Abstract
Extant African great apes and humans are thought to have diverged from each other in the Late Miocene. However, few hominoid fossils are known from Africa during this period. Here we describe a new genus of great ape (Nakalipithecus nakayamai gen. et sp. nov.) recently discovered from the early Late Miocene of Nakali, Kenya. The new genus resembles Ouranopithecus macedoniensis (9.6–8.7 Ma, Greece) in size and some features but retains less specialized characters, such as less inflated cusps and better-developed cingula on cheek teeth, and it was recovered from a slightly older age (9.9–9.8 Ma). Although the affinity of Ouranopithecus to the extant African apes and humans has often been inferred, the former is known only from southeastern Europe. The discovery of N. nakayamai in East Africa, therefore, provides new evidence on the origins of African great apes and humans. N. nakayamai could be close to the last common ancestor of the extant African apes and humans. In addition, the associated primate fauna from Nakali shows that hominoids and other non-cercopithecoid catarrhines retained higher diversity into the early Late Miocene in East Africa than previously recognized.
Keywords: hominoid evolution
Recent molecular studies suggest that the divergence between humans and chimpanzees occurred at 7–5 Ma and that the divergence of gorillas occurred at 9–8 Ma (1–4). Consequently, the Late Miocene (11–5 Ma) is the crucial period for understanding the origins of African great apes and humans, but unfortunately the African hominoid fossil record is extremely poor after 13 Ma (5, 6). A rare exception is Samburupithecus kiptalami (9.6 Ma), discovered from Samburu Hills, northern Kenya (7, 8). However, its dental morphology is so specialized that its phyletic position is unclear. Although there is a variety of Late Miocene hominoids in Eurasia, the majority of them are likely to belong to the Pongo clade (9, 10). Ouranopithecus macedoniensis has often been considered to resemble African great apes (11, 12), but it is known only from the Late Miocene of Greece, and its phyletic relationships are not yet agreed on (13). Because of the poor nature of the hominoid fossil record in Africa, some authors have suggested that a Eurasian Miocene hominoid returned to Africa in the Late Miocene to become the last common ancestor of the African great apes and humans (11, 14). Our recent fieldwork, however, has recovered a diverse primate fauna, including large-bodied hominoids, from the early Late Miocene of Nakali, Kenya (15). Here we report a new genus of great ape from Nakali and provide information on its geological, geochronological, and paleontological context. Among the known fossil hominoids, this great ape resembles Ouranopithecus, from the Late Miocene (9.6–8.7 Ma) of Greece (16, 17), in size and some morphological features, but a number of differences lead us to assign the Nakali material to a different new genus. Nakalipithecus nakayamai and the associated primate fauna provide important new evidence for understanding the origins of the extant African ape and human clades, as well as catarrhine evolution in general in the Middle to Late Miocene Africa. [Since our article was submitted, Suwa et al. (18) published an article on Chororapithecus abyssinicus from Chorora, Ethiopia (10.7–10.1 Ma). We do not yet know how N. nakayamai relates to C. abyssinicus.]
Systematics
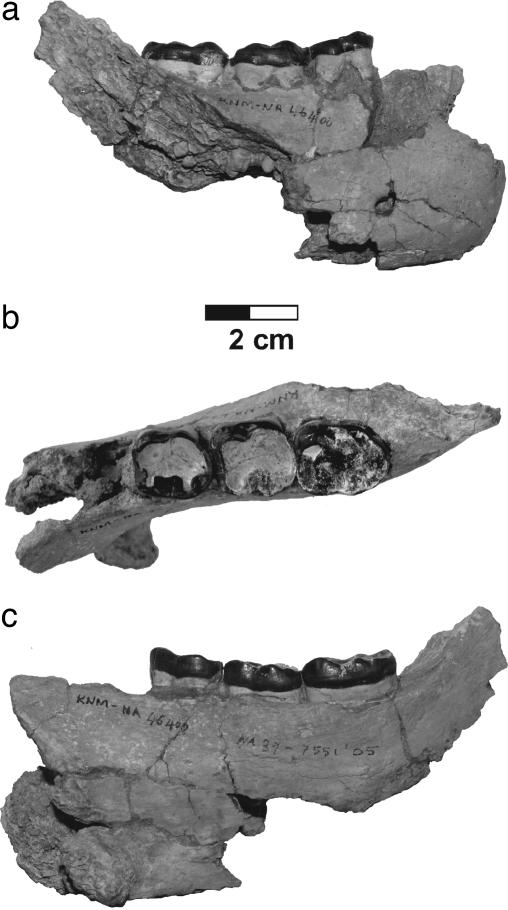
Order Primates Linnaeus, 1758; suborder Anthropoidea Mivart, 1864; infraorder Catarrhini Geoffroy, 1812; superfamily Hominoidea Gray, 1825; genus Nakalipithecus gen. nov. Type species: N. nakayamai gen. et sp. nov. Etymology: Nakali, the area where the fossils were discovered, and pithekos, ape in Greek. Generic diagnosis: as for the species N. nakayamai gen. et sp. nov. Holotype: KNM-NA46400, a right mandibular fragment with M1–M3 (Fig. 1). The repository is the National Museums of Kenya. Hypodigm: the type specimen; left I1 (KNM-NA47592); right C* (KNM-NA47594); right P3 (KNM-NA46431); left P4 (KNM-NA46430); right M1 (KNM-NA47591); right I2 (KNM-NA46425); left P3 (KNM-NA46423); right P4 (KNM-NA46424); right M3 (KNM-NA46429); left M3 (KNM-NA46436); left dp4 (KNM-NA46435) (Figs. 2 and 3 and Table 1). Locality: Nakali, 40 km west of Maralal, along the eastern edge of the Rift Valley, Kenya. Horizon: Upper Member of the Nakali Formation. Age: early Late Miocene (9.9–9.8 Ma). Etymology: After the late Katsuhiro Nakayama, who contributed much to the geological aspects of the expedition.
