Abstract
The reactivities of mononuclear nonheme iron(IV)–oxo complexes bearing different axial ligands, [FeIV(O)(TMC)(X)]n+ [where TMC is 1,4,8,11-tetramethyl-1,4,8,11-tetraazacyclotetradecane and X is NCCH3 (1-NCCH3), CF3COO− (1-OOCCF3), or N3− (1-N3)], and [FeIV(O)(TMCS)]+ (1′-SR) (where TMCS is 1-mercaptoethyl-4,8,11-trimethyl-1,4,8,11-tetraazacyclotetradecane), have been investigated with respect to oxo-transfer to PPh3 and hydrogen atom abstraction from phenol O H and alkylaromatic C
H and alkylaromatic C H bonds. These reactivities were significantly affected by the identity of the axial ligands, but the reactivity trends differed markedly. In the oxidation of PPh3, the reactivity order of 1-NCCH3 > 1-OOCCF3 > 1-N3 > 1′-SR was observed, reflecting a decrease in the electrophilicity of iron(IV)–oxo unit upon replacement of CH3CN with an anionic axial ligand. Surprisingly, the reactivity order was inverted in the oxidation of alkylaromatic C
H bonds. These reactivities were significantly affected by the identity of the axial ligands, but the reactivity trends differed markedly. In the oxidation of PPh3, the reactivity order of 1-NCCH3 > 1-OOCCF3 > 1-N3 > 1′-SR was observed, reflecting a decrease in the electrophilicity of iron(IV)–oxo unit upon replacement of CH3CN with an anionic axial ligand. Surprisingly, the reactivity order was inverted in the oxidation of alkylaromatic C H and phenol O
H and phenol O H bonds, i.e., 1′-SR > 1-N3 > 1-OOCCF3 > 1-NCCH3. Furthermore, a good correlation was observed between the reactivities of iron(IV)–oxo species in H atom abstraction reactions and their reduction potentials, Ep,c, with the most reactive 1′-SR complex exhibiting the lowest potential. In other words, the more electron-donating the axial ligand is, the more reactive the iron(IV)–oxo species becomes in H atom abstraction. Quantum mechanical calculations show that a two-state reactivity model applies to this series of complexes, in which a triplet ground state and a nearby quintet excited-state both contribute to the reactivity of the complexes. The inverted reactivity order in H atom abstraction can be rationalized by a decreased triplet-quintet gap with the more electron-donating axial ligand, which increases the contribution of the much more reactive quintet state and enhances the overall reactivity.
H bonds, i.e., 1′-SR > 1-N3 > 1-OOCCF3 > 1-NCCH3. Furthermore, a good correlation was observed between the reactivities of iron(IV)–oxo species in H atom abstraction reactions and their reduction potentials, Ep,c, with the most reactive 1′-SR complex exhibiting the lowest potential. In other words, the more electron-donating the axial ligand is, the more reactive the iron(IV)–oxo species becomes in H atom abstraction. Quantum mechanical calculations show that a two-state reactivity model applies to this series of complexes, in which a triplet ground state and a nearby quintet excited-state both contribute to the reactivity of the complexes. The inverted reactivity order in H atom abstraction can be rationalized by a decreased triplet-quintet gap with the more electron-donating axial ligand, which increases the contribution of the much more reactive quintet state and enhances the overall reactivity.
Keywords: biomimetics, high-valent iron–oxo intermediate, nonheme iron enzymes, oxygen activation
High-valent iron(IV)–oxo species have been implicated as the key reactive intermediates in the catalytic cycles of oxygen-activating iron enzymes (1–6). In heme enzymes, proximal ligands are proposed to tune the reactivity of the iron(IV)–oxo porphyrin π-cation radical intermediates, collectively designated as Compounds I (Cpd I) (7, 8). In particular, the role of the axial cysteinate ligand of cytochrome P450 has received much attention because of the enzyme's ability to hydroxylate unactivated C H bonds (7–10). In recent reports, Ogliaro et al. (10) and Green and coworkers (11–14) demonstrated that the thiolate ligand in closely related chloroperoxidase increases the basicity of the iron(IV)–oxo moiety such that the Fe–O unit becomes protonated at neutral pH upon reduction by one electron to the Cpd II state. Green speculated that this increased basicity is a strategy that allows hydrogen atom abstraction by Cpd I to occur at a lower redox potential so that the surrounding enzyme environment can be protected from oxidative destruction.
H bonds (7–10). In recent reports, Ogliaro et al. (10) and Green and coworkers (11–14) demonstrated that the thiolate ligand in closely related chloroperoxidase increases the basicity of the iron(IV)–oxo moiety such that the Fe–O unit becomes protonated at neutral pH upon reduction by one electron to the Cpd II state. Green speculated that this increased basicity is a strategy that allows hydrogen atom abstraction by Cpd I to occur at a lower redox potential so that the surrounding enzyme environment can be protected from oxidative destruction.
What is required to test this intriguing hypothesis is more direct experimental evidence that systematically shows the effect of axial ligands on the reactivity of a high-valent iron–oxo species. Although there are several studies demonstrating that axial ligands affect the reactivities of iron(IV)–oxo centers in biomimetic Cpd I analogs (15, 16), only one of these studies reported the effect of a range of ligands on reaction rates (15), but no clear correlation between reactivity and some property of the axial ligand was discerned. More recently, with the synthesis of the first nonheme iron(IV)–oxo complex, [FeIV(O)(TMC)(NCCH3)]2+ (where TMC is 1,4,8,11-tetramethyl-1,4,8,11-tetraazacyclotetradecane) (17), we found that the axial CH3CN ligand can be replaced with anions and that these anions affected the reactivity of the iron(IV)–oxo unit (18–20). In this article, we report a systematic study that documents a dramatic axial ligand effect on the ability of a nonheme iron(IV)–oxo unit to carry out oxygen atom transfer to PPh3 versus its ability to effect hydrogen atom abstraction of C H and O
H and O H bonds. These observations are interpreted in light of theoretical results that shed light on how high-valent iron(IV)–oxo species can activate C
H bonds. These observations are interpreted in light of theoretical results that shed light on how high-valent iron(IV)–oxo species can activate C H bonds.
H bonds.
