7-deficient MEFs (data not shown). Therefore, the apoptotic response induced by CQ is independent of the autophagy pathway. Since CQ treatment could override the prosurvival effects of inhibitors of apoptosis (Figure 3, B and C) and the requirement for Bax and Bak (Figure 3D), we wondered whether the combined inhibition of both apoptosis and autophagy might impair CQ-induced death. To test this notion, 4-HT–treated wild–type and Atg7-deficient MEFs engineered to overexpress Myc-ERTAM were pretreated with the broad-spectrum caspase inhibitor qVD-fmk for 1 hour and then were treated for 24 hours with CQ. Interestingly, despite evidence of cytoplasmic vacuolization (data not shown), CQ-induced cell death was significantly reduced by caspase inhibition in Atg7-deficient cells (Figure 6C). Taken together, these results suggest that CQ kills Myc-overexpressing cells by blocking the end-stage of autophagy, namely lysosomal protein degradation, and that this induces a p53-dependent apoptotic cell death. In the absence of apoptosis, Myc-overexpressing cells can still succumb to death following CQ treatment by switching to a caspase-independent death. However, if both autophagy and apoptosis are blocked, CQ can no longer effectively kill.
Discussion
There are very few agents that have a proven ability to prevent cancer, and their mechanisms of action are often obscure. We reasoned that possible agents would include those that augment DNA damage response pathways, particularly since DNA damage is evident in the precancerous state, where it likely promotes additional mutations that give rise to frank malignancies (43). We focused our chemoprevention studies on the antimalarial drug CQ, which can activate Atm and p53 in the absence of detectable DNA damage (12) and which is tolerated long term in patients suffering from malaria, with few side effects.
Previous studies have highlighted the importance of Atm as a guardian against Myc-induced tumorigenesis (9–11); hence we asked whether CQ could affect Myc-induced lymphoma development. Indeed, administration of CQ to mice once every 5 days dramatically reduced lymphoma development in Eμ-Myc transgenic mice. Since Eμ-Myc mice are often regarded as a murine model for human Burkitt lymphoma (44), this suggested that CQ could be a useful drug in preventing this disorder. Intriguingly, an epidemiologic trial supports this notion. Specifically, in an attempt to investigate whether malaria was a causative agent in the high incidence of Burkitt lymphoma in equatorial Africa, a trial was performed in which CQ was provided to a large section of the country of Tanzania for 5 years, with instructions for children to take 1 dose every 2 weeks (45). During the time period of the study, the incidence of endemic Burkitt lymphoma in the region decreased by approximately 75%, and the tumor incidence then returned to the old baseline within 2 years after completion of the trial. The striking tumor prevention effects of CQ were not further explored at the time, since the major hypothesis of the study, a linkage between malaria incidence and Burkitt lymphoma, was not adequately supported by the results. However, the profound effects we observed in the Eμ-Myc mice treated with CQ now provide a different perspective on the results of this epidemiologic study and suggest that CQ may have been a useful prevention/therapeutic agent in this disease, even as a single agent given once every 2 weeks.
Exploring the mechanism of CQ action provided some unexpected and intriguing insights. At the doses of CQ used, which are similar to those used to prevent malaria, a p53-dependent pathway and increased cell death were observed. Our studies and those of Thompson and colleagues (22) have indicated that in the context of Myc overexpression, CQ induces an apoptotic cell death resulting from ineffective autophagic protein degradation and partial lysosomal permeabilization. Autophagy is a complex adaptive cellular response that enhances cell survival in the face of nutrient limitation or other cellular stresses that include protein accumulation (29, 46). Some studies have suggested that CQ enhances cell death by blocking fusion of autophagosomes with lysosomes (33, 34) while others suggest that it functions as a lysosomotropic agent by inhibiting the acid-dependent degradation of autophagosome cytosolic contents, resulting in an accumulation of autophagic vesicles that cannot be cleared (22, 47, 48). Our results are consistent with the latter mechanism. It is noteworthy that Myc expression itself also affected the autophagic response with respect to LC3 modification by PE. In driving cell growth and proliferation, we can speculate that Myc affects autophagy as a mechanism to deal with increased metabolism.
