Abstract
Aposematic coloration, or warning coloration, is a visual signal that acts to minimize contact between predator and unprofitable prey. The conditions favoring the evolution of aposematic coloration remain largely unidentified. Recent work suggests that diet specialization and resultant toxicity may play a role in facilitating the evolution and persistence of warning coloration. Using a phylogenetic approach, we investigated the evolution of larval warning coloration in the genus Papilio (Lepidoptera: Papilionidae). Our results indicate that there are at least four independent origins of aposematic larval coloration within Papilio. Controlling for phylogenetic relatedness among Papilio taxa, we found no evidence supporting the hypothesis that either diet specialization or chemical specialization facilitated the origin of aposematic larvae. However, there was a significant relationship between the signal environment and the evolution of aposematic larvae. Specifically, Papilio lineages feeding on herbaceous or narrow-leaved plants, regardless of the plants' taxonomic affiliation, were more likely to evolve aposematic larvae than were lineages feeding only on trees/shrubs or broad-leaved plants. These results demonstrate that factors other than diet specialization, such as the signal environment of predator–prey interactions, may play a large role in the initial evolution and persistence of aposematic coloration.
Keywords: caterpillar, diet specialization, Lepidoptera, Papilio
Prey species often have different defensive strategies to avoid predation. These defenses can be structural, chemical, or behavioral, and they can occur in one or multiple modalities. Regardless of the precise mechanism, they function by increasing the likelihood of prey survivorship during a predation event. Aposematic, or warning, coloration is one such defensive strategy used by noxious organisms to visually communicate their toxicity or distastefulness to potential predators (1, 2). An aposematic pattern confers survival benefits to the prey because it is both easier for the predator to learn and less likely to be forgotten (3–6). These benefits are believed to have facilitated the evolution of aposematic coloration from ancestrally cryptic patterns (7, 8).
The functional benefits of aposematic coloration are well documented, yet, despite these advantages, understanding how and when aposematic coloration evolves remains more elusive. There are many examples of noxious or otherwise unprofitable prey that are weakly aposematic or even cryptic [e.g., toads (9) and crickets (10)] and other examples of nonnoxious or otherwise profitable prey exhibiting bright coloration [e.g., frogs (11) and birds (12)]. Given this variation across many systems, many researchers have attempted to identify the specific parameters responsible for the evolution of aposematic coloration (reviewed in refs. 13 and 14). The majority of these can be classified as one of two types: factors contributing to unprofitability or noxiousness of the prey and factors contributing to the efficacy of the visual communication between predator and prey.
With regard to prey noxiousness, both empirical and theoretical investigations indicate that prey toxicity has the greatest role in the evolution of aposematic coloration because noxiousness is necessary for procuring the benefits of aposematic coloration (reviewed in ref. 13). Prey can become noxious by consuming other organisms with defensive compounds (e.g., refs. 15 and 16). By specializing on a particular toxic diet, the consumer becomes noxious and more likely to evolve aposematic coloration as a defensive strategy (reviewed in ref. 13). Diet specialization, in which a consumer feeds on a limited set of related organisms, allows the consumer to tailor its metabolism to efficiently capitalize on the specific toxins shared by a suite of related hosts. Recent investigations suggest that diet specialization on toxic organisms promotes the evolution of aposematic coloration in poison arrow frogs (17, 18) although this pattern has not been demonstrated in other taxa. Another parallel, but uninvestigated, aspect of noxiousness is chemical specialization, or consuming organisms with a certain chemical profile regardless of their taxonomic affiliation. This is a common phenomenon in phytophagous insects and may influence the evolution of aposematism (19, 20). Specialization, either dietary or chemical, may increase the noxiousness of prey, which in turn may promote the evolution of aposematism.
The evolution of aposematic coloration may also be determined by the signal environment. Aposematic coloration is a visual signal whose efficacy depends on environmental factors affecting the transmission and reception of the signal. The signal environment is a combination of the elements contributing to the usefulness of a signal, including incident light, background complexity, and receivers (e.g., potential mates and predators) (21). Changes in the signal environment, such as background cues and predator guilds, should influence the evolution of defensive coloration (22). In contrast to the study of cryptic coloration, this prediction has received little attention in the literature on aposematic coloration (14). However, theoretical and empirical studies from the psychology literature have demonstrated the significance of background cues and predator species identity in two important predator functional benefits of coloration: aversion learning and memory retention (23–25). These differences in the signal environment, above and beyond other variations in diet or host toxicity, should also affect aposematic coloration evolution. For phytophagous insects, predator–prey interactions often occur on the host plant of the prey (26), and thus the signal environment of these interactions may vary with the physical properties of the larval host plant, such as growth form and leaf size.