Fig. 1.
Type specimen of N. nakayamai (KNM-NA46400, right mandible with M1–M3). Buccal (a), occlusal (b), and lingual (c) views are shown.
Fig. 2.
Isolated cheek teeth of N. nakayamai. (a) KNM-NA46424, right P4. (b) KNM-NA46435, left dp4. (c) KNM-NA46436, left M3. (d) KNM-NA46429, right M3. (e) KNM-NA46431, right P3. (f) KNM-NA46430, left P4. (e) KNM-NA46423, right P3. (h) KNM-NA47591, right M1.
Fig. 3.
Isolated canine and incisors of N. nakayamai. (a) Distal view. (b–d) Lingual view. (a and b) KNM-NA47594, right C*. The arrowhead in a indicates the well developed lingual cusplet. (c) KNM-NA47592, left I1. (d) KNM-NA46425, right I2.
Table 1.
Dental measurements (in millimeters) of N. nakayamai
| Accession no. | Tooth type | Length | Breadth |
|---|---|---|---|
| Max | Perp | ||
| KNM-NA46423 | Left P3 | 12.4 | 8.2 |
| MD | BL | ||
| KNM-NA46425 | Right I2 | 6.5 | 9.1 |
| KNM-NA46424 | Right P4 | 10.4 | 14.0 |
| KNM-NA46400 | Right M1 | 15.6 | 14.0 |
| Right M2 | 16.2 | 15.8 | |
| Right M3 | 19.5 | 15.1 | |
| KNM-NA46429 | Right M3 | 15.8 | 13.2 |
| KNM-NA46436 | Left M3 | 16.0 | 12.7 |
| KNM-NA46435 | Left dp4 | 11.4 | 9.4 |
| KNM-NA47592 | Left I1 | 10.8 | 8.6 |
| KNM-NA47594 | Right C* | 10.7 | 10.5 |
| KNM-NA46431 | Right P3 | 9.3 | 11.1 |
| KNM-NA46430 | Left P4 | 9.5 | 11.9 |
| KNM-NA47591 | Right M1 | 12.4 | 13.4 |
Max, maximum; Perp, perpendicular; MD, mesiodistal; BL, buccolingual.
Specific Diagnosis.
N. nakayamai is a large hominoid similar in dental size to female gorillas and orangutans. The mandible has a well developed inferior transverse torus that extends posteriorly to mid-M1. The thickness/height index of the mandibular body is ≈45% at M1. M1 is relatively large compared with M2. M3 is considerably larger than M2 and mesiodistally elongated relative to the buccolingual breadth. Molars have thick enamel, relatively flat dentine/enamel junction, peripherally positioned and voluminous cusps, and reduced but still marked cingula. I2 is mesiodistally narrow and buccolingually thick with a tall, slender crown relative to the mesiodistal dimension. P3 has weak bilateral compression, being semitriangular in occlusal outline with a lingual crest lingually oriented and a wide distal fovea. P4 is broad with voluminous cusps being peripherally positioned. M1 is relatively elongated [mesiodistal length (MD)/buccolingual breadth (BL) = 92.5%] with a short but marked cingular remnant on the mesiolingual corner of the protocone. I1 is low-crowned and mesiodistally wide with the strongly curved distal margin, relatively elevated lingual cingulum, and the lingual surface featured by a thick, rounded crest running from the cingulum toward the incisive edge, and a few other vertical wrinkles. The upper canine is low-crowned and buccolingually as broad as mesiodistally long, showing a continuous and prominent lingual cingulum that is elevated with a remarkably well developed lingual cusplet. Upper premolars are elongated, having peripherally positioned and voluminous cusps with weak heteromorphy.
Differential Diagnosis.
N. nakayamai differs from the other known Miocene and extant hominoids in having an upper canine (presumed female) that is low-crowned and as broad as long and is bordered basally by a highly elevated and clearly differentiated, continuous lingual cingulum that develops an extremely large lingual cusplet. N. nakayamai is distinguished from Proconsul, Ugandapithecus, Afropithecus, and Nacholapithecus by its large size, mesiodistally elongated upper premolars with reduced cusp heteromorphy, reduced cingulum on upper molar, bilaterally less compressed P3, and low-crowned I1. N. nakayamai differs from Kenyapithecus wickeri and Equatorius africanus in its large size, mesiodistally elongated upper premolars, relatively higher mandibular body, and less compressed P3. N. nakayamai is distinguished from Samburupithecus by less inflated molar cusps, which are positioned more peripherally, more spacious occlusal foveae and basins, and reduced upper molar cingulum. N. nakayamai differs from Ouranopithecus in the following features: upper canine (presumed female) slightly higher-crowned with a higher position of the mesial shoulder; molars having less thick enamel and less inflated cusps that result in more spacious occlusal basins and foveae; better developed molar cingula; P3 with more marked buccal and lingual cingula and slightly longer mesiobuccal slope [compared with similar-sized (presumed female) P3s of O. macedoniensis]; upper premolars more elongated; P3 having more marked buccal cingula and styles and a better developed mesial transverse crest; P4 cusps less inflated; and dp4 having a mesiodistally shorter mesial fovea, a larger distal fovea, more rounded distobuccal margin of the crown (straight and oblique in Ouranopithecus), and more buccally placed hypoconulid. N. nakayamai is distinguished from Ankarapithecus, Sivapithecus, and Khoratpithecus by its large size (excluding Sivapithecus parvada), more gracile mandibular body, better developed molar cingula (excluding Khoratpithecus), more peripherally positioned molar cusps, and weaker bilateral compression of P3. N. nakayamai is different from Dryopithecus in its large size, thick molar enamel with flatter dentine/enamel junction, and upper premolars mesiodistally elongated relative to the buccolingual breadth.