An intriguing result from our study was that CQ was effective at killing Myc-overexpressing cells even in the presence of caspase inhibition or overexpressed Bcl-2 or Bcl-XL, which prevent Myc-induced apoptosis (26, 27). Even more striking, CQ was also effective at killing MEFs lacking both Bax and Bak, which together are required for all forms of apoptosis (28, 49). The death in Bax/Bak-deficient cells from CQ treatment may result from the fact that they retain a normal lysosomal membrane permeabilization despite failing to undergo mitochondrial membrane permeabilization (50). Regardless, the result represents an important consideration in the treatment of cancers in which the apoptotic pathway is disabled, particularly advanced follicular lymphoma, which is driven by Bcl-2 and Myc overexpression (51).
It appears that CQ typically facilitates apoptotic cell death in Myc-overexpressing cells via lysosomal changes. Importantly, however, CQ-induced lysosomal changes can lead to a p53-dependent cell death in the absence of apoptosis, though blockade of both apoptosis and autophagy abrogates CQ-induced cell death (Figure 5). Recent studies have highlighted a role for p53 in regulating autophagy, perhaps through effects on the lysosomal protein DRAM or on the AMPK/TSC1/TSC2 signaling pathway (52, 53). Our study indicates that CQ inhibits a late step in autophagy. Therefore, while triggers of the autophagy pathway, such as genotoxic stress and starvation, may play a role in cell survival (52), others (e.g., CQ) that derail the autophagy pathway and result in ineffective clearance are toxic and can induce a cell death response that is dependent upon p53. While CQ clearly affects events at the lysosome, the fact that it can induce p53-dependent cell death independent of either apoptosis or autophagy, but not both, suggests the involvement of other signaling pathways and p53 targets. Interestingly, a recent study suggests that protection from caspase-independent cell death reflects an increase in, and a dependence upon, autophagy (54). Our results suggest that autophagy is not required for cell survival following caspase inhibition; however, since cells still vesiculate in the presence of CQ under defective apoptosis and autophagic conditions, it is possible that they ultimately would succumb to death.
CQ was also effective in reducing spontaneous tumor formation in mice lacking Atm, a finding that potentially has significant implications for patients with the cancer-prone disorder AT. First, it appears that CQ may be useful in the prevention or perhaps treatment of lymphomas that arise in AT patients. Malignancies that arise in AT patients are particularly difficult to treat because of the special sensitivities of AT patients to cytotoxic agents used to treat cancer. CQ provides a novel consideration for treatment of lymphomas in AT patients and has the advantage that it is not typically cytotoxic and may thus be relatively well tolerated. Further, Atm-deficient mice treated with CQ on a weekly basis had no apparent side effects from the chronic intermittent treatment, and the therapeutic benefit might even be greater on a different dosage schedule or in combination with other agents (32). In addition to cancer predisposition, patients with AT also exhibit neurodegeneration, immunologic abnormalities, insulin resistance, abnormal intracellular redox control, and signs of premature aging (55). Interestingly, abnormalities in autophagy and lysosomal function have been linked to all of these processes (56, 57), raising the intriguing possibility that some or all of the clinical abnormalities in the AT disorder could be linked to alterations in autophagy regulation. Such possibilities provide fertile areas for continued study and potential novel opportunities for treatment or prevention of the many tragic clinical features of this disease.
Collectively, the data presented herein suggest that disruption of lysosomal function by CQ provides a potentially useful therapeutic intervention in certain cancer settings. The apparently increased sensitivity of oncogene-expressing cells to CQ treatment would provide a natural therapeutic index. The challenge will be to figure out how to optimally use this approach in the prevention and/or treatment of clinical disease. Questions of optimal dose and schedule, use in combination with cytotoxic or biologic agents, and appropriate genetic background or histopathology of the tumor will all need to be addressed. Perhaps most important is the fact that these studies provide proof of principle for developing antitumor therapies based on the modulation of autophagic pathways and opportunities for novel drug discovery, whether based on CQ itself or targeting other steps in the pathway.
Methods
Mice and tumor analyses.