Results and Discussion
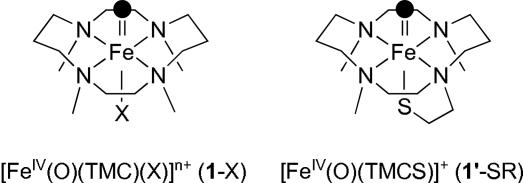
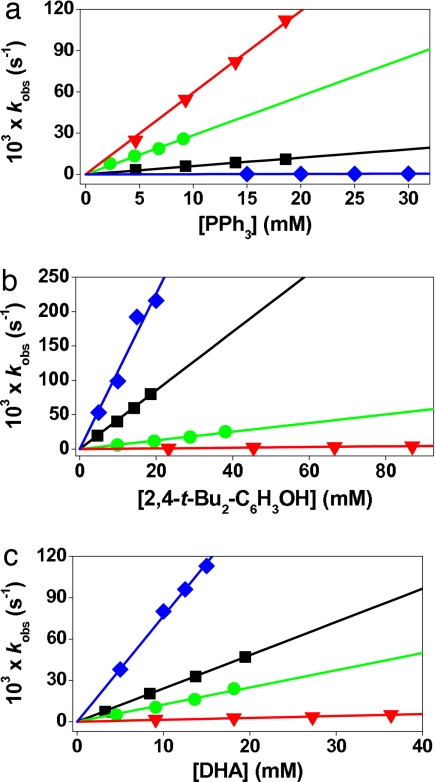
Mononuclear nonheme iron(IV)–oxo complexes bearing different axial ligands, [FeIV(O)(TMC)(X)]n+ (1-X) [where X is NCCH3 (1-NCCH3), CF3COO− (1-OOCCF3), or N3− (1-N3)] (see the structure in Fig. 1), were prepared by reacting their iron(II) complexes, [FeII(TMC)(X)]n+, with 1.2 eq of PhIO in CH3CN at 25°C and characterized as previously reported (17–19). Upon addition of PPh3 to the solutions, the intermediates reverted back to the starting iron(II) complexes [supporting information (SI) Fig. 7], yielding Ph3PO quantitatively. Second-order rate constants extracted from these experiments afforded the reactivity order of 1-NCCH3 > 1-OOCCF3 > 1-N3 (Table 1 and Fig. 2a), suggesting that the axial ligand modulates the reactivity of the Fe O unit in oxygen atom transfer in a systematic fashion.
O unit in oxygen atom transfer in a systematic fashion.
Fig. 1.
Schematic structures of [FeIV(O)(TMC)(X)]n+ (1-X) and [FeIV(O)(TMCS)]+ (1′-SR).
Table 1.
Second-order rate constants and KIE values determined in substrate oxidations by [FeIV(O)(TMC)(X)]n+ (1-X) and [FeIV(O)(TMCS)]+ (1′-SR)
| Substrate | C H BDE,† kcal/mol H BDE,† kcal/mol |
k2, M−1·s−1 |
|||
|---|---|---|---|---|---|
| 1-NCCH3 | 1-OOCCF3 | 1-N3 | 1′-SR | ||
| PPh3 | N/A | 5.9 | 2.9 | 0.61 | [0.016] |
| 2,4-t-Bu2C6H3OH | N/A | 0.050 | 0.63 | 4.3 | [12] |
| Xanthene | 75.5 | 0.39 | 7.6 | 9.6 | |
| (KIE)‡ | (16) | (20) | (17) | ||
| DHA | 77 | 0.14 [0.20] | 1.3 | 2.4 [4.9] | [7.5] |
| (KIE)‡ | (10) | (19) | (17) | ||
| CHD | 78 | 0.12 | 1.2 | 1.4 | |
| Fluorene | 80 | n.d.§ | 0.051 | 0.15 | |
| Ep,c in CH3CN at 25°C | −0.32 | −0.50 | −0.60 | ||
| (1:1 CH3CN/CH3OH at −30°C) | (−0.44) | (−0.62) | (−0.66) | (−1.00) | |
Rate constants were determined at 0°C in CH3CN for 1-X or 1:1 CH3CN/MeOH for 1′-SR. Square brackets designate values obtained in 1:1 CH3CN/CH3OH at 0°C. The spectral changes were monitored at 820 nm for 1-NCCH3, 835 nm for 1-OOCCF3 and 850 nm for 1-N3 and 1′-SR. Rate constants are averaged by three determinations, and standard deviation is <10% of the given values. N/A, not applicable.
‡KIE values were determined at 25°C for 1-NCCH3 and at 0°C for 1-OOCCF3 and 1-N3. See SI Table 3 for kobs constants determined in the reactions.
§Not determined due to the low reactivity of the intermediates in the oxidation of fluorene.
Fig. 2.
Second-order rate constants determined in the reactions of [FeIV(O)(TMC)(X)]n+ (0.5 mM) at 0°C with PPh3 (a), 2,4-t-Bu2C6H3OH (b), and DHA (c). Blue diamonds indicate 1′-SR; black squares indicate 1-N3; green circles indicate 1-OOCCF3; and red inverted triangles indicate 1-NCCH3.
The effect of the axial ligands on hydrogen atom abstraction by 1-X also was investigated in the oxidation of C H and O
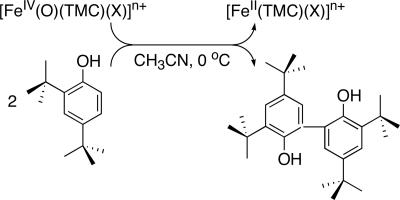
H and O H bonds. Addition of 2,4,6-tri-tert-butylphenol to 1-X caused the decay of the intermediates with the concurrent formation of the corresponding phenoxyl radical as detected by UV–visible and EPR spectroscopies (data not shown) (21, 22). In the case of 2,4-di-tert-butylphenol (2,4-t-Bu2C6H3OH) as substrate, we observed the formation of 2,2′-dihydroxy-3,3′,5,5′-tetra-tert-butylphenol (≈100% based on the intermediates used) (Scheme 1); 2 eq of phenol are oxidized per molecule of 1-X to give the starting [FeII(TMC)(X)]n+ complex and the coupling product from the 2,4-di-tert-butylphenoxyl radical (23). Pseudo-first-order fitting of the kinetic data allowed us to determine kobs values, and second-order rate constants were determined by plotting first-order rate constants against phenol concentration (Table 1 and Fig. 2b). This analysis also revealed a significant axial ligand effect, resulting in a reactivity order of 1-N3 > 1-OOCCF3 > 1-NCCH3, which is opposite to that observed for PPh3 oxidation.
H bonds. Addition of 2,4,6-tri-tert-butylphenol to 1-X caused the decay of the intermediates with the concurrent formation of the corresponding phenoxyl radical as detected by UV–visible and EPR spectroscopies (data not shown) (21, 22). In the case of 2,4-di-tert-butylphenol (2,4-t-Bu2C6H3OH) as substrate, we observed the formation of 2,2′-dihydroxy-3,3′,5,5′-tetra-tert-butylphenol (≈100% based on the intermediates used) (Scheme 1); 2 eq of phenol are oxidized per molecule of 1-X to give the starting [FeII(TMC)(X)]n+ complex and the coupling product from the 2,4-di-tert-butylphenoxyl radical (23). Pseudo-first-order fitting of the kinetic data allowed us to determine kobs values, and second-order rate constants were determined by plotting first-order rate constants against phenol concentration (Table 1 and Fig. 2b). This analysis also revealed a significant axial ligand effect, resulting in a reactivity order of 1-N3 > 1-OOCCF3 > 1-NCCH3, which is opposite to that observed for PPh3 oxidation.