Geology and Geochronology of Nakali
The Nakali Formation is divided into the Upper, Middle, and Lower Members. Hominoid fossils were recovered from the volcanic mud flow (lahar) deposits of the Upper Member at two sites [supporting information (SI) Text and SI Figs. 4 and 5]. Multistory fluvial channel fill deposits are rarely found in the Upper Member, and no soil intervals are present. These features suggest a rapid sediment accumulation during deposition of the upper Nakali Formation. 40Ar-39Ar ages of anorthoclase from pumices in the uppermost part of the Lower Member are 9.82 ± 0.09 and 9.90 ± 0.09 Ma, whereas one grain from pyroclastic flow deposits from the Middle Member is 10.10 ± 0.12 Ma (SI Figs. 4, 6, and 7 and SI Table 2). The uppermost level of the Lower Member and the lowest level of the Upper Member document a reversed magnetic polarity (SI Figs. 4, 8, and 9). This reversed polarity interval, combined with the 40Ar-39Ar ages, can be reasonably correlated with Chron C5n.1r, dated at 9.88–9.92 Ma (19). Consequently, the hominoid bed is located within the C5n.1n chronozone [9.74–9.88 Ma (19)]. Given the rapid sediment accumulation, the age of the hominoid bed is most probably 9.80–9.88 Ma.
Description
The type specimen (KNM-NA46400) is a right mandibular fragment with M1–M3 (Fig. 1). It is of female gorilla size, and, among the presently known Miocene hominoids, it is as large as Samburupithecus, Ouranopithecus, and S. parvada, all of which are known from the early Late Miocene. The alveolar plane slopes down posteriorly to the P3/P4 level and makes a moderate superior transverse torus. The inferior transverse torus is very well developed. It is thick and robust and extends posteriorly to the mid-M1 level. The body height can be estimated as ≈44 mm at the M1 level. The body thickness is 19.8 mm at the same level. The lateral aspect of the body is vertical below M1, and there is a single mental foramen two-thirds of the body height down from the alveolar margin below P4/M1. The molars are heavily worn. In M1 and M2 the buccal cusps are completely worn away, leaving a very large and hollowed area of dentine exposure. The lingual cusps are also worn, but they still retain some occlusal relief. M3 preserves the occlusal morphology better than the anterior molars. The cusps appear to be low and voluminous. The crown is mesiodistally elongated relative to the buccolingual breadth and tapers slightly near the distal margin. The buccal cingulum is relatively reduced on the lower molars, but there are weak cingular remnant on the mesiobuccal aspect of the protoconid and marked remnants between the buccal cusps. M1 is relatively large [M1/M2 ratio in area (MD × BL) = 85%]. M3 is considerably larger than M2 (M3/M2 ratio = 115%).
There are five isolated permanent lower teeth assigned to N. nakayamai (Figs. 2 and 3). A right (KNM-NA46429) and a left (KNM-NA46436) M3 are slightly worn and are considerably smaller than the M3 in the type mandible. They are ≈70% of the latter in crown area, suggesting the presence of high levels of intraspecific variation (possibly sexual dimorphism) in N. nakayamai. Otherwise, they exhibit a similar morphological pattern to the larger M3. The cusps are massive and rather peripherally positioned. The occlusal foveae and basin are moderately spacious. The enamel is thick with a low dentine/enamel relief (SI Figs. 10 and 11). The crown tapers slightly near the distal end. The buccal cingulum is interrupted on the buccal aspect of the protoconid but is prominent distally, surrounding the buccal aspect of the hypoconid. KNM-NA46424 is a right P4 that matches in size the lower molars of the type mandible. The occlusal outline is asymmetrical with a protruding distolingual corner. The two main cusps are massive and peripherally located. The distal fovea is much larger than the mesial fovea. The buccal surface of the crown is decorated basally with a discontinuous but prominent cingulum. KNM-NA46423 is a left P3. It is relatively small and corresponds in size to the smaller M3s. The crown is short and broad with a semitriangular occlusal outline. The mesiobuccal slope is short and is decorated with short, marked cingula both mesially and distally. The lingual crest of the protoconid runs down lingually, forming an approximately right angle with the distal crest of the protoconid. Consequently, the distal fovea is quite wide. The basal portion of the lingual crest is slightly swollen, from which a short crest runs distally. The lingual cingulum is prominent and is continuous along the whole lingual aspect. KNM-NA46425 is a right I2. The crown is high and mesiodistally narrow. In lingual view, the mesial margin is nearly straight, whereas the distal margin is strongly convex distally. The marginal crests are weak, and there is no distinct lingual cingulum at the base of the lingual aspect.
