Abstract
Hypothalamic malonyl-CoA has been shown to function in global energy homeostasis by modulating food intake and energy expenditure. Little is known, however, about the regulation of malonyl-CoA concentration in the central nervous system. To address this issue we investigated the response of putative intermediates in the malonyl-CoA pathway to metabolic and endocrine cues, notably those provoked by glucose and leptin. Hypothalamic malonyl-CoA rises in proportion to the carbohydrate content of the diet consumed after food deprivation. Malonyl-CoA concentration peaks 1 h after refeeding or after peripheral glucose administration. This response depends on the dose of glucose administered and is blocked by the i.c.v. administration of an inhibitor of glucose metabolism, 2-deoxyglucose (2-DG). The kinetics of change in hypothalamic malonyl-CoA after glucose administration is coincident with the suppression of phosphorylation of AMP kinase and acetyl-CoA carboxylase. Blockade of glucose utilization in the CNS by i.c.v. 2-DG prevented the effects of glucose on 5′AMP-activated protein kinase, malonyl-CoA, hypothalamic neuropeptide expression, and food intake. Finally, we showed that leptin can increase hypothalamic malonyl-CoA and that the increase is additive with glucose administration. Leptin-deficient ob/ob mice, however, showed no defect in the glucose- or refeeding-induced rise in hypothalamic malonyl-CoA after food deprivation, demonstrating that leptin was not required for this effect. These studies show that hypothalamic malonyl-CoA responds to the level of circulating glucose and leptin, both of which affect energy homeostasis.
Keywords: acetyl-CoA carboxylase, AMP kinase, carnitine palmitoyl-transferase 1c, fatty acid synthase
An interconnected endocrine and neuroendocrine system controls food intake and energy expenditure (1). Recently, a new pathway for maintaining energy homeostasis has become evident that relies on an ancient nutrient-sensing pathway whereby the CNS directly monitors energy needs by sampling cellular adenine nucleotide levels and responds by directing hunger and peripheral energy expenditure (2, 3). Neural cellular energy status is monitored through AMPK, which directly senses the [AMP]/[ATP] ratio. The AMPK system provides a rapid means of detecting energy status not reliant directly on endocrine signals. The activation of AMPK leads to the inhibition of the key regulatory enzyme of fatty acid synthesis, acetyl-CoA carboxylase (ACC). The activity of this enzyme is an indicator of energy surplus and is thought to be one of the mechanisms by which energy homeostasis is mediated. Several enzymes that are involved in fatty acid metabolism have been implicated in the CNS control of energy homeostasis including AMPK (4–7), ACC (8, 9), fatty acid synthase (FAS) (8, 10), carnitine palmitoyl-transferase 1 (CPT1) (11, 12), and stearoyl-CoA desaturase 1 (13). Because fatty acid synthesis is a process that occurs primarily during energy surplus, it is not surprising that this system has evolved as a means to regulate energy homeostasis.
The product of ACC, malonyl-CoA, is known to regulate energy metabolism in muscle and liver through the inhibition of mitochondrial fatty acid oxidation. While malonyl-CoA serves as the basic chain-elongating unit of fatty acid synthesis, it also serves a signaling role as an allosteric inhibitor of CPT1, an outer mitochondrial membrane enzyme, by blocking entry of fatty acids into the mitochondrion for fatty acid oxidation (14). It has been proposed that malonyl-CoA in the CNS is an effector molecule for the metabolic regulation of energy homeostasis (15). Inhibition of FAS, which carries out the succeeding step in fatty acid synthesis, by pharmacologic (8) or genetic (10) means in the CNS elevates malonyl-CoA and suppresses food intake, resulting in a lean phenotype. This suppression of food intake can be reversed by treating mice with an inhibitor of ACC, suggesting that malonyl-CoA is responsible (8, 16). Furthermore, removal of hypothalamic malonyl-CoA by overexpressing malonyl-CoA decarboxylase results in increased body weight (17) and ultimately obesity (18). These findings strongly suggest that malonyl-CoA is an important component in satiety signaling. The mechanism of action of malonyl-CoA has not been fully elucidated. Evidence suggests that the brain-specific CPT1c may be involved, because it binds to malonyl-CoA and the disruption of its gene results in a phenotype consistent with a role as a CNS malonyl-CoA target protein (11).
