Abstract
It has been hypothesized that plant underground storage organs (USOs) played key roles in the initial hominin colonization of savanna habitats, the development of the distinctive skull and tooth morphology of the genus Australopithecus, and the evolution of the genus Homo by serving as “fallback foods” exploited during periods of food shortage. These hypotheses have been tested mostly by morphological, isotopic, and microwear analyses of hominin bones and teeth. Archaeological evidence of USO digging technology is equivocal. Until now relevant data from studies of chimpanzees, useful in behavioral models of early hominins because of their phylogenetic proximity and anatomical similarities, have been lacking. Here we report on the first evidence of chimpanzees using tools to dig for USOs, suggesting that exploitation of such resources was within the cognitive and technological reach of the earliest hominins. Consistent with scenarios of hominin adaptation to savannas, these data come from Ugalla (Tanzania), one of the driest, most open and seasonal chimpanzee habitats. USOs are, however, exploited during the rainy season, well after the period of most likely food shortage, contradicting the specific prediction of fallback food hypotheses. The discovery that savanna chimpanzees use tools to obtain USOs contradicts yet another claim of human uniqueness and provides a model for the study of variables influencing USO use among early hominins.
Keywords: fallback foods, Plio-Pleistocene hominins, USO foraging
There are sporadic reports of chimpanzees (Pan troglodytes) consuming the underground storage organs (USOs) of plants (1–3). However, the infrequency of USO consumption by chimpanzees in contrast to its regularity by tropical human foragers has typically been viewed as an important behavioral distinction between the taxa (4, 5), and it has been hypothesized that access to USOs played a key role in the initial hominin colonization of savanna habitats as well as in the evolution of the genera Australopithecus and Homo (5–7). This article reports on the first evidence of chimpanzee tool use to obtain USOs, expanding the breadth of the impressive behavioral complexity already known for wild chimpanzees (4, 8, 9). These data come from the savanna woodland of Ugalla, Tanzania, one of the driest and most open and seasonal habitats in which chimpanzees are found (10, 11). However, inconsistent with “fallback food” scenarios (5, 7), USOs are exploited during the rainy season, well after the period of most likely food shortage. Additionally, the Ugalla data, which include evidence of an expedient organic technology, suggest that incorporation of USOs into the hominin diet need not have required a technology that preserved in the archaeological record. This finding renders the archaeological record an unlikely source to test hypotheses concerning the early stages of hominin USO exploitation.
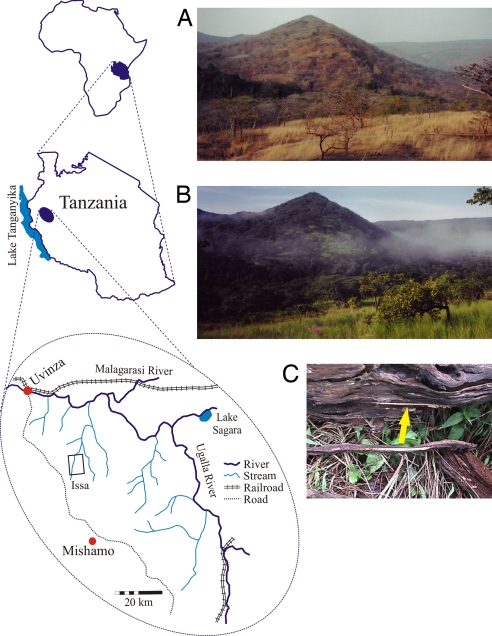
The vegetation of the Ugalla region is “miombo” savanna woodland dominated by trees of the genera Brachystegia and Julbernardia (Fabaceae) (10, 11) (Fig. 1). Two percent of the region is evergreen forest concentrated in patches along plateau edges and in narrow strips along the (mostly seasonal) streams. Woodland canopy is generally open, and the understory is grassy, with fauna including hartebeest, roan antelope, eland, elephant, zebra, and four potential predators of chimpanzees: lion, leopard, spotted hyena, and African wild dog (12, 13). The population density of chimpanzees is low, ≤0.1/km2 (10), because of the marginal quality of such a dry and seasonal habitat (14). Annual rainfall during the study period was 955 mm, with a total of 20 mm falling from June through October (12). In terms of climate, vegetation physiognomy, and associated fauna such savanna woodland as represented at Ugalla is intermediate between forest and open savanna or bushland.