Five permanent upper teeth are assigned to N. nakayamai (Figs. 2 and 3). KNM-NA47591 is a right M1 crown. This molar is relatively elongated mesiodistally and is low-crowned with low and massive cusps. The cusps are peripherally positioned as in the lower molars. There is a very short but distinct cingular remnant on the mesiolingual corner of the protocone. Two upper premolars, a right P3 (KNM-NA46431) and a left P4 (KNM-NA46430), are buccolingually narrow and mesiodistally elongated. The cusps are peripherally located. The cusp heteromorphy is not extensive. In the P3 the buccal moiety is slightly longer than the lingual moiety. The paracone is moderately higher than the protocone. The mesial transverse crest is developed, but the distal transverse crest is obscure. Styles are developed at the base of the mesial and distal crests of the paracone. The buccal cingula are discontinuous but prominent. There is a shallow, short groove at the distal end of the lingual aspect of the crown. The P4 is oval in occlusal outline. The paracone is only slightly higher than the protocone. The mesial transverse crest is continuous, but the distal transverse crest is interrupted by a longitudinal groove. KNM-NA47592 is a left I1. The crown is low and is mesiodistally wide and buccolingually thick. The lingual aspect is bordered basally by a high lingual cingulum that becomes continuous with the mesial and distal marginal crests. The lingual surface is crenulated with a thick, rounded crest rising from the lingual cingulum and a few other vertical wrinkles. The distal margin of the crown is markedly curved and meets the incisive edge at a blunt angle. KNM-NA47594 is a right upper canine. The crown is low and is as long as broad, having short mesial crest and groove. The lingual cingulum is elevated to a very high position (≈40% of the lingual crown height) and prominently differentiated, being continuous along the lingual aspect of the crown. An extremely large tubercle is developed on the lingual cingulum. Light wear facets are observed at the basal part of the mesial crest, at the apex, along the distal crest, and on the lingual cingulum distal to the lingual tubercle apex.
In addition to the permanent teeth, there is one left dp4 (KNM-NA46435). The mesial end of the crown is slightly narrowed buccolingually. The buccal cingulum is strongly reduced to form a short and weak groove on the mesiobuccal face of the protoconid and very faint depressions at the bases of the buccal grooves. The five main cusps are massive and low. The protoconid is positioned slightly mesially relative to the metaconid. The protoconid and metaconid are linked by a thick mesial transverse crest, which runs slightly obliquely. The hypoconulid is smaller than the hypoconid and is positioned close to the latter. The entoconid is as small as the hypoconulid, and these two cusps are connected to each other by a low distal transverse crest. The distal fovea is relatively broad and is only slightly oblique. The talonid basin, coarsely crenulated with several wrinkles, is long and narrow and is incised with fine intercuspal grooves.
Comparisons with Other Hominoids
N. nakayamai is distinguished from the East African Early Miocene hominoids such as Proconsul, Ugandapithecus, and Afropithecus in its much larger dental size and having mesiodistally elongated upper premolars with reduced cusp heteromorphy, bilaterally less compressed P3, and low-crowned I1. In Proconsul and Ugandapithecus, whereas the superior transverse torus is developed, the inferior transverse torus is absent or very weak in contrast to the well developed inferior transverse torus of N. nakayamai, and the molar cingula are better-developed in these Early Miocene taxa, especially on the upper molars, differing from the reduced and discontinuous condition in N. nakayamai. Afropithecus has an inferior transverse torus better-developed than the superior one, but not to the extent observed in N. nakayamai. In addition, compared with the molar size, the mandibular body of Afropithecus is relatively taller than that of N. nakayamai.
The early Middle Miocene East African hominoids such as Kenyapithecus, Equatorius, and Nacholapithecus are baboon-sized animals (20, 21), somewhat smaller than N. nakayamai. They differ from N. nakayamai in having bilaterally compressed P3 crowns and broader upper premolars. Nacholapithecus differs from N. nakayamai in having stronger cusp heteromorphy of upper premolars (21). Kenyapithecus and Equatorius are distinguished from N. nakayamai in having a lower and thicker mandibular body.
A few isolated teeth of hominoids are known from the Ngorora Formation (≈12.5 Ma) in Tugen Hills, ≈80 km southwest of Nakali (5). The Ngorora premolar (P4) is assigned to Proconsul sp. Compared with the Nakali P4 (KNM-NA46424), the crown is considerably smaller, and the cusps are relatively higher and more crystalline and are more centrally located. The Ngorora molar (M1 or M2) is the size of those of female gorillas. Hill and Ward (5) considered that the best match is with modern chimpanzee second molars, although the Ngorora molar is larger. In our observation the cusp configuration also appears reminiscent of that of modern gorillas, but the cusps are lower. The occlusal foveae and basin are more spacious than those of the Nakali upper molar. In addition, micro-computed tomography scanning has revealed that the enamel of the Ngorora molar is not as thick as in N. nakayamai (Y.K., unpublished data). Recently, Pickford and Senut (22) reported a lower molar of a chimpanzee-sized hominoid from Ngorora. Its phylogenetic relationship is still uncertain, but it is too small to belong to the same taxon with N. nakayamai.