Scheme 1.
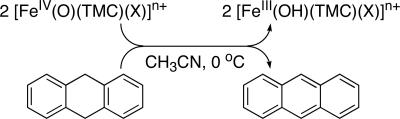
9,10-Dihydroanthracene (DHA) also was oxidized by 1-X to form anthracene as the sole product in ≈80% yield based on the stoichiometry shown in Scheme 2. The product yield was not dependent on the axial ligands, as observed in the 2,4-t-Bu2C6H3OH oxidation reactions. Unlike for the phenol oxidation, two molecules of 1-X are required to oxidize one molecule of DHA, so 1-X acts as an one-electron oxidant in this case, and the hydroxoiron(III) species that is formed is apparently not a powerful enough oxidant to abstract a hydrogen atom from DHA (19, 20). That an iron(III) species is the iron product of DHA oxidation was indicated by the oxidation of 2,4-t-Bu2C6H3OH added after the reactions of 1-X with DHA were completed, affording half as much biphenol product as the reactions of 1-X with 2,4-t-Bu2C6H3OH in the absence of DHA. The second-order rate constants derived from an analysis of the DHA oxidation reactions exhibited a reactivity order similar to that for phenol oxidation (Table 1 and Fig. 2c). Taken together, the above results demonstrate that the reactivity of 1-X is significantly affected by the axial ligands, X. Interestingly, the effect of X is opposite in H atom abstraction with respect to oxo-transfer reactions, i.e., 1 e− vs. 2 e− oxidations. Thus, more detailed investigations were carried out to understand the mechanism(s) and the origin of the axial ligand effect(s) in the oxidation of C H and O
H and O H bonds by mononuclear nonheme iron(IV)–oxo complexes.
H bonds by mononuclear nonheme iron(IV)–oxo complexes.
Scheme 2.
Oxidation of Phenol O H Bonds.
H Bonds.
The oxidation of phenols can in principle occur via an H atom abstraction pathway or by a proton-coupled electron transfer mechanism (24, 25). The latter is disfavored by a comparison of two substrates having similar O H bond strengths and pKa values but different steric demands, namely 2,6-t-Bu2C6H3OH [O
H bond strengths and pKa values but different steric demands, namely 2,6-t-Bu2C6H3OH [O H bond dissociation energy (BDE) = 81.65 kcal/mol; pKa = 11.70 in aqueous solution] and 2,4-t-Bu2C6H3OH (O
H bond dissociation energy (BDE) = 81.65 kcal/mol; pKa = 11.70 in aqueous solution] and 2,4-t-Bu2C6H3OH (O H BDE = 81.85 kcal/mol; pKa = 11.64 in aqueous solution) (25, 26). We observed a 175-fold rate difference in their reactions with 1-N3, 3.6 × 10−4 s−1 for 2,6-t-Bu2C6H3OH and 6.3 × 10−2 s−1 for 2,4-t-Bu2C6H3OH at 0°C. We interpret this large difference in reactivity to reflect a steric effect that implicates O
H BDE = 81.85 kcal/mol; pKa = 11.64 in aqueous solution) (25, 26). We observed a 175-fold rate difference in their reactions with 1-N3, 3.6 × 10−4 s−1 for 2,6-t-Bu2C6H3OH and 6.3 × 10−2 s−1 for 2,4-t-Bu2C6H3OH at 0°C. We interpret this large difference in reactivity to reflect a steric effect that implicates O H bond cleavage as the rate-determining step (23–25, 27).
H bond cleavage as the rate-determining step (23–25, 27).
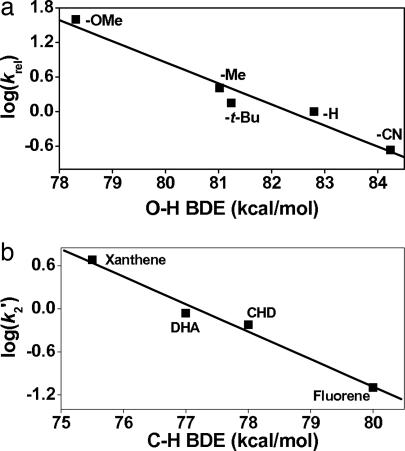
Further mechanistic studies with various para-substituted 2,6-di-tert-butylphenols (p-Y-2,6-t-Bu2-C6H2OH) revealed that the electronegativity of the para-substituents influences the reaction rates significantly as well (SI Table 2). A plot of the relative rates as a function of σp+ shows a good Hammett correlation with ρ values of −1.5 for 1-N3, −2.3 for 1-OOCCF3, and −3.2 for 1-NCCH3 (SI Fig. 8). Such a linear relationship has been used as evidence for an H atom abstraction mechanism in the oxidations of phenol O H bonds by [MnV(O)(Cz)] (where Cz is corrolazine) (23) and [(L)RuVI(O)2]2+ (25). Further support for this notion derives from the plot of log krel against phenol O
H bonds by [MnV(O)(Cz)] (where Cz is corrolazine) (23) and [(L)RuVI(O)2]2+ (25). Further support for this notion derives from the plot of log krel against phenol O H BDE, which also afforded a good linear correlation with slopes of −0.36 for 1-N3, −0.42 for 1-OOCCF3, and −0.55 for 1-NCCH3 (Fig. 3a for 1-N3; SI Fig. 9 for 1-OOCCF3 and 1-NCCH3) (23, 25, 28).
H BDE, which also afforded a good linear correlation with slopes of −0.36 for 1-N3, −0.42 for 1-OOCCF3, and −0.55 for 1-NCCH3 (Fig. 3a for 1-N3; SI Fig. 9 for 1-OOCCF3 and 1-NCCH3) (23, 25, 28).
Fig. 3.
Correlations of reaction rates with BDE. (a) Plot of log krel of [FeIV(O)(TMC)(N3)]+ (1-N3) against O H BDE of p-Y-2,6-t- Bu2C6H3OH in CH3CN at 25°C. (b) Plot of log k′2 of [FeIV(O)(TMC)(N3)]+ (1-N3) against C
H BDE of p-Y-2,6-t- Bu2C6H3OH in CH3CN at 25°C. (b) Plot of log k′2 of [FeIV(O)(TMC)(N3)]+ (1-N3) against C H BDE of substrates. Second-order rate constants, k2, were determined at 25°C and then adjusted for reaction stoichiometry to yield k′2 based on the number of equivalent target C
H BDE of substrates. Second-order rate constants, k2, were determined at 25°C and then adjusted for reaction stoichiometry to yield k′2 based on the number of equivalent target C H bonds of substrates (e.g., four for DHA and CHD and two for xanthene and fluorene).
H bonds of substrates (e.g., four for DHA and CHD and two for xanthene and fluorene).
Oxidation of Activated C H Bonds.
H Bonds.