It is important to establish a physiological role for malonyl-CoA and to position this regulatory mechanism in the appropriate context. Hypothalamic malonyl-CoA fluctuates between food-deprived and refed states, suggesting that it is physiologically relevant (16); however, the regulation of hypothalamic malonyl-CoA is not well understood. Malonyl-CoA is synthesized de novo from glucose. Glucose inhibits food intake, and blockage of glucose utilization (19) or uptake increases food intake (20) although the precise role for neuronal glucose sensing is not well understood (21). These observations are consistent with a role for malonyl-CoA and suggest that hypothalamic glucose sensing may be a major regulatory mechanism for malonyl-CoA.
Here we report the effects of CNS glucose metabolism and leptin signaling on changes in hypothalamic malonyl-CoA and its downstream consequences. Evidence shows that both glucose metabolism by the CNS and leptin increase the level of malonyl-CoA in the hypothalamus; however, the effect of glucose on malonyl-CoA is independent of the leptin signaling pathway.
Results
Dietary Carbohydrate and Peripheral Glucose Administration Modulate Hypothalamic Malonyl-CoA Concentration.
Previously we reported that the hypothalamic malonyl-CoA level rises rapidly (≤2 h) upon refeeding food-deprived mice, a response that is coincident with suppressed food intake (16) and elevated peripheral energy expenditure due to increased muscle fatty acid oxidation (22, 23). To understand the dynamics, requirements, and regulation of the hypothalamic malonyl-CoA signaling pathway, we first determined the kinetics of the change in hypothalamic malonyl-CoA content after an abrupt increase in blood glucose, the ultimate source of malonyl-CoA. We used a standard glucose tolerance protocol in which 1 g of glucose per kilogram of body weight was administered i.p. to mice that had been deprived of food for 20 h. Plasma and hypothalamus were collected every 30 min over the next 2 h. Plasma glucose peaked at 30 min, and hypothalamic malonyl-CoA peaked at 1 h (Fig. 1A). The 30-min lag in malonyl-CoA is consistent with a delay in the transport of blood glucose to the brain from the site of injection and translocation across membrane barriers.
Fig. 1.
Kinetic analysis of hypothalamic malonyl-CoA in food-deprived mice after i.p. glucose administration or feeding. (A) Mice deprived of food for 20 h were given an i.p. injection of glucose (1 g/kg of body weight). Plasma was collected every 30 min, and glucose was determined. Hypothalami were collected every 30 min, and malonyl-CoA concentration was determined (n = 4 per time point). (B) Mice deprived of food for 20 h were given ad libitum high-carbohydrate/low-fat chow, low-carbohydrate/high-fat diet, or an i.p. injection of glucose (8 g/kg of body weight). Plasma was collected hourly, and glucose was determined. Hypothalami were collected hourly, and malonyl-CoA concentration was determined. Malonyl-CoA was plotted on the basis of fold induction from food-deprived levels (n = 4 per time point).
We next compared the effect of glucose administration on hypothalamic malonyl-CoA with the response to refeeding. Mice deprived of food for 20 h were given glucose i.p. (without feeding) or were refed a high-carbohydrate/low-fat diet (10% of calories from fat). To verify the role of the dietary carbohydrate, a comparison was also made to the response provoked by a low-carbohydrate/high-fat diet (60% of calories from fat). As shown in Fig. 1B mice refed the high-carbohydrate/low-fat diet responded similarly to those given glucose by i.p. injection, with peak hypothalamic malonyl-CoA occurring at ≈1 h followed by decreases at 2–3 h. The low-carbohydrate/high-fat diet group, however, exhibited little change in blood glucose and a modest change in hypothalamic malonyl-CoA (Fig. 1B). Thus, the effect of glucose injected i.p. on hypothalamic malonyl-CoA mimicked that of refeeding a carbohydrate-rich diet to food-deprived mice. Of note, in all cases plasma free fatty acids fell dramatically upon both refeeding the high-carbohydrate/low-fat diet and glucose injection, whereas the response to the low-carbohydrate/high-fat diet was somewhat attenuated (Fig. 1B).