To evaluate the efficacy of CQ as a chemopreventative agent, Atm-null, p53-null (C57BL/6J background; The Jackson Laboratory) or Eμ-Myc transgenic mice (C57BL/6J) were injected every 5 days, starting at the age of 4 weeks, with either PBS or 3.5 mg/kg CQ (Sigma-Aldrich). All mice were observed daily for signs of morbidity and the development of lymphoma and analyzed weekly for their differential blood counts by retroorbital bleeds. Atg7+/– mice were generated from Atg7flox/flox mice (generously provided by Keiji Tanaka and Masaaki Komatsu, Tokyo Metropolitan Institute of Medical Science, Bunkyo-ku, Tokyo, Japan; ref. 35) by crossing a male ATG7flox/flox mouse to a female EIIaCre deleter strain. This Cre recombinase–expressing knockin mouse model specifically expresses high levels of Cre recombinase in oocytes (58). The resulting heterozygous F1s were then identified by PCR for the loss of the floxed Atg7 allele using the oligonucleotides 5′-TGGCTGCTACTTCTGCATGATGT-3′ and 5′-TTAGCACAGGGAACAGCGCTCATGG-3′. Atg7+/– mice were then backcrossed to C57BL/6J mice to segregate the Atg7+/– allele from the EIIaCre allele. Finally, to generate paired Atg7+/+ and Atg7–/– MEFs, timed matings of Atg7+/– mice were performed and the embryos were collected on E13.5.
Cell culture.
Wild-type, p53–/–, Arf–/–, and Atm–/– E13.5 MEFs were prepared and cultured as previously described (17, 18). Early passage (p2) Bax/Bak–/– MEFs were kindly provided by Stanley Korsmeyer (Howard Hughes Medical Institute, Dana-Farber Cancer Institute, Boston, Massachusetts, USA). Early passage (p2) Atg7+/+ and Atg7–/– MEFs were cultured as described (35). Retroviral infections were performed as previously described (2, 18). Primary bone marrow–derived pre–B cell cultures were generated from 6-week-old C57BL/6 wild-type or Eμ-Myc transgenic mice, as previously described (59). B cells were maintained in culture on S17 stromal cells (kindly provided by Kenneth Dorshkind, University of California, Riverside, California, USA) in medium containing IL-7 (10 U/ml), as previously described (59). HeLa cells, 1070SK normal human foreskin fibroblasts (ATCC), and HCT116 and p53–/–-HCT116 colon cancer cells (kindly provided by Bert Vogelstein, Johns Hopkins University, Baltimore, Maryland, USA) were all maintained in DMEM culture medium containing 10% FBS and 1% penicillin/streptomycin.
zVAD-fmk and qVD-fmk (MP Biomedicals) were dissolved as 100-mM stock solutions in DMSO. Prior to serum starvation (0.1% FBS), cells were treated for 1 h with either zVAD (50 μM) or qVD (20 μM) before addition of CQ (50 μM; Sigma-Aldrich). 3-MA (Fluka) was used alone (2 mM) or in combination with CQ (50 μM).
Electron microscopy.
Cells were left untreated or were treated with CQ (50 μM) and collected by centrifugation, washed in PBS, and fixed using 2% phosphate-buffered glutaraldehyde. Samples were then postfixed with 1% osmium tetroxide, embedded in Spurr, and sectioned. The sections were stained with uranyl acetate and lead citrate and viewed in a JEOL 1200 electron microscope.
FACS and magnetic-activated cell sorting of B cells.
To obtain bone marrow and splenic B cells, single-cell suspensions were prepared, followed by a red cell lysis using an ammonium chloride/potassium bicarbonate solution. Cell suspensions were then incubated with B220 microbeads and enriched by magnetic-activated cell sorting (MACS) according to the manufacturer’s instructions (Miltenyi Biotech). Rates of proliferation of B220+ cells were determined by using a flow kit according to the manufacturer’s instructions (BD Biosciences — Pharmingen). Briefly, animals were injected i.p. with 100 ml of 10 mg/ml BrdU in sterile PBS. Animals were sacrificed 12 h after injection, and B220+ cells from bone marrow and spleen were harvested. Cells (1 × 106) were used for the BrdU proliferation assays, and 5 × 105 cells were stained with either propidium iodide (Sigma-Aldrich) alone for viability analyses or with propidium iodide and Annexin V FITC antibody (Roche Applied Sciences) for apoptosis analyses, as previously described (18). Following incubation, cells were washed, resuspended in PBS, and analyzed by FACS.