Fig. 1.
The Ugalla region, western Tanzania. The open rectangle indicates the main study area, Issa, during the research period. (A) Typical Ugalla “miombo” woodland physiognomy during the dry season. (B) The same view during the rainy season. Tool IS-W-001 was removed from a fallen log at site 5, as illustrated by the refit of tool to the log (C).
We argue that chimpanzees adapting to such extreme (for them) conditions can be used as models for investigating particular aspects of early hominin behavioral ecology. The analogy is not based simply on the close genetic relationship between chimpanzees and hominins. Some early hominins were similar in encephalization and body size to chimpanzees (15), and the environmental challenges they faced in savanna woodlands would have been broadly similar (16). The behavior of savanna-living chimpanzees is thus relevant to assessing the hypothesis that USO consumption was a key factor in the morphological and technological evolution of Australopithecus and early Homo. In particular, Australopithecus possessed craniodental anatomy well adapted to managing the “highly repetitious masticatory stress cycles” (ref. 17, p 77, and ref. 18) associated with USO chewing and include a deep mandibular corpus, variable sagittal cresting of the cranium, anteriorly placed zygomae, reduced anterior teeth, postcanine megadonty, and thick cheektooth enamel (19). Additionally, it has been suggested that Pleistocene bone artifacts from South Africa were used by Australopithecus robustus and/or Homo erectus to dig up edible USOs (20), but this hypothesis has been challenged recently (21).
Results
Eleven USO digging sites were discovered during a field study conducted by R.A.H.-A. at Ugalla from August 2001 to June 2003 (12). Sites were identified based on the coincidence of multiple holes in the ground and partially consumed USOs, and chimpanzees are linked to the production of these holes through multiple lines of indirect evidence: vocalizations, feces, knuckle-prints, and the expelled fibrous wadges of consumed USOs. Ten digging sites were found immediately below chimpanzee nests, and the 11th was only ≈300 m from a repeatedly used nesting site. Finally, the diagnostic chimpanzee spoor were the only mammalian traces identified at the sites, strengthening the inference that chimpanzees were the sole excavators of the holes.
One of the digging sites was thrice reused; each of the other sites was used only once. The number of holes per site ranged from one to 96 (n = 10 sites). The areas of sites containing more than one hole ranged from 6 to 300 m2. Holes were measured at eight sites. Depths ranged from 30 to 250 mm, and site mean depths ranged from 55 mm (SD = 37) to 151 mm (SD = 62) (Table 1). Table 2 summarizes the taxa and uses of USOs excavated from these holes. Two species and the congeners of two others are consumed as food by humans; the remaining two species are of unknown edibility, but one of them and the congeners of the other have documented medicinal properties exploited by traditional peoples (references in Table 2). The medicinal use of plants by chimpanzees is known from other study sites (22), but more data are required to assess its specific application and significance at Ugalla.
Table 1.