Although it was evident that an increase in blood glucose was associated with a transient rise in hypothalamic malonyl-CoA, we sought to determine whether the rise in hypothalamic malonyl-CoA is proportional to blood glucose concentration. To address this question, mice were deprived of food for 20 h and then given i.p. injections of increasing levels of glucose. Plasma glucose was determined at 30 min, and malonyl-CoA was measured at 1 h (their respective peak levels). Hypothalamic malonyl-CoA increased in a glucose-dependent manner (Fig. 2A). Concurrently, food-deprived mice were refed either the high-carbohydrate/low-fat diet or the low-carbohydrate/high-fat diet. Both diets produced a higher hypothalamic malonyl-CoA with a somewhat lower relative blood glucose level than glucose injection alone. Consistent with the observations above (Fig. 1B), feeding appears to exert an additional effect on hypothalamic malonyl-CoA (Fig. 2B). i.p. injection of glucose (4 g/kg of body weight) to low-carbohydrate/high-fat fed animals increased both blood glucose and hypothalamic malonyl-CoA, suggesting that the effect of the combination of glucose and refeeding is additive (Fig. 2B).
Fig. 2.
Dependence of hypothalamic malonyl-CoA on glucose. (A) Mice deprived of food for 20 h were given 1, 2, 4, and 8 g of glucose or vehicle per kilogram of body weight. Plasma was collected at 30 min after injection, and glucose was determined. Hypothalami were collected at 1 h, and malonyl-CoA concentration was determined (n = 4 per group) (P > 0.002; malonyl-CoA ANOVA). (B) Mice deprived of food for 20 h were fed a high-carbohydrate/low-fat diet and vehicle, a low-carbohydrate/high-fat diet and vehicle, or low-carbohydrate/high-fat diet and 4 g of glucose per kilogram of body weight. Plasma was collected at 30 min after injection, and glucose was determined (red bars). Hypothalami were collected at 1 h, and malonyl-CoA concentration was determined (blue bars) (n = 4 per group). (C) Mice deprived of food for 20 h were given glucose at 4 g/kg of body weight at times 0, 1, and 2 h repetitively. Glucose was measured every half-hour, and hypothalamic malonyl-CoA was measured hourly (n = 4 per time point).
The effect of repetitive glucose injections on hypothalamic malonyl-CoA was also assessed. Mice deprived of food for 20 h were given i.p. injections of the intermediate dose of glucose (4 g/kg of body weight) at times zero, 1 h, and 2 h. Blood glucose exhibited an increase at 30 min and began to decrease during the next 30 min (Fig. 2C). With each subsequent glucose injection the clearance of blood glucose became less effective, apparently because of the exhaustion of glucose-induced insulin secretion (results not shown), and did not rise significantly after the injection at 2 h. Hypothalamic malonyl-CoA reached a maximum of ≈1.4 μM (Fig. 2C), apparently having reached the maximum achievable with glucose alone.
Central Glucose Metabolism Is Required for Signaling to Increase Hypothalamic Malonyl-CoA.
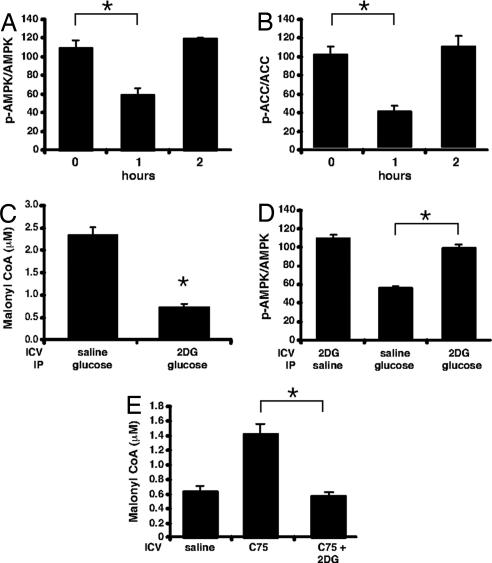
The most plausible explanation for the mechanism by which an increase in blood glucose provokes a rise in hypothalamic malonyl-CoA is as follows: (i) that increased glucose flux into the hypothalamus leads to an increase in malonyl-CoA and an increase in glucose oxidation leading to a decreased [AMP]/[ATP] ratio and thereby (ii) inhibition/deactivation of AMPK and thereby (iii) release of ACC from inhibitory restraint (dephosphorylation), producing an increase in hypothalamic malonyl-CoA concentration. To test this hypothesis we assessed the phosphorylation states of AMPK and ACC, as indicators of their activities, in mice deprived of food for 20 h or deprived of food and then given glucose i.p. Within 1 h after glucose administration the level of phospho-AMPK and phospho-ACC declined and then returned to the level of food-deprived controls by 2 h (Fig. 3 A and B). This kinetic pattern of AMPK and ACC phosphorylation correlated closely with the kinetics of hypothalamic malonyl-CoA concentration, which increased at 1 h and then decreased by 2 h after glucose administration (Fig. 1B). Our findings for the kinetic effect of glucose administration on phospho-AMPK are consistent with another study although the effects on malonyl-CoA were not reported (4).