Swallowtail butterflies (Lepidoptera: Papilionidae) are an exemplary system for evaluating the influences of noxiousness and signal environment on the origin and maintenance of aposematic coloration. Swallowtails, particularly the genus Papilio, are well characterized with regard to their natural history because they have been a model system for studying behavior, ecology, evolution, and physiology of insects. The genus Papilio occurs on all continents except Antarctica, is widely distributed across multiple habitats, and comprises ≈200 species, representing more than one-third of all Papilionidae. Papilio species vary in their larval dietary specialization: at least five taxa (Papilio nobilis, Papilio machaon hippocrates, Papilio birchalli, Papilio pilumnus, and Papilio esperanza) have only a single plant species in their diet. Other species have much broader diets, including Papilio zelicaon, which feeds on at least 45 different plant species, and Papilio rutulus, which feeds on plants in 11 different families (27). The diets of Papilio larvae also vary in their chemical profiles. Papilio hosts include plants with several known noxious chemicals including alkaloids, phenolics, and terpenoids (28). Coumarins, which are a type of phenolic compound, are particularly well studied in relation to their toxic properties and physiological effects on swallowtail larvae (e.g., refs. 29–33). Many Papilio larvae are unpalatable to both vertebrate and invertebrate predators presumably because of host plant chemical defenses that are either sequestered or present in the gut (34–37). Predators learn to avoid aposematic Papilio larvae after experience and sometimes release them unharmed in the process of predator education (36). Papilio larvae are all cryptic to predators from a distance, but at close range species have either an aposematic or a nonaposematic defensive strategy (38). The diet breadth of genus Papilio includes >400 species of plants in at least 20 families, including a diversity of plant growth forms and leaf sizes (27). With this wealth of available information, Papilio is an ideal system to evaluate the factors influencing the evolution of aposematism.
Accurate phylogenies are powerful frameworks for comparative biology and the study of adaptation. However, this historical perspective is not generally used in the study of aposematism (39). Numerous phylogenetic analyses on the relationships among Papilio species have provided a wealth of genetic data available for phylogenetic hypothesis testing (40–43). Here we incorporate phylogenetic information to investigate the evolution of aposematism in Papilio larvae. Using phylogenetic parametric bootstrapping (44), we evaluate the number of times aposematism has arisen within the genus Papilio. Additionally, using concentrated changes tests (45) and phylogenetic independent contrasts (46), we explicitly test hypotheses concerning the evolution of aposematism as it relates to diet specialization, chemical specialization, and signal environment.
Results
Phylogenetic Reconstructions and Origins of Aposematism in Papilio Larvae.
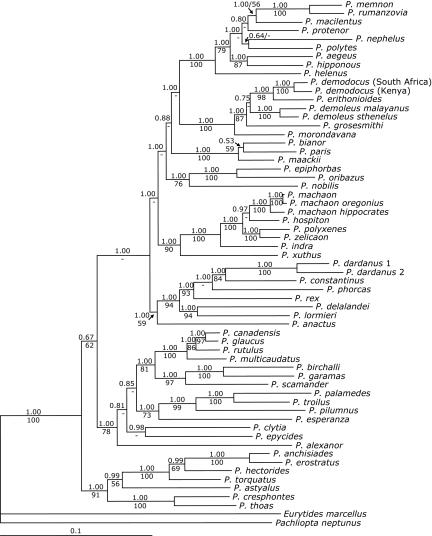
Both parsimony and Bayesian reconstructions were congruent with previous studies (Fig. 1) (40–43), although the Bayesian analyses showed greater resolution among some taxa than the parsimony approach. Parsimony reconstructions of larval morphology suggest five independent origins of aposematic larvae (Fig. 1). A sixth origin may also have occurred within Papilio demodocus populations of South Africa. However, we were unable to determine the larval morphology of the adult specimen, so the P. demodocus specimen from South Africa was treated as nonaposematic for all subsequent analyses (see Discussion for further details concerning aposematism in P. demodocus).
Fig. 1.
Relationships among Papilio species in this study. Numbers above branches reflect Bayesian posterior probabilities, and numbers below branches represent maximum parsimony bootstrap support. See the text for details of tests. Branch lengths represent consensus branch lengths from Bayesian analysis.
Using parametric bootstrapping analyses, we rejected the one-origin (P < 0.001), two-origin (P < 0.001), and three-origin (P < 0.001) hypotheses of aposematic larvae, but we did not reject the hypothesis of four origins (P = 0.132). For the latter case, the taxa constrained as sisters, Papilio clytia and Papilio alexanor, are both aposematic as larvae, but the larval morphology is superficially different between these two taxa and may represent two separate origins of aposematism.
Noxiousness and Aposematism.
Although some of our measures of diet specialization were sometimes associated with lineages in which warning coloration evolved, the concentrated changes tests revealed no relationship between feeding on a single host plant family and the evolution of aposematic coloration (Table 1). Additionally, in our independent contrast analyses, we found no relationship between any of our measures of diet specialization and the evolution of aposematic larvae (Table 2). Feeding on reduced numbers of host plant families, genera, or species did not predict the evolution of aposematism.