Among the African Miocene hominoids, N. nakayamai is nearly contemporaneous with S. kiptalami, known from Samburu Hills, ≈70 km north of Nakali. The type maxilla of S. kiptalami (KNM-SH 8531) and the type mandible of N. nakayamai are of similar dental size and are as large as those of female gorillas. The upper premolars of Samburupithecus resemble those of N. nakayamai in being elongated mesiodistally, but the cusps are more inflated and positioned more centrally. In the upper molars of Samburupithecus the cusps are quite strongly inflated and positioned centrally so that the occlusal foveae and basins are extremely restricted (7). In these features, Samburupithecus seems to be strongly specialized compared with other Miocene and extant apes. There is an upper molar included in the present hypodigm of N. nakayamai, which has less inflated and more peripherally positioned cusps and more spacious foveae and basins. In addition, the dentine/enamel junction of Samburupithecus shows a higher relief relative to the flatter dentine/enamel junction of N. nakayamai. These features clearly distinguish N. nakayamai from Samburupithecus.
The other contemporaneous Miocene hominoids are all known from Eurasia: Dryopithecus from western and central Europe, Ankarapithecus from Turkey, Sivapithecus from South Asia, Lufengpithecus from southwestern China, and Khoratpithecus from Thailand (23). They are smaller than N. nakayamai, except for Ouranopithecus (24, 25) and S. parvada (26). Among the Late Miocene Eurasian hominoids, Ankarapithecus, Sivapithecus, Lufengpithecus, and Khoratpithecus are broadly considered to belong to the Pongo clade (9, 10). Although the specimens of Lufengpithecus are heavily deformed, Ankarapithecus, Sivapithecus, and Khoratpithecus have more robust mandibular bodies, which become very thick posteriorly and form a very wide extramolar sulcus between the M3 and ascending ramus. The remaining part of the body of the Nakali mandible (KNM-NA46400) shows that it is not as robust as in these Eurasian Miocene hominoids. In this aspect, it is more similar to the condition in Ouranopithecus. In fact, the body height, thickness, and height/thickness index at M1 in the Nakali mandible are very close to those in the larger mandibles of Ouranopithecus. In N. nakayamai, M1 is relatively large with the M1/M2 ratios in area (MD × BL) being 85%. A relatively enlarged M1 is characteristic of extant great apes, Australopithecus, and some Middle to Late Miocene Eurasian hominoids such as Ouranopithecus (24, 25) and Dryopithecus (27), whereas the relative M1 size is considerably smaller in the Early and Middle Miocene hominoids of East Africa. Ankarapithecus, Lufengpithecus, Khoratpithecus, and Sivapithecus are intermediate in this feature (SI Table 3). N. nakayamai has a M3 considerably larger than M2 (M3/M2 = 115%). In this feature it is similar to Ouranopithecus (24, 25) (SI Fig. 12) and geologically older Sivapithecus (Sivapithecus indicus) from Chinji (12.7–11.2 Ma) and S. parvada (≈10 Ma) (28). Geologically younger Sivapithecus (Sivapithecus sivalensis), Dryopithecus, Lufengpithecus, and possibly Ankarapithecus have a less enlarged M3 (27, 29). Khoratpithecus piriyai is quite different from N. nakayamai and other hominoids, both extinct and extant, in having M3 exceptionally larger than M2 [RIN765, M3/M2 = 147% (10)], even exceeding those of the Early and Middle Miocene hominoids that show M3/M2 ratios as large as or even larger than in N. nakayamai (SI Table 3). In Ankarapithecus and Sivapithecus the buccal cingulum of the lower molars is absent, as in extant Pongo. In N. nakayamai the buccal cingulum is reduced and discontinuous, compared with Early Miocene taxa like Proconsul, but the cingular remnants are rather marked on the mesiobuccal aspect of the protoconid and between buccal cusps. In Ouranopithecus the buccal cingulum is more reduced, although weak remnants are still observed.
As in the case for the Early to early Middle Miocene African hominoids, the P3 morphology distinguishes N. nakayamai from the majority of the Middle to Late Miocene Eurasian hominoids, whose P3s are more compressed bilaterally and have a more distally oriented lingual crest of the protoconid, resulting in a much narrower distal fovea. In contrast, Ouranopithecus and N. nakayamai share P3s that are less compressed and semitriangular in occlusal outline with a low main cusp, from which the lingual crest runs almost directly lingually and delimits a very broad distal basin. The P3 of N. nakayamai, however, develops the lingual and buccal cingula more strongly than in Ouranopithecus, and the mesiobuccal slope is slightly less vertical relative to similar-sized (presumed female) P3s of Ouranopithecus. Some Sivapithecus specimens from stratigraphically younger localities are reported to have relatively broad P3s (27), but, compared with P3s of N. nakayamai and Ouranopithecus, their crowns are still more compressed bilaterally with a narrow distal basin. Dryopithecus from younger localities are said to have very short, sometimes triangular P3s (27), but Dryopithecus is clearly different from N. nakayamai in its smaller size and thinly enameled molars with high dentine penetration (23). The upper premolars, which are buccolingually broad relative to the mesiodistal length, are different from the relatively long and narrow upper premolars of N. nakayamai. Although the molar cingula are rather prominent in the older species of Dryopithecus (Dryopithecus fontani), they are reduced in younger species such as Dryopithecus brancoi (23), which are contemporaneous with N. nakayamai.