Fig. 3.
Dependence of hypothalamic AMPK phosphorylation status on plasma glucose level. Mice deprived of food for 20 h were given an i.p. injection of glucose (4 g/kg of body weight). Hypothalami were collected in RIPA buffer, and SDS/PAGE was performed with total AMPK and phosphospecific AMPK (A) or total ACC and phosphospecific ACC antibodies (B) shown as the ratio of phosphoprotein/total (n = 3 per group). (C) Mice deprived of food for 20 h were given 2 μl of vehicle or 5 mM 2-deoxyglucose i.c.v. and an i.p. dose of vehicle or glucose (4 g/kg of body weight). Hypothalami were collected at 1 h, and malonyl-CoA was determined (n = 4 per group). (D) Mice deprived of food for 20 h were given 2 μl of vehicle or 5 mM 2-deoxyglucose i.c.v. and an i.p. dose of vehicle or glucose (4 g/kg of body weight). Hypothalami were collected at 1 h, and SDS/PAGE was performed with total AMPK and phosphospecific AMPK antibodies shown as above (n = 3 per group). (E) Mice deprived of food for 20 h were given vehicle or 2 μl of C75 (5 mg/ml) or C75 and 5 mM 2-deoxyglucose i.c.v. Hypothalami were collected at 2 h. *, P < 0.002.
To verify that peripherally administered glucose acts centrally, 2-deoxyglucose (2-DG) was administered by i.c.v. injection to block glucose metabolism by the CNS. 2-DG undergoes phosphorylation by hexokinase to produce 2-DG-6-phosphate, a potent inhibitor of glucose-6-phosphate metabolism and the glycolytic pathway. Thus, downstream events including those required to produce malonyl-CoA from peripherally administered glucose are disrupted. 2-DG was administered i.c.v. to food-deprived mice subjected to i.p. peripheral glucose injection, and then 1 h later hypothalamic malonyl-CoA concentration was measured. As illustrated in Fig. 3C, 2-DG completely blocked the glucose-induced increase in hypothalamic malonyl-CoA and also blocked the glucose-induced suppression of AMPK phosphorylation (Fig. 3D). Furthermore, by using a pharmacologic inhibitor of FAS, hypothalamic malonyl-CoA was markedly increased in 20-h food-deprived mice, and 2-DG completely blocked this effect (Fig. 3E). These findings show that peripherally administered glucose requires its metabolism in the CNS to activate the signaling pathway that gives rise to an increase in hypothalamic malonyl-CoA.
The Central Metabolism of Glucose Modulates Anorexigenic and Orexigenic Neuropeptide Expression and Food Intake.
Previous investigations in this laboratory (16) showed that changes in the level of hypothalamic malonyl-CoA alter the expression of neuropeptides that control food intake. To determine the effect of peripherally administered glucose on the expression of key orexigenic and anorexigenic neuropeptides, food-deprived mice were given an i.p. injection of glucose, and 1 h later hypothalamic mRNA was isolated. The levels of POMC, CART, NPY, and AgRP mRNA were then determined by quantitative RT-PCR. As illustrated in Fig. 4A, peripheral administration of glucose led to an increase in the level of hypothalamic POMC mRNA (anorexigenic) and a suppression of hypothalamic NPY and AgRP mRNA (orexigenic), as would be expected. Furthermore, the i.c.v. injection of 2-DG prevented these effects, indicating that the glucose effect was centrally mediated (Fig. 4A). CART levels were not changed, indicating it may be regulated in a different manner.
Fig. 4.
Inhibition of central glucose utilization prevents the response to feeding. (A) Mice deprived of food for 20 h were treated as described. Hypothalami were recovered, and RNA was subjected to quantitative RT-PCR for the neuropeptides listed. (B) Mice deprived of food for 20 h were treated as described, and food intake was monitored for 2 h. *, P < 0.05.
Consistent with the rapid effect of glucose on the expression of the orexigenic and anorexigenic neuropeptides, peripheral administration of glucose rapidly suppressed food intake (Fig. 4B). The effect on food intake was, in part, the result of the central metabolism of the peripherally administered glucose because the i.c.v. injection of 2-DG partially blocked the effect (Fig. 4B).