Table 1.
Concentrated changes tests results
| Test | Predictor | No. of occurrences* | P value |
|---|---|---|---|
| 1 | Single host plant family | 4 | 0.42 |
| 2 | Alkaloids in diet | 3 | 0.95 |
| 3 | Phenolics in diet | 5 | 0.94 |
| 4 | Terpenoids in diet | 3 | 0.58 |
| 5 | Triterpenoids in diet | 2 | 0.61 |
| 6 | Coumarins in diet | 5 | 0.55 |
| 7 | Herbs in diet | 3 | 0.03 |
| 8 | No trees in diet | 3 | 0.02 |
| 9 | Narrow-leaved plants in diet | 4 | 0.005 |
*The number of origins of aposematism associated with the corresponding predictor character state; e.g., for test 2, three of the five origins of aposematism occurred within lineages that fed on plants containing alkaloids.
Table 2.
Phylogenetic independent contrasts results
| Test | Predictor | t (df) | r value | P value |
|---|---|---|---|---|
| 1 | Number of families | 0.38 (50) | 0.054 | 0.35 |
| 2 | Number of genera | −0.33 (47) | −0.049 | 0.37 |
| 3 | Number of species | −0.18 (47) | −0.027 | 0.43 |
| 4 | Number of anti-feedant classes | −1.09 (47) | −0.160 | 0.14 |
r is the Pearson product–moment correlation coefficient, and P values are for one-tailed tests.
The evolution of larval aposematic coloration was not predicted by the presence of alkaloids, phenolics, terpenoids, triterpenoids, or coumarins according to the concentrated changes tests (Table 1). Also, in the phylogenetic independent contrasts, we found no support for a relationship between the number of chemical compounds in the diet and aposematic coloration (Table 2).
Signal Environment and Aposematism.
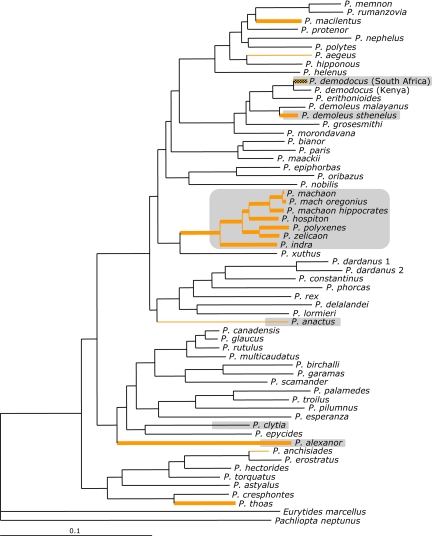
All three tests for a relationship between the signal environment and the evolution of aposematic coloration were significant (Table 1). Herb-feeding (P = 0.03) and an absence of trees in diet (P = 0.02) both predicted the evolution of aposematic larvae. Three of the five reconstructed origins of aposematism were associated with lineages that fed on herbs and lacked trees in their diet (Fig. 2). Additionally, feeding on narrow leaves (0- to 20-mm leaves) was also correlated with the evolution of aposematic coloration in Papilio larvae (P = 0.005) (Table 1). Four of the five reconstructed origins of aposematism were associated with lineages whose diet included narrow-leaved plants (Fig. 2).
Fig. 2.
Ancestral state reconstructions on Bayesian phylogeny. Thickened branches reflect lineages with herbs in diet, and orange shaded branches indicate narrow leaves in diet. Shaded boxes indicate taxa with aposematic larvae.
Discussion
Aposematic coloration is a visual signal whose transmission and reception are greatly affected by the signal environment of the interaction. This environment may aid or limit the spread and maintenance of aposematic coloration. Within Papilio, aposematic coloration has evolved independently a minimum of four times (Fig. 2). Our results demonstrate that neither diet specialization (number of families, genera, or species) nor chemical specialization (presence/absence of particular toxic compounds) is strongly associated with the evolution of aposematic signaling in Papilio (Tables 1 and 2). However, host plant growth form and leaf width, our proxies for the signal environment of the interaction between prey and predator, do affect the evolution of aposematic larvae within Papilio. The presence of herbs, the absence of trees, and the presence of narrow-leaved plants in larval diet are all correlated with the evolution of aposematic coloration (Table 1 and Fig. 2).
Although diet specialization on noxious host plants and toxic chemicals has occurred multiple times within the genus Papilio, our study demonstrates that specialization alone does not result in the evolution of aposematic coloration in this genus. Papilio larval diets include toxic plants, Papilio species vary in their amount of specialization (ref. 28 and this study), and aposematic Papilio larvae are chemically defended and known to deter predator attack (34–37). However, unlike similar studies involving poison arrow frogs (17, 18), we found no support for a link between diet or chemical specialization and the evolution of aposematism in Papilio. Specialist lineages of Papilio were equally likely to evolve aposematism as generalist lineages.