Characteristics of the Ugalla USO digging sites
| Site | Site area,* m2 | No. of holes | Maximum diameter, mm | Minimum diameter, mm | Depth, mm | Species† | Tool(s)‡ |
|---|---|---|---|---|---|---|---|
| 1 | 28 | 9 | 226 ± 68 (90–350) | 207 ± 59 (90–300) | 108 ± 47 (50–210) | Dolichus kilimandscharicus | |
| 2 | n/a | 1 | n/m | n/m | n/m | Unidentified | |
| 3 | 7 | 4 | n/m | n/m | n/m | D. kilimandscharicus | |
| 4 | n/a | 1 | 280 | 220 | 180 | Unidentified | |
| 5 | 6 | 4 | 116 ± 42 (60–155) | 66 ± 20 (45–90) | 55 ± 37 (30–110) | Tacca leontopetaloides | IS-S-001, IS-S-002, IS-W-001, IS-W-002 |
| 6 | 12 | 4 | 221 ± 107 (95–320) | 171 ± 84 (65–260) | 151 ± 62 (80–215) | Raphionacme welwitschii | IS-S-003 |
| 7 | 300 | 62 | 146 ± 41 (60–260) | 115 ± 30 (60–200) | 65 ± 29 (30–150) | Brachystegia bussei, Smilax sp. | |
| 8 | 17.5 | 7 | 200 ± 82 (100–300) | 154 ± 70 (50–250) | 127 ± 76 (50–250) | B. bussei | |
| 9 | 142.5 | 30 | 139 ± 52 (70–250) | 110 ± 45 (50–230) | 99 ± 53 (30–200) | B. bussei, Smilax sp. | |
| 10 | n/m | >1 | n/m | n/m | n/m | B. bussei | |
| 11 | 277.5 | 96 | 97 ± 33 (40–180) | 79 ± 27 (35–170) | 81 ± 39 (30–190) | Fadogia quarrei | IS-B-001, IS-B-002 |
Data are ±SD with ranges in parentheses. n/a, not applicable; n/m, not measured.
*Minimum polygon area encompassing all holes identified at a site.
†Although B. bussei does not have USOs sensu stricto, its young roots are used to store nutrients. Only the roots of very young saplings (up to 20 cm high) of this tree species were dug up; these taproots resembled in shape small carrots.
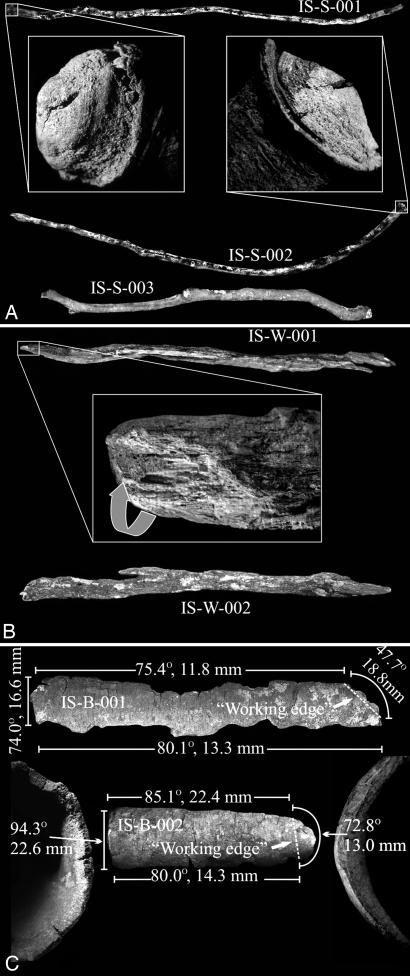
‡See Fig. 2 for an illustrated key to tool specimen catalog numbers.
Table 2.
Taxa and uses of USOs exploited by Ugalla chimpanzees
| Family | Species* | Uses† |
|---|---|---|
| Fabaceae | B. bussei | E |
| Fabaceae | D. kilimandscharicus | M |
| Rubiaceae | F. quarrei | cM |
| Asclepiadaceae | R. welwitschi | cE, cM |
| Smilacaceae | Smilax sp. | cE, cM |
| Taccaceae | T. leontopetaloides | E, M |
E, edible and documented nutritional consumption; cE, congeners edible and documented nutritional consumption; M, documented medicinal ingestion; cM, documented medicinal ingestion of congener.
*One species was not identified. Ugalla chimpanzees also eat the USOs of Costus macranthus (Zingiberaceae), but it was not included in our analyses because its USOs are barely subsurficial and were simply plucked manually from the ground, without excavation of soils.