Effect of Glucose on Hypothalamic Fatty Acid Metabolism.
It was recently suggested that fatty acids, i.e., fatty acyl-CoAs, in the hypothalamus suppress food intake (12, 18, 24, 25). It is evident from numerous physiological studies, including the present report (Fig. 1B), that plasma free fatty acids rise during food deprivation—a condition that provokes hunger—and fall during refeeding—a condition that provokes satiety. Thus, plasma free fatty acids do not qualify as satiety factors. We are left with two possibilities as sources of hypothalamic fatty acyl-CoAs, i.e., de novo fatty acid synthesis or lipolysis-derived fatty acids in the CNS.
To determine whether the level of fatty acyl-CoAs in the hypothalamus is affected by food deprivation and refeeding, we quantified long-chain fatty acyl-CoAs by mass spectrometry. Food-deprived animals were refed either 0.5 g or 3 g of standard lab chow. Hypothalami from food-deprived and refed mice were rapidly (≤30 sec) excised, flash-frozen, and analyzed. Refeeding caused a small but statistically significant rise in hypothalamic fatty acyl-CoA (C18:0 and C18:1) levels upon refeeding (Fig. 5). Glucose administration gave similar results (results not shown). Although these increases are not as great as those reported by others (18, 24), they are not inconsistent with the notion that de novo synthesized fatty acids (derived from glucose) serve as satiety factors. Administration of a potent inhibitor of FAS i.c.v. or a hypothalamic deletion of FAS, and thus CNS fatty acid synthesis, provokes an increase in hypothalamic malonyl-CoA and a decrease of food intake (8, 10, 16). Thus, the source of fatty acyl-CoAs to inhibit food intake cannot be de novo fatty acid synthesis in the CNS.
Fig. 5.
Feeding-induced changes in long-chain hypothalamic fatty acyl-CoAs. Mice were deprived of food for 20 h or refed 0.5 or 3 g of laboratory chow. Data represent five mice pooled per analysis; three pooled groups were analyzed. *, P < 0.02.
Leptin Induces a Sustained Increase in Hypothalamic Malonyl-CoA.
The anorectic hormone leptin, like centrally metabolized glucose, targets two of the same signaling intermediates, i.e., AMPK and ACC, that provoke changes in hypothalamic malonyl-CoA (4). Furthermore, food deprivation and refeeding are known to reciprocally alter leptin expression in a manner consistent with the orexigenic and anorexigenic effects of food deprivation and refeeding on hypothalamic malonyl-CoA. These findings suggest that leptin, like centrally metabolized glucose, might increase hypothalamic malonyl-CoA. Indeed, leptin has been shown to lower hypothalamic AMPK activity (4, 5) and alter food intake in a manner consistent with the malonyl-CoA hypothesis (15). Therefore, we determined the effect of leptin (administered i.c.v.), with or without concurrent peripheral glucose administration, on the hypothalamic malonyl-CoA level. Food-deprived mice were given leptin by i.c.v. injection and/or glucose by i.p. injection, after which hypothalami were analyzed for malonyl-CoA hourly over the next 3 h. Peripheral glucose treatment provoked the usual rise in hypothalamic malonyl-CoA, which peaked at 1 h and then declined rapidly to baseline within 3 h (Figs. 1 and 6A). Leptin treatment alone increased hypothalamic malonyl-CoA progressively at 2 and 3 h, and the combination of glucose and leptin produced an even greater, additive increase of hypothalamic malonyl-CoA (Fig. 6A). The response to leptin was concentration-dependent, with the maximal response occurring at 200 ng of leptin (Fig. 6B). These findings are consistent with those of Kahn's laboratory on hypothalamic AMPK activity (4) and indicate that leptin can function, at least in part, via the hypothalamic malonyl-CoA signaling mechanism.
Fig. 6.
Leptin increases hypothalamic malonyl-CoA. (A) Mice deprived of food for 20 h were treated as described with 2 μg of leptin and 4 g of glucose per kilogram of body weight. Food-deprived mice and mice deprived of food and refed for 2 h served as positive and negative controls, respectively. Hypothalamic malonyl-CoA was measured at 0, 1, 2, and 3 h after treatment. (B) Mice deprived of food for 20 h were injected i.p. with 4 g of glucose per kilogram of body weight and i.c.v. with vehicle or 20, 200, or 2,000 ng of leptin. Hypothalamic malonyl-CoA was measured at 3 h after treatment. (C) Mice deprived of food for 20 h were injected i.p. with 4 g of glucose per kilogram of body weight and i.c.v. with vehicle, 200 ng of leptin, or 200 ng of leptin plus 2-deoxyglucose. (n = 4 per group.) *, P < 0.001; #, P < 0.04; ##, P < 0.01.
