Abstract
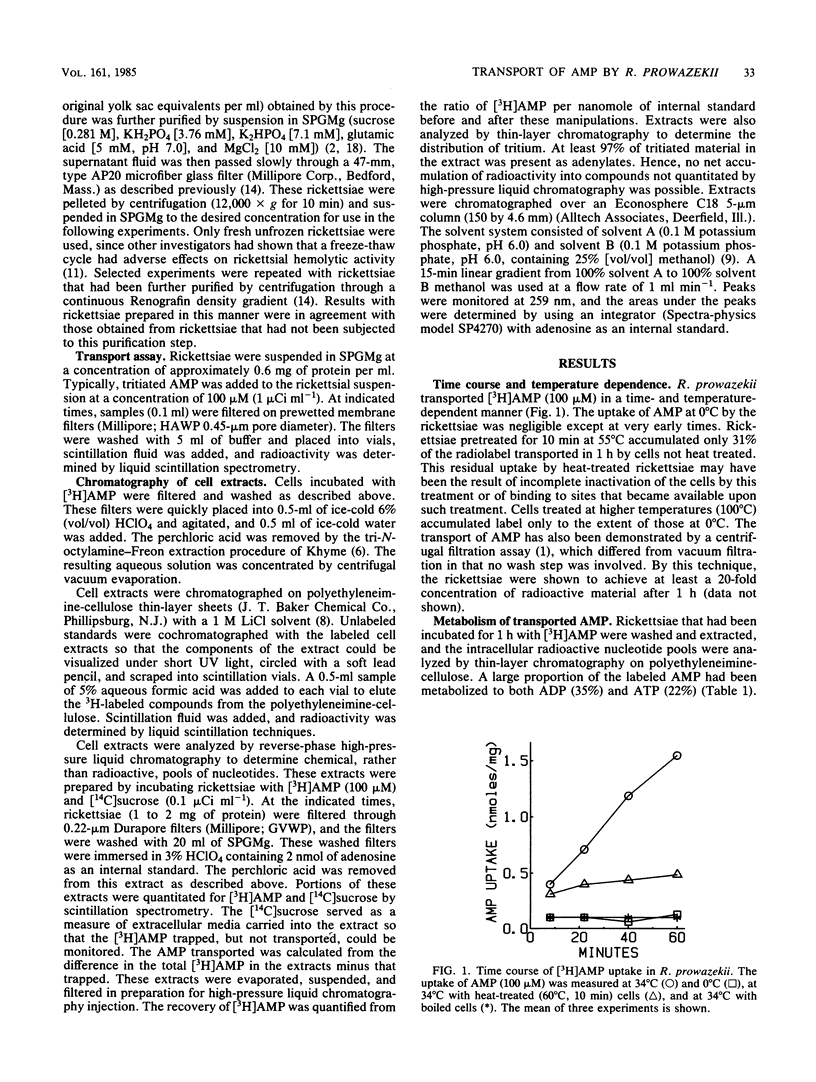