†There are no previous reported cases of ingestion or use by chimpanzees of USOs belonging to the genera excavated at Ugalla, but all have previously documented uses among humans as food or medicine (26–32), except for B. bussei, which was also found to be edible and of pleasant taste (R.A.H.-A. and J.M., unpublished observations).
All of the digging sites appeared in the rainy season (November to May). A 17.5-month phenological study in Ugalla revealed that more edible fruits and terrestrial herbaceous vegetation were available to chimpanzee consumers during the rainy season than during the dry season (12). Although interannual data need to be collected and assessed, a hypothesis of USOs as seasonal fallback foods for the Ugalla chimpanzees is inconsistent with current data. The overall low resource density (for chimpanzees) of savannas may favor exploitation of marginal or difficult-to-obtain resources whenever they are available, or similar behavior may not yet have been seen in forested habitats. Alternatively, the hardness of the Ugalla soils during the dry season might be a factor in preventing chimpanzee USO foraging then [Ugalla soils can be difficult to breach manually even in the rainy season (our personal observations)].
Three sites yielded seven digging tools, which can be grouped into three types (Fig. 2). Three are sticks. Only the larger, presumably stronger proximal end of each stick shows evidence of use. The broken, smaller distal end is devoid of any adhering sediment and appears to be unused in digging activities. Two sticks (IS-S-001 and IS-S-002) are deciduous twigs, each with an abscission layer forming a callus at its proximal end, ≈10 mm from its terminal apex. The callus on each acted as a barrier against which sediment is banked, as did the bark edge on specimen IS-S-003. The encrusted sediment, visible with the naked eye, provides evidence of the use of the sticks as tools for digging. Microscopic analysis of the digging ends of the stick tools revealed neither working polish nor any nonmorphological striations or pits, suggesting a short working life for each piece. IS-W-001 and IS-W-002 are pieces of tree trunk, of driftwood-like appearance, removed from a decaying fallen log 50 cm from the closest hole at site 5. Their removal is evidenced by R.A.H.-A.'s being able to refit the pieces to the log in the field (Fig. 1). As with the stick specimens, each of the tree trunk pieces displays a differential distribution of adhering sediment, with it embedded along the length and width of a naturally formed longitudinal trough [≈50 mm × ≈20 mm (maximum) × ≈3 mm (maximum)] on the inner surface of IS-W-002. IS-W-001 has thick sediment cover on the tip of its inner surface, partially in a naturally formed trough, for a length of ≈10 mm. On its opposite surface a polish, supporting a single nonmorphological striation, has been developed, presumably from its use in digging. Two other specimens (IS-B-001 and IS-B-002), recovered from site 11, are long fragments of tree outer bark (rhytidome). Their use as digging tools is inferred based on discernible variation in the thickness and angles of each piece around its full circumference. Each specimen possesses a single worn edge of greater thinness and of more acute angularity than its remainder (Fig. 2C). Other edges on each piece are defined by distinguishable individual layers of periderm, whereas the worn edges lack clearly separate layers. We interpret these disparities as moderate use-wear from digging. However, because the pieces are overall relatively thin, only a few layers of periderm thick at their thickest, the wear pattern seems to show evidence of minimal use by chimpanzees. The pieces are flimsy and would disintegrate under any force much greater than brief scraping of surface soil. Taken together, analyses of the digging tools reveal an expedient technology, probably limited largely to breaching the hard surface of compacted sediments. Chimpanzees then likely used their hands to enlarge the holes and extract the desired USOs, as evidenced by shallowness of the measured holes and emanating finger drag marks at some digging sites. [USO extraction holes created by chimpanzees using their hands at Tongo (3) were estimated at depths of 50 cm; however, the soil there was sandy and easy to dig.]
Fig. 2.