Further insight into the mechanism of C H bond activation by nonheme iron(IV)–oxo complexes was obtained by investigating the reactions of 1-X with a range of substrates having weak C
H bond activation by nonheme iron(IV)–oxo complexes was obtained by investigating the reactions of 1-X with a range of substrates having weak C H bonds, specifically xanthene (75.5 kcal/mol), DHA (77 kcal/mol), 1,4-cyclohexadiene (78 kcal/mol), and fluorene (80 kcal/mol) (29, 30), from which second-order rate constants were determined. For these reactions, the reactivity order was found to be 1-N3 > 1-OOCCF3 > 1-NCCH3 (Table 1 and SI Fig. 10), as found for DHA oxidation (Fig. 2c). The log (k′2) values also correlated linearly with the C
H bonds, specifically xanthene (75.5 kcal/mol), DHA (77 kcal/mol), 1,4-cyclohexadiene (78 kcal/mol), and fluorene (80 kcal/mol) (29, 30), from which second-order rate constants were determined. For these reactions, the reactivity order was found to be 1-N3 > 1-OOCCF3 > 1-NCCH3 (Table 1 and SI Fig. 10), as found for DHA oxidation (Fig. 2c). The log (k′2) values also correlated linearly with the C H BDE values of the substrates, giving a slope of approximately −0.4 for each iron(IV)–oxo complex studied (Fig. 3b for 1-N3 and SI Fig. 11 for 1-OOCCF3 and 1-NCCH3). As expected, the rate constants decrease with the increase of the C
H BDE values of the substrates, giving a slope of approximately −0.4 for each iron(IV)–oxo complex studied (Fig. 3b for 1-N3 and SI Fig. 11 for 1-OOCCF3 and 1-NCCH3). As expected, the rate constants decrease with the increase of the C H BDE of substrates; this linear relationship between the reaction rates and the BDE of substrates implicates an H atom abstraction as the rate-determining step for the oxidation (31–39).
H BDE of substrates; this linear relationship between the reaction rates and the BDE of substrates implicates an H atom abstraction as the rate-determining step for the oxidation (31–39).
Further evidence for an H atom abstraction mechanism was obtained from a measurement of kinetic isotope effects (KIE) in the oxidation of DHA and xanthene. KIE values of 10–20 (average 17) were obtained in all of the reactions, consistent with C H bond cleavage being the rate-determining step (Table 1 and SI Table 3) (33–37). These large KIE values exceed the semiclassical limit (i.e., KIE < 7) and suggest a tunneling behavior in the reaction mechanism or another phenomenon that creates these high values (see below). For comparison, even larger KIE values of 30–50 were reported in the oxidations of ethylbenzene and benzyl alcohol by [FeIV(O)(N4Py)]2+ [where N4Py is N,N-bis(2-pyridylmethyl)-N-bis(2-pyridyl)methylamine] (38, 39) and in the cleavage of the target C
H bond cleavage being the rate-determining step (Table 1 and SI Table 3) (33–37). These large KIE values exceed the semiclassical limit (i.e., KIE < 7) and suggest a tunneling behavior in the reaction mechanism or another phenomenon that creates these high values (see below). For comparison, even larger KIE values of 30–50 were reported in the oxidations of ethylbenzene and benzyl alcohol by [FeIV(O)(N4Py)]2+ [where N4Py is N,N-bis(2-pyridylmethyl)-N-bis(2-pyridyl)methylamine] (38, 39) and in the cleavage of the target C H bond of taurine by the FeIV
H bond of taurine by the FeIV O intermediate of taurine:α-ketoglutarate dioxygenase (40). The good correlation between reaction rates and BDE of substrates and large KIE values in the oxidation of alkylaromatics support the notion that the C
O intermediate of taurine:α-ketoglutarate dioxygenase (40). The good correlation between reaction rates and BDE of substrates and large KIE values in the oxidation of alkylaromatics support the notion that the C H bond oxidation by 1-X occurs via an H atom abstraction mechanism.
H bond oxidation by 1-X occurs via an H atom abstraction mechanism.
The Reactivity of [Fe(IV)(O)(TMCS)]+ (1′-SR).
The reactivity trends noted above were further tested by extending the series in some experiments to include [FeIV(O)(TMCS)]+ (1′-SR) (where TMCS is 1-mercaptoethyl-4,8,11-trimethyl-1,4,8,11-tetraazacyclotetradecane) (see the structure in Fig. 1) (20), because 1′-SR has an axial thiolate, the most electron-donating ligand thus far shown to coordinate to an iron(IV)–oxo center. However, 1′-SR differs from the 1-X series in having its axial ligand introduced as a mercaptoethyl tail that replaces one of the TMC methyl groups. Furthermore, the generation of the iron(IV)–oxo complex requires different reaction conditions, mainly the use of CH3OH as solvent and a requirement for lower temperature due to the greater instability of 1′-SR (20). To be able to compare the reactivity of 1′-SR with the 1-X series, 1′-SR was first generated in CH3OH solvent as previously described (20), and the resulting solution was then diluted by an equal volume of CH3CN before the addition of substrates. Reaction of 1′-SR with PPh3 afforded a k2 of 0.016 M−1·s−1, a value that is clearly the smallest of any found in the 1-X series (Table 1 and Fig. 2a). In contrast, the reactions of 1′-SR with 2,4-t-Bu2C6H3OH and DHA afforded k2 values of 12 and 7.5 M−1·s−1, respectively, values that are larger than any found in the 1-X series (Table 1 and Fig. 2c). As a control, DHA oxidations were carried out for 1-NCCH3 and 1-N3 in 1:1 CH3OH/CH3CN, and the second-order rate constants obtained did not differ significantly from those obtained in pure CH3CN solvent (Table 1). These results corroborate the electrophilicity trend for phosphine oxidation observed in the 1-X series and the opposite trend for H atom abstraction.
Electrochemical Measurements.