Although female upper canines of Miocene hominoids are low-crowned and have a well developed lingual cingulum in general, the upper canine of N. nakayamai is distinguished from them by the combination of the following features: crown as broad as long, a well differentiated and elevated lingual cingulum, and an extremely large lingual cusplet on the cingulum, showing a tendency for premolarization. In the mesiodistal and buccolingual dimensions, the upper canine of N. nakayamai is similar to the smaller (presumably female) canines of Ouranopithecus. In N. nakayamai, however, the upper canine is slightly higher-crowned with the mesial shoulder more elevated from the cervix. The upper canines of Ouranopithecus are heavily worn and/or damaged, but in some specimens, such as RPl128 (presumed male) and RPl775 [sex disputed (30, 31)], there seems to be a swelling on the basal part of the lingual aspect. It does not appear, however, to be so clearly differentiated as in N. nakayamai. In presumedly female specimens (NKT 89, RPl208), the relatively well preserved upper canines do not have such a swelling, and the lingual cingulum is more weakly expressed than in N. nakayamai. Some upper canines (but not all) of Sivapithecus have the long axis of the root cross section strongly rotated externally so that the maximum crown dimension is oriented more or less buccolingually. Consequently, the upper canine crown is sometimes buccolingually broadened, but it does not show the characteristic lingual morphology of N. nakayamai (SI Fig. 13).
Because C. abyssinicus from Ethiopia was published after our article was submitted, we do not yet know how N. nakayamai relates to C. abyssinicus. As far as we can recognize from the descriptions and figures in ref. 18, N. nakayamai differs from C. abyssinicus in the following features: The distal portion of M3 is well developed, having large hypoconid, entoconid, and hypoconulid and a large distal fovea with a faint tubercle on the distal cingulum, so that the buccal and lingual margins of the crown run approximately parallel to each other except for the distal end. The buccal cingulum on M3 is much more strongly expressed. The size ranges of the Nakali and Chorora specimens suggest that C. abyssinicus could be larger than N. nakayamai.
Paleoenvironments
The known faunal assemblage of Nakali (SI Table 4) is similar to that of Samburu Hills (Namurungule Formation) (32, 33). These early Late Miocene faunas of East Africa contain components similar to the Pikermian Biome, which was established on seasonal sclerophyllous evergreen woodlands covering Greece, Turkey, Iran, and the Sahara (34). This suggests that N. nakayamai, Samburupithecus, and Ouranopithecus may have lived under more or less similar ecological circumstances.
Nevertheless, there were probably some differences in paleoenvironments among Nakali, Samburu Hills, and Greece. Stable isotopic studies have indicated that the paleoenvironment of Nakali was C3-plant-dominant whereas that of Samburu Hills was in an intermediate stage between C3-plant-dominant and C4-plant-dominant conditions (refs. 35 and 36 and T. Cerling, personal communication). The presence of small non-cercopithecoid catarrhines and a colobine monkey (SI Table 4) supports the interpretation that the paleoenvironment of Nakali was more forested than at the Samburupithecus site. The stable isotopic study by Quade et al. (37) suggests that C3/C4 transition did not occur in Greece. This is likely because of the higher latitudes of the region, as is the case of North America, where C3/C4 transition is not observed in the area above 37° N (38). It is therefore difficult to compare the paleoenvironments between Greece and Nakali based on stable isotopic studies. However, Ouranopithecus has more inflated cusps and thicker enamel, with the occlusal surface becoming more flatly worn compared with N. nakayamai. In other words, Ouranopithecus is probably more specialized in its molar morphology to adapt to hard/abrasive-object feeding than N. nakayamai. These differences suggest that Ouranopithecus may have lived in a drier and more open, and perhaps more seasonal, paleoenvironment than Nakali.
Discussion
The discovery of N. nakayamai has great importance for research on African great ape and human origins because of the poor nature of the hominoid fossil record in Africa after the mid-Miocene. An exception is S. kiptalami, the type maxilla of which was discovered from Samburu Hills by the Kenya–Japan Joint Project team in 1982 (39). Despite subsequent fieldwork through more than two decades, no additional hominoid specimen has been recovered from the Samburu Hills. The Nakali Expedition, however, has revealed that Samburupithecus is not the only hominoid that survived in Africa during 10–9 Ma. Nakali is close to Samburu Hills, both geographically and chronologically, but N. nakayamai is clearly different from Samburupithecus in a number of morphological features. In addition, the Nakali material includes an isolated P3 (KNM-NA46434) that is quite dissimilar to that of either N. nakayamai or Samburupithecus, suggesting the presence of another, more primitive large hominoid taxon at Nakali (SI Table 4). It has, therefore, turned out that the diversity of large-bodied hominoids in the early Late Miocene of East Africa (10–9 Ma) was higher than previously recognized.
Because of the paucity of African hominoid fossils in the later Miocene and the presence of some Eurasian apes that show affinities to extant great apes, it has been suggested that some Eurasian Miocene hominoid may have come back to Africa in the early Late Miocene to become the last common ancestor of extant African great apes and humans (11, 13). However, the Nakali and Samburu Hills primate faunas show that three large hominoids and two small non-cercopithecoid catarrhines coexisted with a small colobine monkey at ≈10 Ma, even within a relatively small area in Kenya. The primate fauna from the slightly older Ngorora localities (≈12.5 Ma) probably includes three large hominoids, a small non-cercopithecoid catarrhine, and a primitive cercopithecoid (5, 38, 40). These findings support the idea that non-cercopithecoid catarrhines survived and kept their diversity through the Middle to Late Miocene in Africa. Consequently, it has become less likely that hominoid primates were extinguished completely in Africa and were reintroduced from Eurasia.