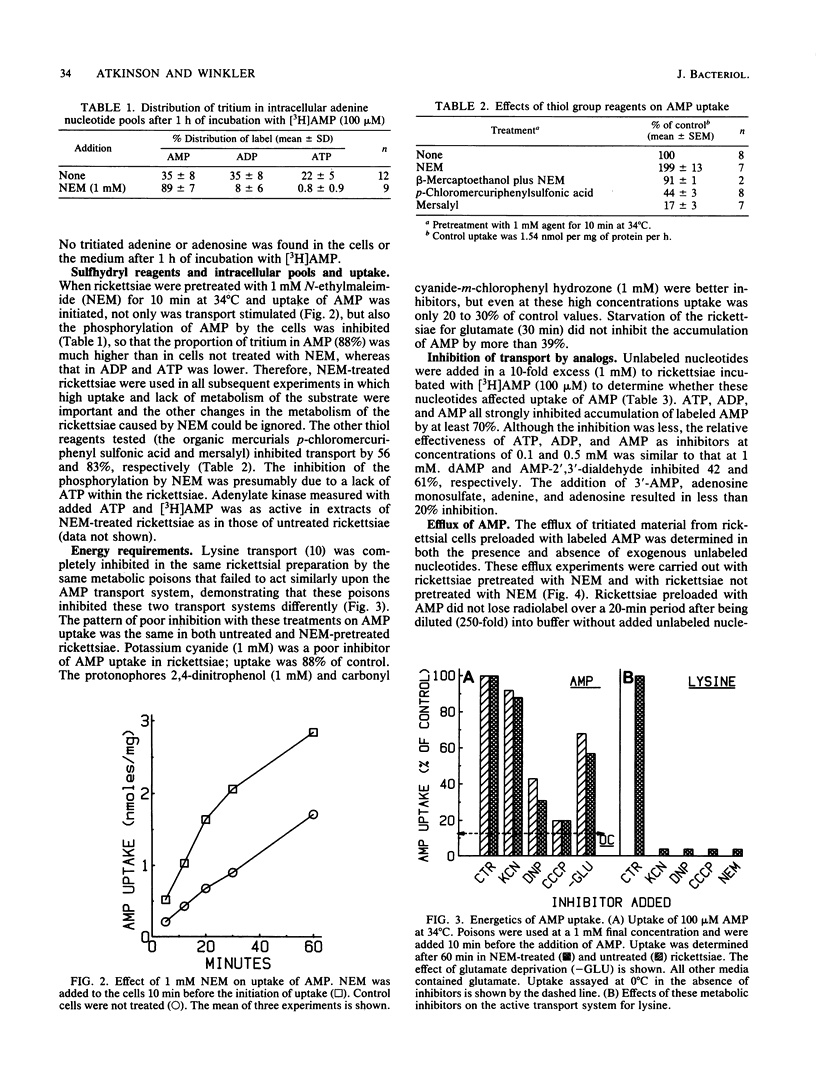
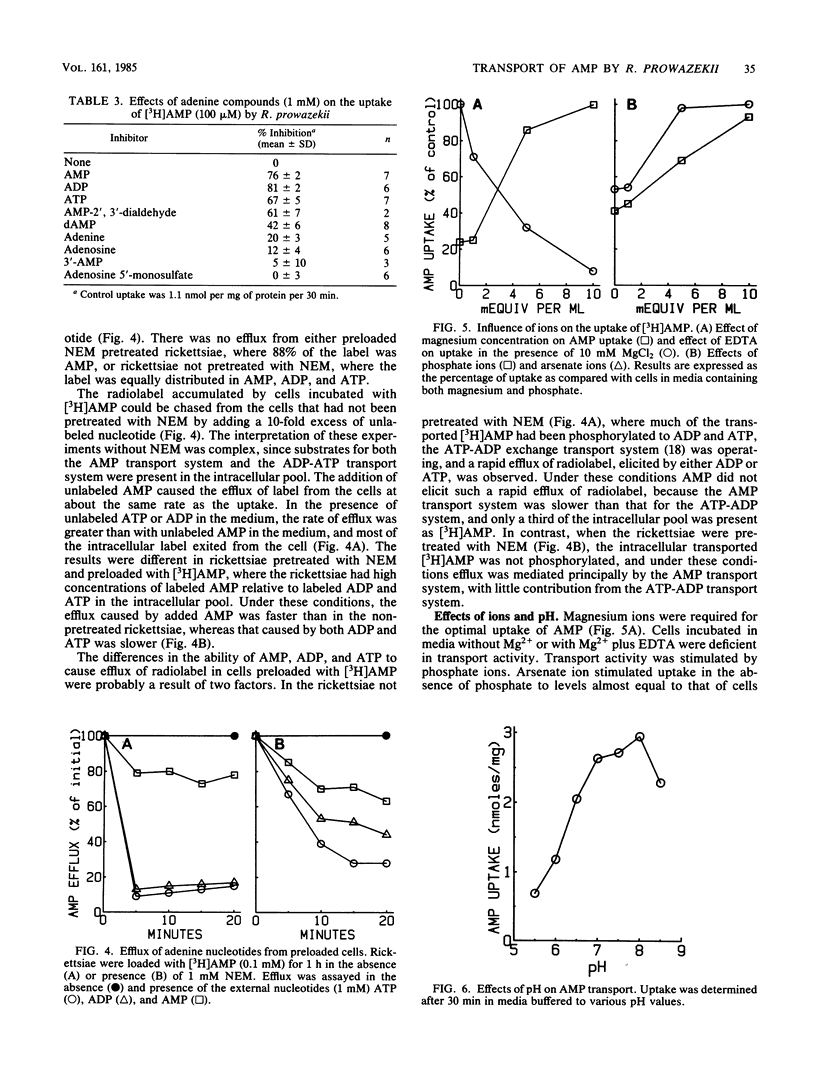