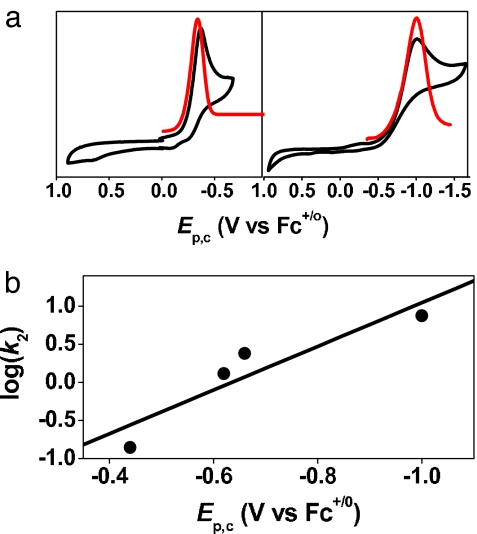
The electrochemistry of the 1-X series was investigated as a means to gain insight into the observed reactivity and provide a more quantitative basis for the effect of the anionic ligands on the iron(IV)–oxo center. Cyclic and differential pulse voltammetry of 1-NCCH3 revealed only one feature, a reductive wave with Ep,c at −0.32 V vs. ferrocene (Fc)+/o (Fig. 4a Left). This wave shifted cathodically upon replacement of CH3CN with anionic ligands to −0.50 V for 1-OOCCF3 and −0.60 V for 1-N3 in CH3CN at 25°C and to −1.00 V for 1′-SR in 1:1 CH3OH/CH3CN at −30°C (Table 1, Fig. 4a Right, and SI Fig. 12). The negative shifts observed are consistent with the introduction of anionic ligands trans to the oxo group, which are more basic than CH3CN and would thus be expected to decrease the redox potential of the iron(IV)–oxo center. Very interestingly, there is a linear correlation between the Ep,c values of the 1-X series and the reaction rates of phenol and DHA oxidation by the intermediates [Fig. 4b for the reaction of DHA and SI Fig. 13 for the reactions of 1,4-cyclohexadiene (CHD), xanthene, and 2,4-t-Bu2C6H3OH]. Strikingly, the complex having the most negative potential exhibits the fastest rates of oxidation within the series.
Fig. 4.
Electrochemical comparisons. (a) Cyclic and differential pulse voltamograms of [FeIV(O)(TMC)(NCCH3)]2+ (1-NCCH3) in CH3CN at 25°C (Left) and [FeIV(O)(TMCS)]+ (1′-SR) in 1:1 CH3OH/CH3CN at −30°C (Right). (b) Plot of log k2 determined in the oxidation of DHA at 0°C against Ep,c values of [FeIV(O)(TMC)(X)]n+ complexes and [FeIV(O)(TMCS)]+ in 1:1 CH3CN/CH3OH measured at −30°C.
The observed Ep,c values for the 1-X series, however, are surprisingly quite negative for oxidants that can carry out the reactions described in the previous sections. However, the electrochemical behavior of nonheme iron(IV)–oxo complexes exhibits some complexity that is not straightforward to interpret. For example, a reduction wave with an Ep,c value of −0.44 V vs. Fc+/o has been observed in the cyclic voltammetry of the closely related [FeIV(O)(N4Py)]2+ complex (41), which has been shown to be reactive enough to oxidize cyclohexane at room temperature (38). However, subsequent spectropotentiometric experiments established its actual redox potential to be significantly more positive, 0.9 V vs. Fc+/o (42). This unusual electrochemical behavior for the FeIV/III couple was in fact first noted by Wieghardt and coworkers (43) in their study of a series of [MIIIFeIII(μ-O)(O2CR)(L)2] complexes (where M is Fe or Cr and L is a tridentate N3 ligand), and was attributed to “strongly kinetically inhibited heterogeneous electron transfer processes at the working electrode (glassy carbon).” Establishing whether this explanation applies to the 1-X series will require an in-depth electrochemical investigation supported by spectroscopic experiments. For the purposes of this study, we focus on the observation that the redox potentials of the 1-X complexes decrease in an order that follows the increase in the basicity of the X ligand. Despite the more negative potentials, the iron(IV)–oxo complexes with more electron donating axial ligands are more reactive in the activation of C H and O
H and O H bonds. This reactivity trend is opposite to that observed for PPh3 oxidation by the 1-X series and requires a compelling rationale, which is provided by DFT calculations discussed in the next section.
H bonds. This reactivity trend is opposite to that observed for PPh3 oxidation by the 1-X series and requires a compelling rationale, which is provided by DFT calculations discussed in the next section.
DFT Calculations.
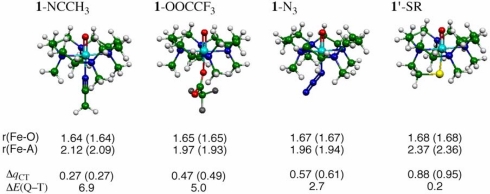
To understand the effect of the axial ligands on the properties of the [FeIV(O)(TMC)(X)]n+ complexes, we carried out DFT calculations using the hybrid functional, B3LYP, and the double-zeta basis set, LACVP (44) (see SI Materials and Methods for details). The so computed geometric features of these complexes are shown in Fig. 5, along with the amount of charge transferred (ΔqCT) from the axial ligand X to the [FeIV(O)(TMC)]2+ moiety. Inspection of the ΔqCT values shows that the DFT calculations support the axial ligand effect as reflected by the experimental Ep,c values. Thus, the DFT results demonstrate that the poorest electron donor among the axial ligands is CH3CN and the strongest is RS− followed by N3−. As such, the organizing quantity of the C H and O
H and O H oxidation reactivities appears to be the electron-releasing property of X, as quantified by ΔqCT in Fig. 5 and the experimental Ep,c quantity in Table 1.
H oxidation reactivities appears to be the electron-releasing property of X, as quantified by ΔqCT in Fig. 5 and the experimental Ep,c quantity in Table 1.
Fig. 5.
Key geometric features of 1-NCCH3, 1-OOCCF3, 1-N3, and 1′-SR optimized at the B3LYP/LACVP level (bond lengths in angstroms) in the triplet (quintet) states, along with the amounts of charge shifted from ligand to the (TMC)FeO moiety (ΔqCT) and the quintet–triplet energy gap (ΔEQ-T, in kcal/mol).
Recent discussions on the reactions of metal-oxo species with C H bonds, particularly the seminal work of Mayer (30, 31), have focused on the notion that the key determinant of the hydrogen atom abstraction rate is the hydrogen affinity of a metal–oxo reagent. This property is equivalent to the BDE of the O
H bonds, particularly the seminal work of Mayer (30, 31), have focused on the notion that the key determinant of the hydrogen atom abstraction rate is the hydrogen affinity of a metal–oxo reagent. This property is equivalent to the BDE of the O H bond that is formed, which is a function of both the redox potential of the M
H bond that is formed, which is a function of both the redox potential of the M O oxidant and the pKa of the nascent O
O oxidant and the pKa of the nascent O H bond based on a thermochemical cycle developed by Bordwell et al. (45) and others (46–48). Thus, a less favorable redox potential can be energetically compensated for by a higher pKa. To address this question directly, we have calculated the BDE (O
H bond based on a thermochemical cycle developed by Bordwell et al. (45) and others (46–48). Thus, a less favorable redox potential can be energetically compensated for by a higher pKa. To address this question directly, we have calculated the BDE (O H) values for all of the [FeIII(OH)(TMC)(X)]n+ complexes and find that BDE(O
H) values for all of the [FeIII(OH)(TMC)(X)]n+ complexes and find that BDE(O H) is essentially independent of the ligand X at 84 ± 1 kcal/mol (see SI Table 4). Therefore, the counterintuitive reactivity trend in H atom abstraction from DHA and CHD does not originate in bond strength effects in the series, and the explanation must be found elsewhere.