It is interesting that, among the known fossil hominoids, N. nakayamai resembles Ouranopithecus from the early Late Miocene of Greece, in size and some dentognathic features. Ouranopithecus has often been suggested to be close to the ancestry of the African great ape and human clade (11, 12, 41, 42), but it was known only from Greece in southeastern Europe. However, the possibility that Ouranopithecus or a very similar genus would be discovered in Africa in the future has not been discounted (43). On the other hand, Nakalipithecus occurs in Africa and shows more primitive features, such as better expressed molar cingula, less inflated molar cusps, and more spacious occlusal basins and foveae. Moreover, the age of Nakali (9.9–9.8 Ma) predates the currently known First Appearance Date of Ouranopithecus (9.6 Ma). More fossil discoveries are needed to elucidate the phylogenetic relationship between the Nakali and the Greek hominoid and the actual circumstances of the hominoid dispersals between Africa and Eurasia. Nevertheless, given the retention of more primitive features and the slightly older age of N. nakayamai, even if the morphological similarities between Nakalipithecus and Ouranopithecus indicate their close phylogenetic relationship rather than the result of convergence, it appears more likely that dispersal may have occurred from Africa to southeastern Europe. There is also another possibility that the morphological similarities of N. nakayamai to Ouranopithecus may have been acquired for similar functional requirements, very likely for dietary adaptations in drier and more open environments. In either case, the discovery of N. nakayamai in the early Late Miocene of East Africa suggests that it is highly probable that large-bodied hominoids survived through the Middle to Late Miocene in Africa, giving rise to the last common ancestor of African great apes and humans. The Chorora findings by Suwa et al. (18) also support this idea. Three large-bodied hominoids are now known from the African early Late Miocene (Nakali and Samburu in Kenya and Chorora in Ethiopia). Although more fossil discoveries and analyses are needed to reveal the phyletic relationships among them and to other hominoids, it is likely that these early Late Miocene African hominoids are more or less close to the last common ancestor of the African great apes and humans, although any of them may not be the last common ancestor itself.
Supplementary Material
Acknowledgments
We thank the Government of Kenya for research permission; B. Onyango, W. Mangao, N. Kanyenze, P. Nzube, D. Mutinda, R. Tayama, and Y. Matsuda for their support during fieldwork; and H. Ishida for logistic support. We are grateful to B. Richmond, T. Harrison, and B. Roser for commenting on the manuscript; to G. Koufos for permitting us to examine the original specimens of O. macedoniensis; and to L. de Bonis and R. Macchiarelli for providing us with O. macedoniensis computed tomography images. We also appreciate assistance from the Japan Society for the Promotion of Science Nairobi Research Station. This expedition was funded by grants from the Japan Society for the Promotion of Science and the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grants 18255006, 18370078, 16370104, 14253006, 15570193, 18370098, 19207019, and 21COE A14, Core-to-Core Program HOPE).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706190104/DC1.
References
- 1.Goodman M, Porter CA, Czelusniak J, Page SL, Schneider H, Shoshani J, Gunnell G, Groves CP. Mol Phylogenet Evol. 1998;9:585–598. doi: 10.1006/mpev.1998.0495. [DOI] [PubMed] [Google Scholar]
- 2.Stauffer RL, Walker A, Ryder OA, Lyons-Weiler M, Blair Hedges S. J Hered. 2001;92:469–474. doi: 10.1093/jhered/92.6.469. [DOI] [PubMed] [Google Scholar]
- 3.Glazko GV, Nei M. Mol Biol Evol. 2003;20:424–434. doi: 10.1093/molbev/msg050. [DOI] [PubMed] [Google Scholar]
- 4.Steiper ME, Young NM. Mol Phylogenet Evol. 2006;2006:384–394. doi: 10.1016/j.ympev.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Hill A, Ward S. Yearb Phys Anthropol. 1988;31:49–83. [Google Scholar]
- 6.Cote SM. C R Palevol. 2004;3:323–340. [Google Scholar]
- 7.Ishida H, Kunimatsu Y, Nakatsukasa M, Nakano Y. Anthropol Sci. 1999;107:189–191. [Google Scholar]
- 8.Sawada Y, Pickford M, Itaya T, Makinouchi T, Tateishi M, Kabeto K, Ishida S, Ishida H. C R Acad Sci Paris. 1998;326:445–451. [Google Scholar]