Our results did demonstrate that the signal environment promotes the evolution of aposematic coloration in Papilio. Aposematic color signals are the products of the visual communication between prey and predator. Like all visual signals, an aposematic signal must be effectively emitted by the prey, transmitted through the environment, and received by the predator (20). In aposematism, the toxic prey may evolve a visual signal that increases both signal efficacy and reliability of a predator's response. In addition, the receiving predator evolves sensory and cognitive machinery to increase signal reception, processing, and discrimination (20). These capabilities promote accurate and consistent decision making by the predator, which results in mutual benefit to both signaling prey and receiving predator. Thus, the evolution of a visual aposematic signal will be heavily mediated by two features of the signal environment: visual background and predator guild.
Visual background is not traditionally regarded as an important component of aposematic coloration because cryptic signals are considered to be more limited by the visual background than conspicuous signals (13). However, the visual background likely plays a key role in the functional benefits of aposematic coloration (23). Predators encode visual background information when learning to avoid unpalatable prey (25), and variation in visual background can interfere with predator discrimination, learning, and memory (24). For Papilio, the visual background of herbs or narrow-leaved plants may have better and more consistent qualities, such as color or luminance contrast, than the visual background of trees or broad-leaved plants. This, in turn, may promote the functional benefits of the aposematic signal. This is a relatively unexplored perspective on the evolution of aposematic coloration and warrants future investigation, especially in the field.
Previous work suggested that aposematic coloration releases prey species to explore more environmental opportunities than a cryptic phenotype because an aposematic signaler is no longer constrained to a particular visual background (13, 47). However, aposematism in Papilio larvae has had the opposite effect, meaning that aposematic lineages are constrained to particular host plant growth forms, specifically narrow-leaved herbs and shrubs. Once warning coloration evolves, Papilio larvae are more restricted to living on small-leaved plants even though there may be suitable broad-leaved host plants available. For example, only two aposematic species, Papilio anactus and P. clytia, feed on trees in natural environments. Additional evidence of this constraint is found within P. demodocus. This species is known to have two forms of larval coloration: the green, cryptic pattern and the striped, aposematic pattern (48), although we did not consider this in our analyses (see Results). These color patterns are differentially associated with broad-leaved trees (cryptic larvae) and narrow-leaved umbellifers (aposematic larvae), and the latter form occurs only in parts of South Africa where the umbellifer hosts occur. Larval color patterns are genetically determined and may be selected against on the alternative hosts by avian predators (48). A contrary case occurs within P. zelicaon, which also has aposematic larvae but has recently colonized cultivated Citrus trees in California (49). However, these populations of P. zelicaon also feed on the introduced herb Foeniculum vulgare (Apiaceae), which may allow aposematism to persist.
Additionally, variation in predator guilds between different signal environments may also influence the evolution of aposematism. Predators vary tremendously in their sensory and cognitive abilities to detect and process visual information. For example, color discrimination in invertebrate and vertebrate predators ranges from monochromatic to tetrachromatic (50, 51). Rate of aversion learning and memory retention is determined by species identity; some species are simply more proficient than others at learning and remembering signals (23). Given that predator community composition and density vary based on habitat, plant diversity, and herbivorous insect diversity (e.g., refs. 52–54), the specific suite of predators in herbs and shrubs or narrow-leaved plants may be more suitable for receiving, learning, and remembering aposematic signals. Structurally complex habitats, those with trees, shrubs, and herbs, generally have more insectivores than those habitats without trees (52). Thus, trees, as compared with shrubs or herbs, may simply have too many predators or too much variation among predator species to promote the establishment of an aposematic phenotype. Because the receiver is part of the signal environment, the number of predators, the type of predators, and their sensory and cognitive capabilities may limit or promote aposematic coloration.
With our results we could not delineate between these two major contextual parameters of the signal environment, visual background and predator guild, so we were unable to discern their relative importance. If visual background is an important determinant in the evolution of aposematism, predation rates on larvae feeding on narrow-leaved trees should not differ from predation on larvae feeding on narrow-leaved herbs. Alternatively, if differences in predator guilds among growth forms influence the evolution of aposematism, predation rates should vary with growth form regardless of leaf size. Besides Papilio, there are other systems where aposematic insect species occur on herbs and shrubs while their cryptic congeners occur on forest trees that warrant future investigation [e.g., chrysomelid beetles, looper moth caterpillars, and ladybird beetles (55)].
Aposematic coloration is the summation of many components: prey toxicity, prey signaling, predator reception, and predator decision. Prey diet specialization and resultant prey toxicity are just two components of this phenomenon. However, it does not always result in the evolution and maintenance of aposematic coloration. Other factors such as visual background and predator community play essential roles in the evolution of aposematic coloration. Here we have strong indirect evidence that the signal environment is essential to consider in the evolution of aposematic coloration.