H) is essentially independent of the ligand X at 84 ± 1 kcal/mol (see SI Table 4). Therefore, the counterintuitive reactivity trend in H atom abstraction from DHA and CHD does not originate in bond strength effects in the series, and the explanation must be found elsewhere.
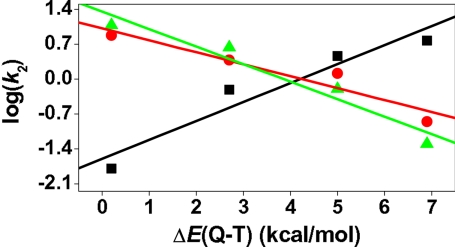
Note that all of the [FeIV(O)(TMC)(X)]n+ complexes (Fig. 5) possess two adjacently lying states: a ground state with a triplet spin quantum number (T) and a low-lying state with a quintet (Q) spin state (44). The two states are very close in energy for all of the complexes, lying within <7 kcal/mol; as X becomes a better electron donor, the ΔEQ-T gap decreases (Fig. 5) and the quintet state becomes more accessible along the reaction trajectory. As explained previously (44), this trend is rooted in the greater destabilizing effect on the localized δ orbital compared with the delocalized σ∗xy orbital as the donor ability of X increases from the neutral to the anionic ligands. Indeed, this tuning of reactivity by the ΔEQ-T parameter is apparent from Fig. 6, which plots the log k2 values for the oxidations of PPh3, DHA, and 2,4-t-Bu2C6H3OH against this quantity. Thus, it is seen that PPh3 oxidation exhibits a reactivity pattern opposite of those of DHA and phenol oxidation. In the P-oxidation series, the reactivity follows the electrophilicity of the reagents; the rate constant is small when X is a good electron donor (e.g., where X is RS−) and large when X is a poor electron donor (e.g., where X is CH3CN). In sharp contrast, the C H and O
H and O H activation rate constants increase as ΔEQ-T decreases, indicating that the greater accessibility of the quintet state enhances the C
H activation rate constants increase as ΔEQ-T decreases, indicating that the greater accessibility of the quintet state enhances the C H and O
H and O H activation rates. Indeed, as shown and rationalized in a previous theoretical study (44), the quintet transition state lies below the triplet one and has much smaller barriers for C
H activation rates. Indeed, as shown and rationalized in a previous theoretical study (44), the quintet transition state lies below the triplet one and has much smaller barriers for C H bond activation (SI Figs. 14 and 15). Therefore, the participation of the quintet species in the overall reaction is expected to increase progressively as X becomes a better electron donor, and the net barrier for C
H bond activation (SI Figs. 14 and 15). Therefore, the participation of the quintet species in the overall reaction is expected to increase progressively as X becomes a better electron donor, and the net barrier for C H activation will now decrease with the increased accessibility of the quintet state.
H activation will now decrease with the increased accessibility of the quintet state.
Fig. 6.
Plots of log k2 values for the reactions of 1-X and 1′-SR with DHA (red open circles), 2,4-t-Bu2C6H3OH (green filled triangles), and PPh3 (black filled squares) against the computed quintet-triplet energy gaps (ΔEQ-T) for 1-X and 1′-SR.
A simple way to understand the effect is to write the free-energy barrier of the combined triplet-quintet reaction (44) as a weighted blend of the individual barriers (Eq. 1),
where wQ is the weight of the quintet reaction in the total reaction and this weight should increase as the quintet-triplet energy gap decreases. The lowering of the barrier due to the participation of the quintet reaction is given by Eq. 2.
It is seen that the blended barrier, ΔG≠, decreases (compared with the large triplet barrier) in proportion to wQ, which depends on the accessibility of the quintet state. It follows therefore that, as the ligand changes from when X is CH3CN to when X is RS−, the quintet reaction will become more important (higher wQ) and the blended barrier will be increasingly reduced compared with the triplet barrier. In this manner, the participation of the quintet state in the C H activation can in principle reverse the reactivity order of 1-X to become an antielectrophilic trend as observed in this study. In a case where the blending coefficient wQ is 1, the relative reactivity will obey the electrophilicity of 1-X, because the barriers on the quintet surface should behave normally and follow electrophilicity. This appears to be the case for oxo-transfer to triphenylphosphine, where the reaction crosses over and proceeds mostly via the transition state of the quintet surface. An alternative two-state reactivity (TSR) scenario is one in which the reaction proceeds by crossover from the triplet ground state to the quintet state where the bond activation takes place. In such a scenario, with a slow spin crossover, the rate of the reaction will be given by the rate constant for the quintet reaction times the spin-inversion probability (τTQ) from the triplet to the quintet, namely,
H activation can in principle reverse the reactivity order of 1-X to become an antielectrophilic trend as observed in this study. In a case where the blending coefficient wQ is 1, the relative reactivity will obey the electrophilicity of 1-X, because the barriers on the quintet surface should behave normally and follow electrophilicity. This appears to be the case for oxo-transfer to triphenylphosphine, where the reaction crosses over and proceeds mostly via the transition state of the quintet surface. An alternative two-state reactivity (TSR) scenario is one in which the reaction proceeds by crossover from the triplet ground state to the quintet state where the bond activation takes place. In such a scenario, with a slow spin crossover, the rate of the reaction will be given by the rate constant for the quintet reaction times the spin-inversion probability (τTQ) from the triplet to the quintet, namely,
Here kQ is the rate constant according to transition state theory, and it increases with the increase of the electrophilicity of 1-X. In contrast, τTQ depends on the Q–T energy gap and increases as this gap decreases; namely, it is the largest for the worst electrophilie in the series 1′-SR, and smallest for the best one, 1-CH3CN. The combination of the two factors can lead to the observed reversal of reactivity and generation of a counterintuitive trend in H atom abstraction.
Clearly, therefore, although the correlations found here are fundamentally important in the connection that they make with the ligand control of reactivity in heme enzymes, theory and spectroscopy suggest that the reactivity scenario might be more complex and more intriguing because of the availability of two closely lying spin states for all of the 1-X reagents (44). The two-state picture does not affect the correlations found here (49) but may provide rationale for other observations, such as the observed KIEs and the stereoselectivity of the reactions with substrates containing stereochemical probes.
Summary
We have demonstrated that the behavior of [FeIV(O)(TMC)(X)]n+ and [FeIV(O)(TMCS)]+ complexes in both oxo-transfer and H atom abstraction reactions depends systematically on the electron-donating ability of the axial ligands, as measured by the electrochemical properties of the complexes. Interestingly, whereas oxo-transfer reactivity follows a trend expected of the electrophilicity of the oxidant, H atom abstraction reactivity follows the opposite trend, which is counterintuitive. DFT calculations provide a plausible framework within which to rationalize these reactivity patterns. It is postulated that the observed reactivity reflects the availability of two closely lying spin states for all of the 1-X reagents, the so-called TSR hypothesis fully detailed in ref. 44, wherein the excited quintet state has a much lower reaction barrier than the ground triplet state. Thus, increasing the electron-donating character of the axial ligand results in a decrease in the triplet-quintet gap and an increased participation of the quintet state in determining the rate of reaction. With respect to the role of the thiolate ligand in cytochrome P450, our studies suggest that the thiolate ligand may serve not only to reduce the redox potential of the high-valent iron center to mitigate unwanted electron transfer events but also to maintain and perhaps even enhance the H atom abstraction ability of the iron–oxo unit for the catalysis of alkane hydroxylation.