Immunoblotting analyses.
MACS-sorted B cells and MEFs were lysed in RIPA buffer and proteins quantified using a BCA assay (Pierce Biotechnology). Proteins (25 or 50 mg/lane) were electrophoretically separated on 4%–12% SDS-PAGE gels (Invitrogen), transferred to membranes (Protran; Schleicher and Schuell), and blotted with antibodies specific for β-actin, Puma (Sigma-Aldrich), c-Myc, p62 (Santa Cruz Biotechnology Inc.), p53 (BD Biosciences — Pharmingen; IC12, Cell Signaling), p19Arf (kindly provided by Charles Sherr, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA), cathepsin D (R&D Systems), and LC3 (polyclonal antibody raised in rabbits). Following incubation with primary antibodies, the blots were then incubated with appropriate anti-mouse, anti-rat, or anti-rabbit immunoglobulin secondary antibodies (Amersham). Bound immunocomplexes were detected by enhanced chemiluminescence (Amersham) or ECL SuperSignal (Pierce Biotechnology).
Immunofluorescence analyses.
For analysis of GFP-LC3B, cells were plated onto MatPek glass bottom dishes 24 h before the start of the experiment. Cells were then labeled with 1 nM Lysotracker (Invitrogen) for 30 min at 37°C, 9% CO2. After labeling, cells were washed 3 times with prewarmed cell culture media and then left untreated or treated with CQ. The images were then collected using a Nikon C1SI with an In Vivo Scientific CO2/temperature-controlled chamber at different time intervals.
Statistics.
Means were compared using the χ2 test. All data analyses were performed using GraphPad Prism curve comparisons.
Supplementary Material
Acknowledgments
We thank Charles Sherr and Martine Roussel (St. Jude Children’s Research Hospital [SJCRH]) for providing p19Arf antibody; Peter McKinnon (SJCRH) for providing Atm+/– mice; Keiji Tanaka for providing the Atg7flox/flox mice; Chunying Yang, Elsie White, Diane Woods, Heather Bailey, Margaret Reis, Pamela Freire, Simon Moshiach, and Silvia Coenen for outstanding technical assistance; Darren Phillips and Stephen Tait for critical review of the manuscript; Linda Mann and Gopal Murti for assistance with electron microscopy; and the staff of the Core Services of the SJCRH FACS facility and the Hartwell Center of SJCRH. This work was supported by National Cancer Institute grants CA76379 (to J.L. Cleveland), CA71387 and ES05777 (M.B. Kastan), the Cancer Center (CORE) support grant CA21765, and the American Lebanese Syrian Associated Charities (ALSAC) of SJCRH.
Footnotes
Nonstandard abbreviations used: AT, ataxia telangiectasia; CQ, chloroquine; ER, estrogen receptor; 4-HT, 4-hydroxytamoxifen; 3-MA, 3-methyladenine; MEF, mouse embryonic fibroblast; PARP, poly-ADP-ribose polymerase; PE, phosphatidylethanolamine.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:79–88 (2008). doi:10.1172/JCI33700
Kirsteen H. Maclean and Frank C. Dorsey contributed equally to this work.
See the related Commentary beginning on page 15.