The USO digging tools from Ugalla. (A) The stick tool specimens. The “working end” of each specimen is that to which sediment adheres (see Insets, showing sediment banked against callus rings of specimens). IS-S-001: weight = 18.5 g, length = 520 mm, circumference = 37 mm maximum/proximal and 20 mm distal. IS-S-002: weight = 26.5 g, length = 520 mm, circumference = 39 mm maximum/proximal and 25 mm distal. IS-S-003: weight = 45.5 g, length = 490 mm, circumference = 59 mm maximum/proximal and 34 mm distal. (B) The wood tool specimens. IS-W-001: weight = 33.6 g, length = 427 mm, maximum circumference = 62 mm. IS-W-002: weight = 33.6 g, length = 312 mm, maximum circumference = 56 mm. (Inset) Working end of IS-W-001, slightly rotated (arrow) from full-specimen view, showing a thick accumulation of sediment in a natural trough. (C) The bark tool specimens. The working end of each bark specimen is that portion that lacks macroscopically apparent layers of periderm in the edge-on view. For each specimen, angle and thickness measurements were taken at 1-cm intervals. Values on the figure represent means for the measured regions of each specimen. IS-B-001: weight = 44.0 g, length = 422 mm, maximum width = 80 mm (a small section of the working end is broken from the larger specimen and not pictured here). IS-B-002: weight = 23.7 g, length = 176 mm, maximum width = 77 mm.
Discussion
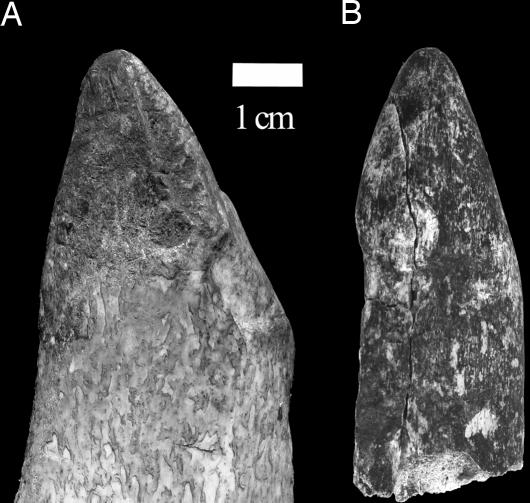
Following our results, if the first use of digging tools by early hominins was to extract shallowly buried USOs, that technology might have consisted of relatively fragile organic implements, minimally modified (if at all) and unlikely to have been curated. Relatively expedient technology also characterizes bone tool assemblages from the A. robustus sites of Swartkrans and Drimolen, which consist mostly of small fragments of broken ungulate limb bones (20, 23). Hypothetically, these Early Pleistocene artifacts from South Africa were used by hominins to extract shallow-rooted USOs of the genera Hypoxis and Scilla (20). Current experimental work shows USO digging with ungulate limb bone fragments for as little as ≤30 min to result in diagnostic wear similar to that on the Swartkrans artifacts (Fig. 3). These examples contrast dramatically with the large wooden digging sticks used by many modern human foragers in Africa to extract deeply buried roots and tubers, such as Vigna spp. and Vatovaea macroryhncha exploited by the Hadza of northern Tanzania [Hadza digging stick mean length = 1,360 mm, mean weight = 583 g] (24). The Ugalla results and those on the bone tools from South Africa suggest that the archaeological record is an unlikely source of evidence to test the hypothesis that the evolution of the robust jaws and dentition of Plio-Pleistocene Australopithecus resulted from an adaptive shift away from a “chimpanzee-like” reliance on herbaceous leaves and piths to USOs in fallback situations (7). It is more likely that data to test this hypothesis directly will be found in the microwear patterns and isotopic composition of early hominin teeth. However, the discovery that savanna chimpanzees use tools to obtain USOs shows that such consumption was within the grasp of chimpanzee-like hominins and provides a model for further study of variables (such as seasonality) influencing USO use among early hominins. The data on USO exploitation by Ugalla chimpanzees joins recent observations of dry habitat-adapted chimpanzees at Fongoli (Senegal) using wooden spears to capture galagos (Galago senegalensis) (25). These tool-assisted behavioral patterns—both considered to be relevant to the evolution of humans—are unique among chimpanzees and emphasize the importance of conserving and studying rare savanna populations.