Materials and Methods
Larval Coloration.
Larval morphology data were collected from published descriptions [supporting information (SI) Table 3]. All Papilio larvae have a very similar bird dropping mimic coloration pattern until the fourth or fifth instar. At that developmental point they exhibit one of three different phenotypes: a bird dropping mimic (cryptic, nonaposematic), a green snake mimic (startle or cryptic, nonaposematic), or a larva with green or black background with orange or yellow dots and black and white bands (aposematic) (Fig. 3). For this study aposematic coloration is defined as a signal pattern that serves to advertise its bearer's unprofitability to potential predators, usually to the benefit of both prey and predator. Behavioral evidence has shown that these aposematic colored Papilio larvae are unpalatable to birds and that the described color pattern increases predator avoidance through learning and memory retention (28, 36, 37, 56, 57). Some authors argue that this larval color pattern is cryptic and not aposematic (58). However, recent behavioral studies indicate that the pattern is cryptic to predators from a distance but has an aposematic function once the predator is in close proximity to the larva (38).
Fig. 3.
Photos of three Papilio larval forms. (a) Bird dropping mimic, Papilio cresphontes. (b) Green P. rutulus. (c) Aposematic P. polyxenes. The photos in a and c were taken by J.C.O., and the photo in b was taken by G. Pohl.
Phylogenetic Reconstructions.
To estimate the phylogenetic relationships among the members of the genus Papilio, we used published sequences of the mitochondrial genes cytochrome oxidase subunits I (COI) and II (COII) and nuclear genes elongation factor 1α (EF-1a) and wingless (wg), although wg sequences were not available for all taxa (40–43). Eurytides marcellus (Papilionidae: Leptocircini) and Pachilopta neptunus (Papilionidae: Troidini) were used as outgroups in all phylogenetic analyses. Using PAUP*4.0b10 (59), we performed 1,000 maximum parsimony bootstrap pseudoreplicates to assess nodal support. We also performed MCMC searches in MrBayes 3.1.2 (60) to estimate clade posterior probabilities. For the Bayesian analyses the data were partitioned according to gene, and each partition was allowed a unique GTR+G+I model of evolution. We ran two searches of four chains each for 1 × 108 generations, and posterior probabilities were assessed after a 5 × 107 generation burn-in. We used parsimony to reconstruct the ancestral state of larval morphology (nonaposematic vs. aposematic) on each of these phylogenetic reconstructions.
Hypothesis Testing Methods.
We tested the hypotheses of one, two, three, and four origins of aposematism by performing parametric bootstrapping (44). For the single-origin test we tested the hypothesis that all Papilio taxa with aposematic larvae (P. alexanor, P. anactus, P. clytia, Papilio demoleus sthenelus, Papilio hospiton, Papilio indra, P. machaon, P. machaon hippocrates, Papilio machaon oregonius, Papilio polyxenes, and P. zelicaon) form an exclusive monophyletic group. We tested the two-origin hypothesis by allowing two clades of aposematic larvae to occur: (i) P. alexanor, P. hospiton, P. indra, P. machaon, P. machaon hippocrates, P. machaon oregonius, P. polyxenes, and P. zelicaon; and (ii) P. anactus, P. clytia, and P. demoleus sthenelus. The three-origin test separated the machaon group (P. hospiton, P. indra, P. machaon, P. machaon hippocrates, P. machaon oregonius, P. polyxenes, and P. zelicaon) from the groups P. alexanor/P. clytia and P. anactus/P. demoleus sthenelus. Finally, the four-origin test differed from the three-origin test only in splitting the P. anactus/P. demoleus sthenelus clade so that P. anactus and P. demoleus sthenelus were not constrained to be sister species. All parametric bootstrapping analyses used Mesquite 1.06 (61) to generate sequence matrices using a GTR+G+I model of DNA evolution and PAUP*4.0b10 (59) to perform parsimony searches.
To evaluate which factors may influence the evolution of aposematism, we performed concentrated changes tests (45) and phylogenetic independent contrasts (46). In all tests we addressed the following question: does a particular aspect of the diet increase the likelihood of the evolution of aposematic larvae? For categorical diet characteristics (i.e., the presence or absence of a particular chemical compound or host plant growth form) we coded the character states as binary, and we performed concentrated changes tests in MacClade 4.08 (62) to determine whether transitions to aposematic larvae occurred in lineages with a particular character state more often than expected by chance. All tests were performed on the inferred Bayesian phylogeny with parsimony-reconstructed ancestral states. Taxa lacking relevant data were pruned from the phylogeny before tests were performed. Significance was assessed with 100,000 simulated character state change reconstructions. We performed nine concentrated changes test—one related to diet specialization, five related to chemical specialization, and three related to signal environment (see sections below for specifics).