References
- 1.Lowe S.W., Sherr C.J. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr. Opin. Genet. Dev. 2003;13:77–83. doi: 10.1016/s0959-437x(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 2.Eischen C.M., Weber J.D., Roussel M.F., Sherr C.J., Cleveland J.L. Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 1999;13:2658–2669. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitt C.A., McCurrach M.E., de Stanchina E., Wallace-Brodeur R.R., Lowe S.W. INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 1999;13:2670–2677. doi: 10.1101/gad.13.20.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giaccia A.J., Kastan M.B. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 1998;12:2973–2983. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- 5.Kastan M.B., Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 6.Siliciano J.D., et al. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 1997;11:3471–3481. doi: 10.1101/gad.11.24.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canman C.E., et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 8.Tibbetts R.S., et al. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev. 1999;13:152–157. doi: 10.1101/gad.13.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pusapati R.V., et al. ATM promotes apoptosis and suppresses tumorigenesis in response to Myc. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1446–1451. doi: 10.1073/pnas.0507367103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shreeram S., et al. Regulation of ATM/p53-dependent suppression of myc-induced lymphomas by Wip1 phosphatase. J. Exp. Med. 2006;203:2793–2799. doi: 10.1084/jem.20061563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maclean K.H., Kastan M.B., Cleveland J.L. Atm deficiency affects both apoptosis and proliferation to augment myc-induced lymphomagenesis. Mol. Cancer Res. 2007;5:705–711. doi: 10.1158/1541-7786.MCR-07-0058. [DOI] [PubMed] [Google Scholar]
- 12.Bakkenist C.J., Kastan M.B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 13.Kitagawa R., Bakkenist C.J., McKinnon P.J., Kastan M.B. Phosphorylation of SMC1 is a critical downstream event in the ATM-NBS1-BRCA1 pathway. Genes Dev. 2004;18:1423–1438. doi: 10.1101/gad.1200304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langdon W.Y., Harris A.W., Cory S., Adams J.M. The c-myc oncogene perturbs B lymphocyte development in E-mu-myc transgenic mice. Cell. 1986;47:11–18. doi: 10.1016/0092-8674(86)90361-2. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson J.A., et al. Targeting ornithine decarboxylase in Myc-induced lymphomagenesis prevents tumor formation. Cancer Cell. 2005;7:433–444. doi: 10.1016/j.ccr.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 16.Littlewood T.D., Hancock D.C., Danielian P.S., Parker M.G., Evan G.I. A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res. 1995;23:1686–1690. doi: 10.1093/nar/23.10.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zindy F., et al. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maclean K.H., et al. c-Myc augments gamma irradiation-induced apoptosis by suppressing Bcl-X(L). Mol. Cell. Biol. 2003;23:7256–7270. doi: 10.1128/MCB.23.20.7256-7270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barlow C., et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 20.Harvey M., McArthur M.J., Montgomery C.A., Jr., Bradley A., Donehower L.A. Genetic background alters the spectrum of tumors that develop in p53-deficient mice. FASEB J. 1993;7:938–943. doi: 10.1096/fasebj.7.10.8344491. [DOI] [PubMed] [Google Scholar]
- 21.Boya P., et al. Inhibition of macroautophagy triggers apoptosis. Mol. Cell. Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amaravadi R.K., et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J. Clin. Invest. 2007;117:326–336. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juin P., Hueber A.O., Littlewood T., Evan G. c-Myc-induced sensitization to apoptosis is mediated through cytochrome c release. Genes Dev. 1999;13:1367–1381. doi: 10.1101/gad.13.11.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Askew D.S., Ashmun R.A., Simmons B.C., Cleveland J.L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991;6:1915–1922. [PubMed] [Google Scholar]
- 25.Evan G.I., et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 26.Fanidi A., Harrington E.A., Evan G.I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Proc. Natl. Acad. Sci. U. S. A. 1992;59:554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- 27.Bissonnette R.P., Echeverri F., Mahboubi A., Green D.R. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992;359:552–554. doi: 10.1038/359552a0. [DOI] [PubMed] [Google Scholar]