We next asked whether the increase in hypothalamic malonyl-CoA induced by i.c.v. leptin depends on central glucose metabolism. Food-deprived mice were given an i.c.v. injection of leptin and an i.p. injection of glucose with or without 2-DG to block central glucose metabolism. 2-DG completely inhibited the leptin-induced rise in hypothalamic malonyl-CoA (Fig. 6C). Similar data were obtained in the absence of exogenous glucose (data not shown). These data support the hypothesis that central glucose utilization is a critical component of this system.
Leptin Is Not Required for the Refeeding- or Glucose-Induced Response of Hypothalamic Malonyl-CoA.
It is clear from this study that leptin can affect the hypothalamic fatty acid biosynthetic pathway; however, is leptin required for the increase in hypothalamic malonyl-CoA? To address this question we deprived leptin-deficient ob/ob mice (Fig. 7A) of food and administered an i.p. injection of glucose (4 g/kg of body weight) or refed for 1 h and measured the hypothalamic malonyl-CoA concentration. Hypothalamic malonyl-CoA of ob/ob mice exhibited the prototypical response to refeeding and glucose identical to that of wild-type mice (Fig. 2). These results suggest that, although leptin can increase hypothalamic malonyl-CoA, leptin is not required.
Fig. 7.
Leptin is not required for the glucose- or feeding-induced increase of hypothalamic malonyl-CoA. (A) Leptin-deficient ob/ob mice deprived of food for 20 h were injected i.p. with glucose at 4 g/kg of body weight or refed high-carbohydrate chow for 1 h. Hypothalamic malonyl-CoA was measured at 1 h after treatment (n = 3 per group). (B) WT mice deprived of food for 20 h were injected i.p. with 4 g of glucose per kilogram of body weight and i.c.v. with vehicle, 200 ng of leptin, or 200 ng of CNTF. Hypothalamic malonyl-CoA was measured at 3 h after treatment. (n = 4 per group.) *, P < 0.01; #, P < 0.001.
Other cytokines can have effects similar to that of leptin, notably the anorexogenic action of CNTF (26). Therefore, the effects of leptin and CNTF were compared. As shown in Fig. 7B, CNTF was equally as effective as leptin in eliciting an increase in hypothalamic malonyl-CoA under otherwise identical experimental conditions. These findings suggest that, like leptin and CNTF, other cytokines with anorectic activity may also produce a hypothalamic malonyl-CoA response and be representative of a more general mechanism for cytokine-induced satiety.
Discussion
Nutrients and adipokines are thought to exert their effects on energy homeostasis in the CNS primarily by short-term and long-term mechanisms, respectively. Recent evidence (4), including results presented here (Figs. 6 and 7), indicates that leptin, like glucose infusion, produces short-term effects mediated by AMPK. Thus, the anorectic signals from leptin and glucose in the hypothalamus (and possibly other sites such as the hippocampus and brainstem) appear to converge at AMPK and are transmitted to ACC, which generates malonyl-CoA (4). Although the molecular basis for the effect of glucose infusion is known (see below), the molecular mechanism by which leptin acts on AMPK remains obscure especially in light of the requirement for STAT3 in leptin signaling (27). Nevertheless, leptin action to increase malonyl-CoA (Figs. 6 and 7) requires glucose metabolism in the CNS, because i.c.v. 2-DG blocks the leptin-induced increase in malonyl-CoA (Fig. 6). The inverse is not true as leptin is not required for the refeeding- or glucose-induced rise in hypothalamic malonyl-CoA (Fig. 7), because these responses occur normally in ob/ob mice that lack functional leptin (Fig. 7). Thus, the upstream leptin- and nutrient-signaling pathways for short-term control differ, but they intersect at AMPK. Presumably, the long-term control by leptin makes use of the traditional JAK/STAT3 pathway involving translocation of STAT3 to the nucleus, transcriptional activation, and translation, processes that require a considerably longer lag time to respond to the adipokine.