For continuous-valued diet characteristics (e.g., total number of plant families in diet) we coded our dependent variable, aposematic larvae, as a binary character (0 = nonaposematic, 1 = aposematic) and performed phylogenetic independent contrasts using the PDAP:PDTREE package (63) in Mesquite 1.06 (61). All independent contrast analyses were performed on the Bayesian phylogeny following Pagel's branch length transformation (64). We pruned trees of outgroup taxa and taxa lacking relevant data before performing independent contrasts. We performed four phylogenetic independent contrast tests—three related to diet specialization and one related to chemical specialization (see sections below for specifics).
Noxiousness: Diet Specialization and Host Plant Chemical Profiles.
We surveyed the available literature to generate a database of Papilio host characteristics. We began by creating a list of known larval host plants for each species of Papilio (SI Table 3). Then we used the larval host plant data to generate a host plant chemical profile from published sources (SI Tables 3 and 4). We reported only compounds known to have toxic and/or anti-feedant properties (65). We categorized the plant compounds both broadly into three structural classes (alkaloids, phenolics, and terpenoids) and more narrowly based on higher levels of potency and toxicity (triterpenoids and coumarins) (65). These data were used to test hypotheses concerning diet and chemical specialization and the evolution of aposematism.
Using a concentrated changes test we evaluated whether diet specialization was related to the evolution of aposematic coloration. Specifically, we tested the hypothesis that feeding on plants from a single family increased the likelihood of evolving aposematic coloration. Using phylogenetic independent contrasts, we further tested for a relationship between aposematic coloration and diet specialization, measured in three different ways: (i) number of plant families in diet, (ii) number of plant genera in diet, and (iii) number of plant species in diet. If diet specialization increases the likelihood of the evolution of aposematic coloration, then aposematic lineages will have diets that are restricted to one host plant family and/or diets that consist of fewer host plant families, genera, or species as compared with nonaposematic larvae.
Using concentrated changes tests, we tested for a relationship between the evolution of aposematic coloration and the following five measures of chemical specialization: (i) diet includes alkaloid-containing plants, (ii) diet includes phenolic-containing plants, (iii) diet includes terpenoid-containing plants, (iv) diet includes triterpenoid-containing plants, and (v) diet includes coumarin-containing plants. If chemical specialization affected the evolution of aposematic coloration in Papilio larvae, then the origins of aposematic coloration would be associated with the presence of a certain toxic compound. We also tested for a correlation between aposematic coloration and number of toxic compounds in the larval diet using phylogenetic independent contrasts: if chemical specialization increases the likelihood of the evolution of aposematic coloration, then aposematic larvae will have fewer chemicals in their diet compared with nonaposematic larvae.
Signal Environment: Host Plant Growth Form and Host Plant Leaf Size.
To test for effects of signal environment on the evolution of aposematism, we used two physical properties of the larval host plant as proxies for the signal environment: growth form and leaf size. From our database of known Papilio host plant records, we compiled available data for host plant growth forms from published sources (SI Table 3). Host plants were categorized into one of the three types based on their ecological description: herb, shrub, or tree. We also accumulated data for average host plant leaf width from herbarium specimens and published sources (SI Tables 3 and 4). We consider a leaf to be the widest feeding surface that a Papilio larva could rest on, which morphologically could be a leaf or a leaflet. Because the average mature Papilio larva is ≈10 mm wide, we broadly categorized leaf size into one of two designations: 0–20.0 mm wide (“narrow”) or >20.0 mm wide (“broad”).
To test for a relationship between the evolution of aposematic coloration and signal environment, we performed three concentrated changes tests. Specifically, we asked whether the evolution of aposematic coloration was predicted by any of the following characteristics of the signal environment: (i) larva feeds on herbs, (ii) larva does not feed on trees, and (iii) larva feeds on narrow leaves (0–20.0 mm). If signal environment influences the evolution of aposematism, then the origins of aposematism should be concentrated on lineages characterized by one or more of the three states listed above.
Supplementary Material
Acknowledgments
We thank J. Bronstein, D. Papaj, E. Snell-Rood, B. Timmermann, and the anomymous reviewers for discussion and helpful comments on the manuscript. G. Pohl kindly provided the P. rutulus image in Fig. 3b. This research was funded by a University of Arizona BIO5 fellowship (to K.L.P.), a Science To Achieve Results (STAR) fellowship from the Environmental Protection Agency (to J.C.O.), and a Natural Sciences and Engineering Research Council (Canada) Discovery Grant (to F.A.H.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705478104/DC1.