- 9.Andrews P, Bernor RL. In: Hominoid Evolution and Climatic Change in Europe. Agustí J, Rook L, Andrews P, editors. Vol 1. Cambridge, UK: Cambridge Univ Press; 1999. pp. 454–487. [Google Scholar]
- 10.Chaimanee Y, Suteethorn V, Jintasakul P, Vidtthayanon C, Marandat B, Jaeger J-J. Nature. 2004;427:439–441. doi: 10.1038/nature02245. [DOI] [PubMed] [Google Scholar]
- 11.Begun DR. Anthropol Sci. 2005;113:53–64. [Google Scholar]
- 12.Andrews P, Harrison T, Delson E, Bernor RL, Martin L. In: The Evolution of Western Eurasian Neogene Mammal Faunas. Bernor RL, Fahlbusch V, Mittmann H-W, editors. New York: Columbia Univ Press; 1996. pp. 168–207. [Google Scholar]
- 13.Moyà-Solà S, Köhler M, editors. J Hum Evol. 1995;29:101–139. [Google Scholar]
- 14.Stewart C-B, Disotell TR. Curr Biol. 1998;8:R582–R588. doi: 10.1016/s0960-9822(07)00367-3. [DOI] [PubMed] [Google Scholar]
- 15.Nakatsukasa M, Kunimatsu Y, Sawada Y, Sakai T, Hyodo H, Itaya T, Saneyoshi M, Tsujikawa H, Mbua E. Am J Phys Anthropol. 2006;129:136. [Google Scholar]
- 16.de Bonis L, Melentis J. C R Acad Sci Paris. 1977;284:1393–1397. [Google Scholar]
- 17.Agustí J, Cabrera L, Garcés M. In: Hominoid Evolution and Climate Change in Europe: Phylogeny of the Neogene Hominoid Primates of Eurasia. de Bonis L, Koufos GD, Andrews P, editors. Vol 2. Cambridge, UK: Cambridge Univ Press; 2001. pp. 2–18. [Google Scholar]
- 18.Suwa G, Kono RT, Katoh S, Asfaw B, Beyene Y. Nature. 2007;448:921–924. doi: 10.1038/nature06113. [DOI] [PubMed] [Google Scholar]
- 19.Cande SC, Kent DV. J Geophys Res. 1995;100:6093–6095. [Google Scholar]
- 20.Pickford M. J Hum Evol. 1985;14:113–143. [Google Scholar]
- 21.Kunimatsu Y, Ishida H, Nakatsukasa M, Nakano Y, Sawada Y, Nakayama K. J Hum Evol. 2004;46:365–400. doi: 10.1016/j.jhevol.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Pickford M, Senut B. Anthropol Sci. 2005;113:95–102. [Google Scholar]
- 23.Begun DR. In: The Primate Fossil Record. Hartwig WC, editor. Cambridge, UK: Cambridge Univ Press; 2002. pp. 339–367. [Google Scholar]
- 24.Koufos GD. Am J Phys Anthropol. 1993;91:225–234. doi: 10.1002/ajpa.1330910208. [DOI] [PubMed] [Google Scholar]
- 25.Koufos GD, de Bonis L. Geobios. 2006;39:223–243. [Google Scholar]
- 26.Kelley J. J Hum Evol. 1988;17:305–324. [Google Scholar]
- 27.Begun DR, Güleç E, editors. Am J Phys Anthropol. 1998;105:279–314. doi: 10.1002/(SICI)1096-8644(199803)105:3<279::AID-AJPA2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 28.Kelley J. In: Interpreting the Past: Essays on Human, Primate, and Mammal Evolution. Lieberman DE, Smith RA, Kelley J, editors. Boston: Brill Academic Publishers; 2005. pp. 123–143. [Google Scholar]
- 29.Kelley J, Plavcan JM. J Hum Evol. 1998;35:577–596. doi: 10.1006/jhev.1998.0253. [DOI] [PubMed] [Google Scholar]
- 30.de Bonis L, Koufos GD, Guy F, Peigné S, Sylvestrou I. C R Acad Sci Paris. 1998;327:141–146. [Google Scholar]
- 31.Kelley J. In: Hominoid Evolution and Climate Change in Europe: Phylogeny of the Neogene Hominoid Primates of Eurasia. de Bonis L, Koufos GD, Andrews P, editors. Vol 2. Cambridge, UK: Cambridge Univ Press; 2001. pp. 269–271. [Google Scholar]
- 32.Tsujikawa H, Nakaya H. Chikyu Monthly. 2005;27:603–611. [Google Scholar]
- 33.Tsujikawa H. Afr Study Monogr Suppl. 2005;32:1–50. [Google Scholar]
- 34.Solounias N, Plavcan JM, Quade J, Witmer L. In: Hominoid Evolution and Climatic Change in Europe: The Evolution of Neogene Terrestrial Ecosystems in Europe. Agustí J, Rook L, Andrews P, editors. Vol 1. Cambridge, UK: Cambridge Univ Press; 1999. pp. 436–453. [Google Scholar]
- 35.Cerling TE, Harris JM, MacFadden BJ, Leakey MG, Quade J, Eisenmann V, Ehleringer JR. Nature. 1997;389:153–158. [Google Scholar]
- 36.Cerling TE, Harris JM, Leakey MG. Oecologia. 1999;120:364–374. doi: 10.1007/s004420050869. [DOI] [PubMed] [Google Scholar]
- 37.Quade J, Solounias N, Cerling TE. Palaeogeogr Palaeoclimatol Palaeoecol. 1994;108:41–53. [Google Scholar]
- 38.Rossie JB, Hill A. Am J Phys Anthropol. 2005;126:178–179. doi: 10.1002/ajpa.20139. [DOI] [PubMed] [Google Scholar]
- 39.Ishida H, Pickford M. C R Acad Sci Paris. 1997;325:823–829. [Google Scholar]
- 40.Hill A, Leakey M, Kingston JD, Ward S. J Hum Evol. 2002;42:75–93. doi: 10.1006/jhev.2001.0518. [DOI] [PubMed] [Google Scholar]
- 41.Andrews P. Nature. 1990;345:664–665. doi: 10.1038/345664a0. [DOI] [PubMed] [Google Scholar]
- 42.Dean D, Delson E. Nature. 1992;359:676–677. doi: 10.1038/359676a0. [DOI] [PubMed] [Google Scholar]
- 43.de Bonis L, Koufos GD. C R Palevol. 2004;3:257–264. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.