Fig. 3.
Comparison of a modern cow humerus (A) fragment used to dig shallowly buried Hypoxis USOs for 30 min on Swartkrans Hill, South Africa, to SKX 5000 (B), an ≈1.8- to 1.0-million-year-old bone artifact from Member 1 of the Swartkrans Formation. Note the similar form and degree of macroscopic wear and polish developed on each specimen, suggesting a relatively expedient digging technology for Early Pleistocene South African hominins compared with that of most modern human foragers. Others (19) have argued that it requires as many as 4 h of digging with a freshly broken modern bone fragment for it to assume the form and wear pattern of the fossil tools. This proposed greater span of time is still relatively brief in comparison to the long-term curation and use of wooden digging sticks by modern human foragers, such as the Hadza (ref. 23 and T.R.P., unpublished observations). The image was produced by Jason Heaton.
Acknowledgments
We thank the Government of Tanzania for permission to work at Ugalla. A number of people have contributed in important ways to our research at Ugalla: Yahya Abeid, Tano Ahmadi, Kurt Benirschke, Chris Boehm, Gianfranco Cassiano, Anthony Collins, Roy Gereau, Axel Hernandez-Aguilar, Busoti Juma, Shadrack Kamenya, William McGrew, Juma Mkondo, Hideshi Ogawa, Alex Piel, Lilian Pintea, Moshi Rajabu, Abdalla Said, Craig Stanford, Fiona Stewart, Are Thune, Samwell Wagala, and Janette Wallis. We are grateful to Alan Walker, William McGrew, Henry Bunn, and an anonymous reviewer for comments that improved this article. R.A.H.-A. thanks Craig Stanford for his support. We thank Jason Heaton for his collaboration on the bone tool digging experiments and for the photographs in Fig. 3, and we thank Axel Hernandez-Aguilar for help with figures. Bob Brain, Francis Thackeray, and Stephanie Potze of the Transvaal Museum granted access and permission to study the Swartkrans fossils. We thank the following for financial support: the L. S. B. Leakey Foundation (R.A.H.-A., J.M., and T.R.P.), the National Science Foundation (R.A.H.-A. and T.R.P.), the Jane Goodall Center at the University of Southern California (R.A.H.-A.), the University of California Committee on Research (J.M.), and the Palaeontology Scientific Trust (T.R.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 19167.
References
- 1.Kortlandt A, Holzhaus E. Primates. 1987;28:473–496. [Google Scholar]
- 2.McGrew WC, Baldwin PJ, Tutin CEG. Am J Primatol. 1988;16:213–226. doi: 10.1002/ajp.1350160304. [DOI] [PubMed] [Google Scholar]
- 3.Lanjouw A. In: Behavioural Diversity in Chimpanzees and Bonobos. Boesch C, Hohmann G, Marchant LF, editors. Cambridge, UK: Cambridge Univ Press; 2002. pp. 52–60. [Google Scholar]
- 4.McGrew WC. Chimpanzee Material Culture: Implications for Human Evolution. New York: Cambridge Univ Press; 1992. [Google Scholar]
- 5.Wrangham RW, Jones JH, Laden G, Pilbeam D, Conklin-Brittain N. Curr Anthropol. 1999;40:567–594. [PubMed] [Google Scholar]
- 6.Hatley T, Kappleman J. Hum Ecol. 1980;8:371–387. [Google Scholar]