References
- 1.Cott HB. Adaptive Coloration in Animals. London: Methuen; 1940. [Google Scholar]
- 2.Guilford T. In: Insect Defenses: Adaptive Mechanism and Strategies of Prey and Predators. Evans DL, Schmidt JO, editors. Albany: State Univ of New York Press; 1990. pp. 23–64. [Google Scholar]
- 3.Gittleman JL, Harvey PH. Nature. 1980;286:149–150. [Google Scholar]
- 4.Roper TJ. Anim Behav. 1990;39:466–473. [Google Scholar]
- 5.Alatalo RV, Mappes J. Nature. 1996;382:485–503. [Google Scholar]
- 6.Roper TJ, Redstone S. Anim Behav. 1987;35:739–747. [Google Scholar]
- 7.Fisher RA. The Genetical Theory of Natural Selection. New York: Dover; 1930. [Google Scholar]
- 8.Sherratt TN, Beatty CD. Am Nat. 2003;162:377–389. doi: 10.1086/378047. [DOI] [PubMed] [Google Scholar]
- 9.Daly JW. Proc Natl Acad Sci USA. 1995;92:9–13. doi: 10.1073/pnas.92.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf S, Brettshneider H, Batesman DW. Afr Zool. 2006;41:75–80. [Google Scholar]
- 11.Warkentin KM. Behav Ecol. 1999;10:251–262. [Google Scholar]
- 12.Dale S, Slagsvold T. Auk. 1996;113:849–857. [Google Scholar]
- 13.Ruxton GD, Sherratt TN, Speed MP. Avoiding Attack: The Evolutionary Ecology of Crypsis, Warning Signals, and Mimicry. Oxford: Oxford Univ Press; 2004. [Google Scholar]
- 14.Mappes J, Marples N, Endler JA. Trends Ecol Evol. 2005;20:593–603. doi: 10.1016/j.tree.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Termonia A, Pasteels JM, Windsoer DM, Milinkovitch MC. Philos Trans R Soc London. 2001;269:1–6. [Google Scholar]
- 16.Daly JW, Kaneko T, Wilham J, Garraffo HM, Soande TF, Espinosa A, Donnelly MA. Proc Natl Acad Sci USA. 2002;99:13996–14001. doi: 10.1073/pnas.222551599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos JC, Coloma LA, Cannatella DC. Proc Natl Acad Sci USA. 2003;100:12792–12797. doi: 10.1073/pnas.2133521100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darst CR, Menendez-Guerrero PA, Coloma LA, Cannatella DC. Am Nat. 2005;165:56–69. doi: 10.1086/426599. [DOI] [PubMed] [Google Scholar]
- 19.Dyer LA. Ecology. 1995;76:1483–1496. [Google Scholar]
- 20.Rothschild M. Phytochemistry. 1993;33:1037. [Google Scholar]
- 21.Endler JA. Philos Trans R Soc London B. 1993;340:215–225. doi: 10.1098/rstb.1993.0060. [DOI] [PubMed] [Google Scholar]
- 22.Endler JA. In: Visual Signals: Signaling and Signal Design in Animal Communication. Espmark Y, Asmundsen T, Rosenqvist G, editors. Trondheim, Norway: Tapir Academic; 2000. pp. 11–46. [Google Scholar]
- 23.Bouton ME. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 24.Pearce JM, Bouton ME. Annu Rev Psychol. 2001;52:111–139. doi: 10.1146/annurev.psych.52.1.111. [DOI] [PubMed] [Google Scholar]
- 25.Skow CD, Jacob EM. Behav Ecol. 2005;17:34–40. [Google Scholar]
- 26.Bernays EA. Ecol Entomol. 1997;22:121–123. [Google Scholar]
- 27.Scott JA. The Butterflies of North America: A Natural History and Field Guide. Stanford, CA: Stanford Univ Press; 1986. [Google Scholar]
- 28.Berenbaum MR. In: Swallowtail Butterflies: Their Ecology and Evolutionary Biology. Scriber JM, Tsubaki Y, Lederhouse RC, editors. Gainesville, FL: Scientific Press; 1995. pp. 27–38. [Google Scholar]
- 29.Berenbaum MR. Ecology. 1981;62:1254–1266. [Google Scholar]
- 30.Berenbaum MR. Annu Rev Entomol. 1990;35:319–343. [Google Scholar]
- 31.Feeny P. In: Swallowtail Butterflies: Their Ecology and Evolutionary Biology. Scriber JM, Tsubaki Y, Lederhouse RC, editors. Gainesville, FL: Scientific Press; 1995. pp. 9–15. [Google Scholar]