In contrast, effects of glucose on AMPK activity in the hypothalamus occur rapidly (Figs. 1–3) in response to minute-to-minute changes in the cellular [AMP]/[ATP] ratio. In neurons, ATP in the fed state is largely derived from glucose metabolism. Glucose is the primary physiological fuel for the brain in the fed state, whereas ketones become primary fuel during prolonged food deprivation or when conditions leading to elevated circulating ketones, e.g., the ketogenic diet, prevail. Glucose rapidly activates or inhibits hypothalamic neuronal firing (28) and has long been known to suppress food intake (19). Although little is known about the molecular mechanism of hypothalamic glucose sensing per se, it is likely that AMPK plays an important role (4, 5). Here we provide evidence that increased glucose flux through the CNS/hypothalamus causes decreased AMPK phosphorylation/activity and thereby increased ACC activity, malonyl-CoA concentration, and fatty acid synthesis in the hypothalamus as depicted in the regulatory sequence shown in Scheme 1.
Scheme. 1.
Experimental design.
A large body of evidence from this (8, 10, 16) and other laboratories (9, 18, 29–31) has demonstrated the relationship between the rate of fatty acid synthesis in the hypothalamus and food intake. Thus, when fatty acid synthesis is activated malonyl-CoA level is increased and food intake is suppressed. This mechanism is consistent with a role for malonyl-CoA as the signaling molecule, possibly through the inhibition of CPT1c (11). It has also been suggested that fatty acids serve as satiety factors (12, 25); however, the fact that the inhibition of FAS (8, 10) or removal of malonyl-CoA (17, 18) produces opposing phenotypes, although both should lead to a reduction in de novo fatty acid synthesis, is inconsistent with this hypothesis. Our model implicates glucose as a major dietary product delivered to and sensed by the hypothalamus. Thus, we suggest that it is the glucose flux into fatty acid synthesis that produces malonyl-CoA, which mediates the satiety response.
Materials and Methods
Animals.
Wild-type and ob/ob mice on the C57BL/6 background were purchased from Harlan Laboratories. Mice were deprived of food for 20 h and given either food (10% of calories from fat, Research Diets D12450B, or 60% calories from fat, Research Diets D12492) or i.p. injection of glucose at the indicated dose. All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and under the approval of the Johns Hopkins Medical School Animal Care and Use Committee.
Analysis of Metabolites.
Blood samples were taken from cut tail tips of conscious mice. Plasma was collected for measurement of glucose (glucose oxidase method, QuantiChrom; Bioassay Systems), insulin (double antibody rat RIA, Linco Research), or nonesterified fatty acids (enzymatic method, NEFA-C; Wako Pure Chemicals Industries). Malonyl-CoA was measured by a malonyl-CoA recycling assay using malonate decarboxylase from Pseudomonas putida as previously described (16). Fatty acyl-CoAs were measured as previously described (32).
Immunoblotting.
Lysates in RIPA buffer were subjected to SDS/PAGE separation. AMPK, phospho-AMPK (T172), ACC, and phospho-ACC were purchased from Cell Signaling Technology and used per the manufacturer's instructions to blot PVDF membranes. A horseradish peroxidase-conjugated secondary antibody was used and visualized by using SuperSignal chemiluminescent substrate (Pierce).
Acknowledgments
This work was supported by National Institutes of Health Grants DK-38418 (to M.D.L.), DK-40936 (to G.I.S.), U24 DK-76169 (to G.I.S.), and P30 DK-45735 (to G.I.S.) and a Distinguished Clinical Scientist Award from the American Diabetes Association.
Footnotes
The authors declare no conflict of interest.