- 7.Laden G, Wrangham RW. J Hum Evol. 2005;49:482–498. doi: 10.1016/j.jhevol.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin CEG, Wrangham RW, Boesch C. Nature. 1999;399:682–685. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- 9.Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin CEG, Wrangham RW, Boesch C. Behaviour. 2001;138:1481–1516. [Google Scholar]
- 10.Kano T. Kyoto Univ Afr Stud. 1972;7:37–129. [Google Scholar]
- 11.Moore J. Tropics. 1994;3:333–340. [Google Scholar]
- 12.Hernandez-Aguilar RA. Los Angeles: Univ of Southern California; 2006. PhD thesis. [Google Scholar]
- 13.Ogawa H, Idani G, Moore J, Pintea L, Hernandez-Aguilar RA. Int J Primatol. 2007;28 in press. [Google Scholar]
- 14.Moore J. In: Topics in Primatology: Human Origins. Nishida T, McGrew WC, Marler P, Pickford MP, de Waal FBM, editors. Tokyo: Univ of Tokyo Press; 1992. pp. 99–118. [Google Scholar]
- 15.McHenry HM, Coffing K. Annu Rev Anthropol. 2000;29:125–146. [Google Scholar]
- 16.Moore J. In: Great Ape Societies. McGrew WC, Marchant LF, Nishida T, editors. Cambridge, UK: Cambridge Univ Press; 1996. pp. 265–292. [Google Scholar]
- 17.Hylander WL. In: Evolutionary History of the “Robust” Australopithecines. Grine FE, editor. New York: de Gruyter; 1988. pp. 55–83. [Google Scholar]
- 18.Teaford MF, Ungar PS. Proc Natl Acad Sci USA. 2000;97:13506–13511. doi: 10.1073/pnas.260368897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grine FE, editor. Evolutionary History of the “Robust” Australopithecines. New York: de Gruyter; 1988. [Google Scholar]
- 20.Brain CK, Shipman P. In: Swartkrans: A Cave's Chronicle of Early Man. Brain CK, editor. South Africa: Transvaal Museum, Pretoria; 1993. pp. 195–215. [Google Scholar]
- 21.Backwell L, d'Errico F. S Afr J Sci. 2003;99:259–267. [Google Scholar]
- 22.Huffman MA, Wrangham RW. In: Chimpanzee Cultures. Wrangham RW, McGrew WC, Heltne PG, editors. Cambridge, MA: Harvard Univ Press; 1996. pp. 129–148. [Google Scholar]
- 23.Keyser A. Natl Geogr. 2000;197:76–83. [Google Scholar]
- 24.Vincent A. World Archaeol. 1984;17:131–148. doi: 10.1080/00438243.1985.9979958. [DOI] [PubMed] [Google Scholar]
- 25.Pruetz JD, Bertolani P. Curr Biol. 2007;17:412–417. doi: 10.1016/j.cub.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 26.Sohni YR, Davis CL, DesChamps AB, Kale PG. Environ Mol Mutagen. 1995;25:77–82. doi: 10.1002/em.2850250111. [DOI] [PubMed] [Google Scholar]
- 27.Marston A, Gafner F, Dossaji SF, Hostettmann K. Phytochemistry. 1988;27:1325–1326. [Google Scholar]
- 28.Ohiomokhare SO, Adelaiye AB, Okwasaba FK. J Neurochem. 2003;87:46. [Google Scholar]
- 29.Peters CR, O'Brien EM, Drummond RB. Edible Wild Plants of Subsaharan Africa. Kent, UK: Royal Botanic Gardens Kew; 1992. [Google Scholar]
- 30.Spennemann DHR. J Ethnobiol. 1994;14:211–234. [Google Scholar]
- 31.Charlson AJ. J Ethnopharmacol. 1980;2:323–335. doi: 10.1016/s0378-8741(80)81014-2. [DOI] [PubMed] [Google Scholar]
- 32.Sautour M, Miyamoto T, Lacaille-Dubois MA. Planta Med. 2006;72:667–670. doi: 10.1055/s-2006-931582. [DOI] [PubMed] [Google Scholar]