- 32.Li WM, Schuler MA, Berenbaum MR. Proc Natl Acad Sci USA. 2003;100:14593–14598. doi: 10.1073/pnas.1934643100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li XC, Baudry J, Berenbaum MR, Schuler MA. Proc Natl Acad Sci USA. 2004;101:2939–2944. doi: 10.1073/pnas.0308691101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisner T, Meinwald YC. Science. 1965;150:1733–1735. doi: 10.1126/science.150.3704.1733. [DOI] [PubMed] [Google Scholar]
- 35.Honda K. Physiol Entomol. 1983;8:173–179. [Google Scholar]
- 36.Järvi T, Sillen-Tullberg B, Wiklund C. Oikos. 1981;36:267–272. [Google Scholar]
- 37.Sillén-Tullberg B. Anim Behav. 1990;40:856–860. [Google Scholar]
- 38.Tullberg BS, Merilaita S, Wiklund C. Proc Biol Sci. 2005;272:1315–1321. doi: 10.1098/rspb.2005.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Härlin C, Härlin M. Evol Ecol. 2003;17:197–212. [Google Scholar]
- 40.Caterino MS, Sperling FAH. Mol Phylogenet Evol. 1999;11:122–137. doi: 10.1006/mpev.1998.0549. [DOI] [PubMed] [Google Scholar]
- 41.Reed RD, Sperling FAH. Mol Biol Evol. 1999;16:286–297. doi: 10.1093/oxfordjournals.molbev.a026110. [DOI] [PubMed] [Google Scholar]
- 42.Zakharov EV, Caterino MS, Sperling FAH. Syst Biol. 2004;53:193–215. doi: 10.1080/10635150490423403. [DOI] [PubMed] [Google Scholar]
- 43.Zakharov EV, Smith CR, Lees DC, Cameron A, Vane-Wright RI, Sperling FAH. Evolution (Lawrence, Kans) 2004;58:2763–2782. doi: 10.1111/j.0014-3820.2004.tb01628.x. [DOI] [PubMed] [Google Scholar]
- 44.Huelsenbeck JP, Hillis DM, Nielsen R. Syst Biol. 1996;45:546–558. [Google Scholar]
- 45.Maddison WP. Evolution (Lawrence, Kans) 1990;44:539–557. doi: 10.1111/j.1558-5646.1990.tb05937.x. [DOI] [PubMed] [Google Scholar]
- 46.Felsenstein J. Evolution (Lawrence, Kans) 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 47.Speed MP, Ruxton GD. Proc Biol Sci. 2005;272:431–438. doi: 10.1098/rspb.2004.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clarke CA, Dickson CGC, Sheppard PM. Evolution (Lawrence, Kans) 1963;17:130–137. [Google Scholar]
- 49.Shapiro AM, Masuda KK. CA Agric. 1980;34:4–5. [Google Scholar]
- 50.Briscoe AD, Chittka L. Annu Rev Entomol. 2001;46:471–510. doi: 10.1146/annurev.ento.46.1.471. [DOI] [PubMed] [Google Scholar]
- 51.Goldsmith TH. Q Rev Biol. 1990;65:281–322. doi: 10.1086/416840. [DOI] [PubMed] [Google Scholar]
- 52.Diaz L. Forest Ecol Manage. 2006;223:54–65. [Google Scholar]
- 53.Jones GA, Sieving KE, Jacobson SK. Conserv Biol. 2005;19:1234–1245. [Google Scholar]
- 54.Symstad AJ, Siemann E, Haarstad J. Oikos. 2001;89:243–253. [Google Scholar]
- 55.Pasteels JM, Gregoire J-C, Rowell-Rahier M. Annu Rev Entomol. 1983;28:263–289. [Google Scholar]
- 56.Wiklund C, Sillén-Tullberg B. Evolution (Lawrence, Kans) 1985;39:1155–1158. doi: 10.1111/j.1558-5646.1985.tb00456.x. [DOI] [PubMed] [Google Scholar]
- 57.Leslie AJ, Berenbaum MR. J Lepidoptera Soc. 1990;44:245–251. [Google Scholar]
- 58.Brower AVZ, Sime KR. J Lepidoptera Soc. 1998;52:206–212. [Google Scholar]
- 59.Swofford DL. PAUP*: Phylogenetic Analysis Using Parsimony (*And Other Methods) Sunderland, MA: Sinauer; 2002. Version 4.0b10. [Google Scholar]
- 60.Ronquist F, Huelsenbeck JP. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 61.Maddison WP, Maddison DR. Mesquite: A Modular System for Evolutionary Analysis. Tucson, AZ: Univ of Arizona; 2005. Version 1.06. [Google Scholar]
- 62.Maddison DR, Maddison WP. MacClade 4.08: Analysis of Phylogeny and Character Evolution. Sunderland, MA: Sinauer; 2005. [DOI] [PubMed] [Google Scholar]
- 63.Midford PE, Garland T, Jr, Maddison WP. PDAP Package of Mesquite. Tucson, AZ: Univ of Arizona; 2005. Version 1.07. [Google Scholar]
- 64.Pagel M. J Theor Biol. 1992;156:431–442. [Google Scholar]
- 65.Isman M. Pesticide Outlook. 2002;13:152–157. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.