Materials and Methods
Preparation and handling of air-sensitive materials were done under an inert atmosphere either on a Schlenk line or in a glove box with solvents dried according to published procedures (50). Iron(II) complexes [FeII(TMC)(X)]n+ (where X is NCCH3, CF3COO−, or N3−) were prepared by adding 1.2 eq of appropriate tetraalkylammonium salts to FeII(TMC)(CF3SO3)2 (17–19). Iron(IV)–oxo complexes [Fe(O)(TMC)(X)]n+ (1-X) were prepared by reacting [FeII(TMC)(X)]n+ (0.5 mM) with 1.2 eq of PhIO (0.6 mM) in CH3CN at ambient temperature (17–19). The salt [FeII(TMCS)](PF6) was prepared as previously described (20, 51). The corresponding iron(IV)–oxo complex [FeIV(O)(TMCS)]+ (1′-SR) was generated by treating a methanol solution of [FeII(TMCS)](PF6) (1 mM) with 1.1 eq of m-chloroperbenzoic acid in the presence of 6 eq of potassium tert-butoxide (20). For reactivity studies of 1′-SR, 1 ml of 1′-SR (1 mM) was generated at 0°C and immediately diluted with chilled acetonitrile to bring the concentration of 1′-SR to 0.5 mM. An appropriate amount of substrate was then added to this solution, and the resulting reaction was monitored by a UV–visible spectrophotometer.
Kinetic studies were performed by adding appropriate amounts of substrates to the solutions of 1-X and 1′-SR, and spectral changes of the intermediates were directly monitored by a UV–visible spectrophotometer. Rate constants, kobs, were determined by pseudo-first-order fitting of the decrease of absorption bands at 820 nm for 1-NCCH3, 835 nm for 1-OOCCF3, and 850 nm for 1-N3 and 1′-SR. Product analysis for the oxidation of PPh3 was performed by injecting reaction solutions directly into HPLC. Product analysis for the oxidation of 2,4-t-Bu2C6H3OH and DHA by 1-X was performed by injecting reaction solutions directly into GC and GC–MS, by following procedures reported by Lansky and Goldberg (23).
All of the geometries were optimized with Jaguar 5.5 (52) at the UB3LYP/LACVP level (UB3LYP/B1) (53, 54).
Complete materials, instrumentation, and methods are provided in SI Materials and Methods, SI Figs. 8–15, and SI Tables 2–4.
Supplementary Material
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation/Ministry of Science and Technology through the Creative Research Initiative Program (W.N.); Science/Engineering Research Center Grant R11-2005-008-00000-0 (to J.K.); Ewha Womans University (W.N.); the BK 21 Program (K.O. and W.S.); Korea Science and Engineering Foundation Basic Research Program Grant R01-2004-000-10988-0 (to W.S.); National Institutes of Health Grant GM-33162 (to L.Q.) and Postdoctoral Fellowship GM-075700 (to T.A.J.); National Science Foundation Grant CHE-0615479 (to J.A.H.); Japan Society for the Promotion of Science Fellowships for Research Abroad (to H.H.); and DIP, the German–Israeli Project Cooperation (S.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709471104/DC1.
References
- 1.Nam W. Acc Chem Res. 2007;40:522–531. doi: 10.1021/ar700027f. [DOI] [PubMed] [Google Scholar]
- 2.Meunier B, de Visser SP, Shaik S. Chem Rev. 2004;104:3947–3980. doi: 10.1021/cr020443g. [DOI] [PubMed] [Google Scholar]
- 3.Groves JT. J Inorg Biochem. 2006;100:434–447. doi: 10.1016/j.jinorgbio.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Costas M, Mehn MP, Jensen MP, Que L., Jr Chem Rev. 2004;104:939–986. doi: 10.1021/cr020628n. [DOI] [PubMed] [Google Scholar]
- 5.Kryatov SV, Rybak-Akimova EV, Schindler S. Chem Rev. 2005;105:2175–2226. doi: 10.1021/cr030709z. [DOI] [PubMed] [Google Scholar]
- 6.Bollinger JM, Jr, Price JC, Hoffart LM, Barr EW, Krebs C. Eur J Inorg Chem. 2005;2005:4245–4254. [Google Scholar]
- 7.Dawson JH. Science. 1988;240:433–439. doi: 10.1126/science.3358128. [DOI] [PubMed] [Google Scholar]
- 8.Green MT, Dawson JH, Gray HB. Science. 2004;304:1653–1656. doi: 10.1126/science.1096897. [DOI] [PubMed] [Google Scholar]
- 9.Behan RK, Green MT. J Inorg Biochem. 2006;100:448–459. doi: 10.1016/j.jinorgbio.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 10.Ogliaro F, de Visser SP, Shaik S. J Inorg Biochem. 2002;91:554–567. doi: 10.1016/s0162-0134(02)00437-3. [DOI] [PubMed] [Google Scholar]
- 11.Green MT. J Am Chem Soc. 2006;128:1902–1906. doi: 10.1021/ja054074s. [DOI] [PubMed] [Google Scholar]
- 12.Behan RK, Hoffart LM, Stone KL, Krebs C, Green MT. J Am Chem Soc. 2006;128:11471–11474. doi: 10.1021/ja062428p. [DOI] [PubMed] [Google Scholar]
- 13.Stone KL, Behan RK, Green MT. Proc Natl Acad Sci USA. 2006;103:12307–12310. doi: 10.1073/pnas.0603159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone KL, Behan RK, Green MT. Proc Natl Acad Sci USA. 2005;102:16563–16565. doi: 10.1073/pnas.0507069102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross Z, Nimri S. Inorg Chem. 1994;33:1731–1732. [Google Scholar]
- 16.Song WJ, Ryu YO, Song R, Nam W. J Biol Inorg Chem. 2005;10:294–304. doi: 10.1007/s00775-005-0641-9. [DOI] [PubMed] [Google Scholar]