- 28.Lindsten T., et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine B., Klionsky D.J. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 30.Ferraro E., Cecconi F. Autophagic and apoptotic response to stress signals in mammalian cells. Arch. Biochem. Biophys. 2007;462:210–219. doi: 10.1016/j.abb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Zaidi A.U., et al. Chloroquine-induced neuronal cell death is p53 and Bcl-2 family-dependent but caspase-independent. J. Neuropathol. Exp. Neurol. 2001;60:937–945. doi: 10.1093/jnen/60.10.937. [DOI] [PubMed] [Google Scholar]
- 32.Carew J.S., et al. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood. 2007;110:313–322. doi: 10.1182/blood-2006-10-050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paludan C., et al. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 34.Shacka J.J., et al. Bafilomycin A1 inhibits chloroquine-induced death of cerebellar granule neurons. Mol. Pharmacol. 2006;69:1125–1136. doi: 10.1124/mol.105.018408. [DOI] [PubMed] [Google Scholar]
- 35.Komatsu M., et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kabeya Y., et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizushima N., et al. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J. Cell Biol. 2001;152:657–668. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pankiv S., et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 39.Bjorkoy G., et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoka V., Turk V., Turk B. Lysosomal cysteine cathepsins: signaling pathways in apoptosis. Biol. Chem. 2007;388:555–560. doi: 10.1515/BC.2007.064. [DOI] [PubMed] [Google Scholar]
- 41.Seglen P.O., Gordon P.B. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc. Natl. Acad. Sci. U. S. A. 1982;79:1889–1892. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petiot A., Ogier-Denis E., Blommaart E.F., Meijer A.J., Codogno P. Distinct classes of phosphatidylinositol 3ι-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J. Biol. Chem. 2000;275:992–998. doi: 10.1074/jbc.275.2.992. [DOI] [PubMed] [Google Scholar]
- 43.Bartek J., Lukas J. DNA damage checkpoints: from initiation to recovery or adaptation. Curr. Opin. Cell Biol. 2007;19:238–245. doi: 10.1016/j.ceb.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Adams J.M., et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- 45.Geser A., Brubaker G., Draper C.C. Effect of a malaria suppression program on the incidence of African Burkitt’s lymphoma. Am. J. Epidemiol. 1989;129:740–752. doi: 10.1093/oxfordjournals.aje.a115189. [DOI] [PubMed] [Google Scholar]
- 46.Ohsumi Y. Molecular dissection of autophagy: two ubiquitin-like systems. Nat. Rev. Mol. Cell Biol. 2001;2:211–216. doi: 10.1038/35056522. [DOI] [PubMed] [Google Scholar]
- 47.Poole B., Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J. Cell Biol. 1981;90:665–669. doi: 10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glaumann H., Ahlberg J. Comparison of different autophagic vacuoles with regard to ultrastructure, enzymatic composition, and degradation capacity — formation of crinosomes. Exp. Mol. Pathol. 1987;47:346–362. doi: 10.1016/0014-4800(87)90018-9. [DOI] [PubMed] [Google Scholar]
- 49.Wei M.C., et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boya P., et al. Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene. 2003;22:3927–3936. doi: 10.1038/sj.onc.1206622. [DOI] [PubMed] [Google Scholar]
- 51.Vaux D.L., Cory S., Adams J.M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 52.Feng Z., Zhang H., Levine A.J., Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc. Natl. Acad. Sci. U. S. A. 2005;102:8204–8209. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crighton D., et al. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126:121–134. doi: 10.1016/j.cell.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 54.Colell A., et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007;129:983–997. doi: 10.1016/j.cell.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 55.Shiloh Y., Kastan M.B. ATM: genome stability, neuronal development, and cancer cross paths. Adv. Cancer Res. 2001;83:209–254. doi: 10.1016/s0065-230x(01)83007-4. [DOI] [PubMed] [Google Scholar]
- 56.Kundu M., Thompson C.B. Macroautophagy versus mitochondrial autophagy: a question of fate? Cell Death Differ. 2005;12(Suppl. 2):1484–1489. doi: 10.1038/sj.cdd.4401780. [DOI] [PubMed] [Google Scholar]
- 57.Kondo Y., Kanzawa T., Sawaya R., Kondo S. The role of autophagy in cancer development and response to therapy. Nat. Rev. Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 58.Williams-Simons L., Westphal H. EIIaCre — utility of a general deleter strain. Transgenic Res. 1999;8:53–54. doi: 10.1023/a:1008994831937. [DOI] [PubMed] [Google Scholar]
- 59.Eischen C.M., Woo D., Roussel M.F., Cleveland J.L. Apoptosis triggered by Myc-induced suppression of Bcl-X(L) or Bcl-2 is bypassed during lymphomagenesis. Mol. Cell. Biol. 2001;21:5063–5070. doi: 10.1128/MCB.21.15.5063-5070.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.