References
- 1.Gao Q, Horvath TL. Annu Rev Neurosci. 2007;30:367–398. doi: 10.1146/annurev.neuro.30.051606.094324. [DOI] [PubMed] [Google Scholar]
- 2.Kahn BB, Alquier T, Carling D, Hardie DG. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Wolfgang MJ, Lane MD. Annu Rev Nutr. 2006;26:23–44. doi: 10.1146/annurev.nutr.25.050304.092532. [DOI] [PubMed] [Google Scholar]
- 4.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, et al. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 5.Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. J Biol Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- 6.Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, et al. J Clin Invest. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, et al. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Loftus TM, Jaworsky DE, Frehywot GL, Townsend CA, Ronnett GV, Lane MD, Kuhajda FP. Science. 2000;288:2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- 9.Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- 10.Chakravarthy MV, Zhu Y, Lopez M, Yin L, Wozniak DF, Coleman T, Hu Z, Wolfgang MJ, Vidal-Puig A, Lane MD, Semenkovich CF. J Clin Invest. 2007;117:2539–2552. doi: 10.1172/JCI31183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolfgang MJ, Kurama T, Dai Y, Suwa A, Asaumi M, Matsumoto S, Cha SH, Shimokawa T, Lane MD. Proc Natl Acad Sci USA. 2006;103:7282–7287. doi: 10.1073/pnas.0602205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obici S, Feng Z, Arduini A, Conti R, Rossetti L. Nat Med. 2003;9:756–761. doi: 10.1038/nm873. [DOI] [PubMed] [Google Scholar]
- 13.Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Proc Natl Acad Sci USA. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGarry JD, Foster DW. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- 15.Wolfgang MJ, Lane MD. J Biol Chem. 2006;281:37265–37269. doi: 10.1074/jbc.R600016200. [DOI] [PubMed] [Google Scholar]
- 16.Hu Z, Cha SH, Chohnan S, Lane MD. Proc Natl Acad Sci USA. 2003;100:12624–12629. doi: 10.1073/pnas.1834402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu Z, Dai Y, Prentki M, Chohnan S, Lane MD. J Biol Chem. 2005;280:39681–39683. doi: 10.1074/jbc.C500398200. [DOI] [PubMed] [Google Scholar]
- 18.He W, Lam TK, Obici S, Rossetti L. Nat Neurosci. 2006;9:227–233. doi: 10.1038/nn1626. [DOI] [PubMed] [Google Scholar]
- 19.Lewis SR, Ahmed S, Khaimova E, Israel Y, Singh A, Kandov Y, Kest B, Bodnar RJ. Physiol Behav. 2006;87:595–601. doi: 10.1016/j.physbeh.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Bady I, Marty N, Dallaporta M, Emery M, Gyger J, Tarussio D, Foretz M, Thorens B. Diabetes. 2006;55:988–995. doi: 10.2337/diabetes.55.04.06.db05-1386. [DOI] [PubMed] [Google Scholar]
- 21.Routh VH. Physiol Behav. 2002;76:403–413. doi: 10.1016/s0031-9384(02)00761-8. [DOI] [PubMed] [Google Scholar]
- 22.Cha SH, Hu Z, Chohnan S, Lane MD. Proc Natl Acad Sci USA. 2005;102:14557–14562. doi: 10.1073/pnas.0507300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cha SH, Rodgers JT, Puigserver P, Chohnan S, Lane MD. Proc Natl Acad Sci USA. 2006;103:15410–15415. doi: 10.1073/pnas.0607334103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam TK, Schwartz GJ, Rossetti L. Nat Neurosci. 2005;8:579–584. doi: 10.1038/nn1456. [DOI] [PubMed] [Google Scholar]
- 25.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Diabetes. 2002;51:271–275. doi: 10.2337/diabetes.51.2.271. [DOI] [PubMed] [Google Scholar]
- 26.Lambert PD, Anderson KD, Sleeman MW, Wong V, Tan J, Hijarunguru A, Corcoran TL, Murray JD, Thabet KE, Yancopoulos GD, Wiegand SJ. Proc Natl Acad Sci USA. 2001;98:4652–4657. doi: 10.1073/pnas.061034298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu XY. Proc Natl Acad Sci USA. 2004;101:4661–4666. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levin BE. Diabetes Nutr Metab. 2002;15:274–280. discussion 281. [PubMed] [Google Scholar]
- 29.Kim EK, Miller I, Landree LE, Borisy-Rudin FF, Brown P, Tihan T, Townsend CA, Witters LA, Moran TH, Kuhajda FP, Ronnett GV. Am J Physiol. 2002;283:E867–E879. doi: 10.1152/ajpendo.00178.2002. [DOI] [PubMed] [Google Scholar]
- 30.Thupari JN, Kim EK, Moran TH, Ronnett GV, Kuhajda FP. Am J Physiol. 2004;287:E97–E104. doi: 10.1152/ajpendo.00261.2003. [DOI] [PubMed] [Google Scholar]
- 31.Kasser TR, Harris RB, Martin RJ. Am J Physiol. 1985;248:R447–R452. doi: 10.1152/ajpregu.1985.248.4.R447. [DOI] [PubMed] [Google Scholar]
- 32.Hammond LE, Neschen S, Romanelli AJ, Cline GW, Ilkayeva OR, Shulman GI, Muoio DM, Coleman RA. J Biol Chem. 2005;280:25629–25636. doi: 10.1074/jbc.M503181200. [DOI] [PubMed] [Google Scholar]