- 17.Rohde JU, In JH, Lim MH, Brennessel WW, Bukowski MR, Stubna A, Münck E, Nam W, Que L., Jr Science. 2003;299:1037–1039. doi: 10.1126/science.299.5609.1037. [DOI] [PubMed] [Google Scholar]
- 18.Sastri CV, Park MJ, Ohta T, Jackson TA, Stubna A, Seo MS, Lee J, Kim J, Kitagawa T, Münck E, Que L, Jr, Nam W. J Am Chem Soc. 2005;127:12494–12495. doi: 10.1021/ja0540573. [DOI] [PubMed] [Google Scholar]
- 19.Rohde JU, Que L., Jr Angew Chem Int Ed. 2005;44:2255–2258. doi: 10.1002/anie.200462631. [DOI] [PubMed] [Google Scholar]
- 20.Bukowski MR, Koehntop KD, Stubna A, Bominaar EL, Halfen JA, Münck E, Nam W, Que L., Jr Science. 2005;310:1000–1002. doi: 10.1126/science.1119092. [DOI] [PubMed] [Google Scholar]
- 21.Altwicker ER. Chem Rev. 1967;67:475–531. [Google Scholar]
- 22.Traylor TG, Xu F. J Am Chem Soc. 1990;112:178–186. [Google Scholar]
- 23.Lansky DE, Goldberg DP. Inorg Chem. 2006;45:5119–5125. doi: 10.1021/ic060491+. [DOI] [PubMed] [Google Scholar]
- 24.Rhile IJ, Mayer JM. J Am Chem Soc. 2004;126:12718–12719. doi: 10.1021/ja031583q. [DOI] [PubMed] [Google Scholar]
- 25.Yiu DTY, Lee MFW, Lam WWY, Lau T-C. Inorg Chem. 2003;42:1225–1232. doi: 10.1021/ic026184v. [DOI] [PubMed] [Google Scholar]
- 26.Habibi-Yangjeh A, Danandeh-Jenagharad M, Nooshyar M. J Mol Model. 2006;12:338–347. doi: 10.1007/s00894-005-0050-6. [DOI] [PubMed] [Google Scholar]
- 27.Lucarini M, Pedrielli P, Pedulli GF, Cabiddu S, Fattuoni C. J Org Chem. 1996;61:9259–9263. [Google Scholar]
- 28.Mulder P, Saastad OW, Griller D. J Am Chem Soc. 1988;110:4090–4092. [Google Scholar]
- 29.Roth JP, Mayer JM. Inorg Chem. 1999;38:2760–2761. doi: 10.1021/ic990251c. [DOI] [PubMed] [Google Scholar]
- 30.Mayer JM. In: Biomimetic Oxidations Catalyzed by Transition Metal Complexes. Meunier B, editor. London: Imperial College Press; 2000. pp. 1–43. [Google Scholar]
- 31.Mayer JM. Acc Chem Res. 1998;31:441–450. [Google Scholar]
- 32.Borovik AS. Acc Chem Res. 2005;38:54–61. doi: 10.1021/ar030160q. [DOI] [PubMed] [Google Scholar]
- 33.Gardner KA, Kuehnert LL, Mayer JM. Inorg Chem. 1997;36:2069–2078. doi: 10.1021/ic961297y. [DOI] [PubMed] [Google Scholar]
- 34.Matsuo T, Mayer JM. Inorg Chem. 2005;44:2150–2158. doi: 10.1021/ic048170q. [DOI] [PubMed] [Google Scholar]
- 35.Lam WWY, Yiu SM, Yiu DTY, Lau TC, Yip WP, Che CM. Inorg Chem. 2003;42:8011–8018. doi: 10.1021/ic034782j. [DOI] [PubMed] [Google Scholar]
- 36.Goldsmith CR, Stack TDP. Inorg Chem. 2006;45:6048–6055. doi: 10.1021/ic060621e. [DOI] [PubMed] [Google Scholar]
- 37.Goldsmith CR, Cole AP, Stack TDP. J Am Chem Soc. 2005;127:9904–9912. doi: 10.1021/ja039283w. [DOI] [PubMed] [Google Scholar]
- 38.Kaizer J, Klinker EJ, Oh NY, Rohde JU, Song WJ, Stubna A, Kim J, Münck E, Nam W, Que L., Jr J Am Chem Soc. 2004;126:472–473. doi: 10.1021/ja037288n. [DOI] [PubMed] [Google Scholar]
- 39.Oh NY, Suh Y, Park MJ, Seo MS, Kim J, Nam W. Angew Chem Int Ed. 2005;44:4235–4239. doi: 10.1002/anie.200500623. [DOI] [PubMed] [Google Scholar]
- 40.Price JC, Barr EW, Glass TE, Krebs C, Bollinger JM., Jr J Am Chem Soc. 2003;125:13008–13009. doi: 10.1021/ja037400h. [DOI] [PubMed] [Google Scholar]
- 41.Sastri CV, Oh K, Lee YJ, Seo MS, Shin W, Nam W. Angew Chem Int Ed. 2006;45:3992–3995. doi: 10.1002/anie.200504422. [DOI] [PubMed] [Google Scholar]
- 42.Collins MJ, Ray K, Que L., Jr Inorg Chem. 2006;45:8009–8011. doi: 10.1021/ic061263i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slep LD, Mijovilovich A, Meyer-Klaucke W, Weyhermüller T, Bill E, Bothe E, Neese F, Wieghardt K. J Am Chem Soc. 2003;125:15554–15570. doi: 10.1021/ja030377f. [DOI] [PubMed] [Google Scholar]
- 44.Hirao H, Kumar D, Que L, Jr, Shaik S. J Am Chem Soc. 2006;128:8590–8606. doi: 10.1021/ja061609o. [DOI] [PubMed] [Google Scholar]
- 45.Bordwell FG, Cheng JP, Ji GZ, Satish AV, Zhang X. J Am Chem Soc. 1991;113:9790–9795. [Google Scholar]
- 46.Tilset M, Parker VD. J Am Chem Soc. 1992;111:6711–6717. [Google Scholar]
- 47.Parker VD, Handoo KL, Roness F, Tilset M. J Am Chem Soc. 1991;113:7493–7498. [Google Scholar]
- 48.Wayner DDM, Lusztyk E, Page D, Ingold KU, Mulder P, Laarhoven LLJ, Aldrich HS. J Am Chem Soc. 1995;117:8737–8744. [Google Scholar]
- 49.de Visser SP, Kumar D, Cohen S, Shacham R, Shaik S. J Am Chem Soc. 2004;126:8362–8363. doi: 10.1021/ja048528h. [DOI] [PubMed] [Google Scholar]
- 50.Armarego WLF, Perrin DD, editors. Purification of Laboratory Chemicals. Oxford: Pergamon; 1997. [Google Scholar]
- 51.Fiedler AT, Halfen HL, Halfen JA, Brunold TC. J Am Chem Soc. 2005;127:1675–1689. doi: 10.1021/ja046939s. [DOI] [PubMed] [Google Scholar]
- 52.Schrödinger Inc. Jaguar 5.5. Portland, OR: Schrödinger; 2004. [Google Scholar]
- 53.Becke AD. J Chem Phys. 1993;98:5648–5652. [Google Scholar]
- 54.Friesner RA, Murphy RB, Beachy MD, Ringnalda MN, Pollard WT, Dunietz BD, Cao Y. J Phys Chem A. 1999;103:1913–1928. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.