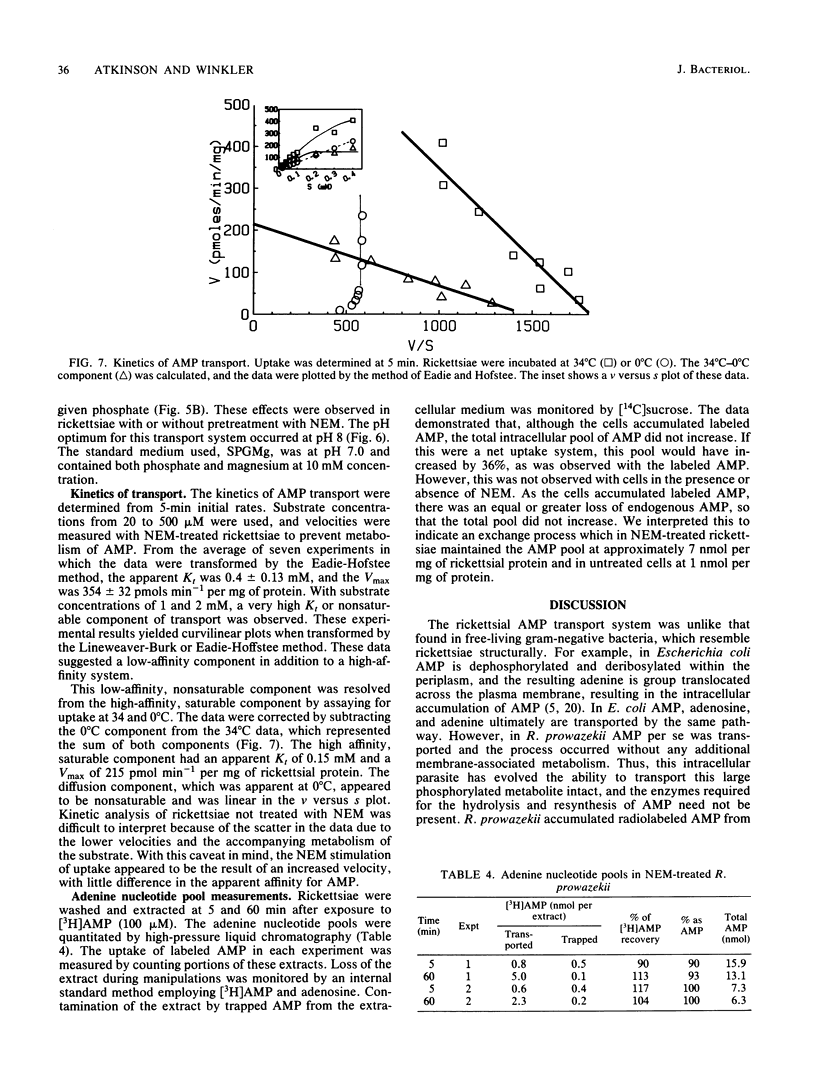
Rickettsia prowazekii possesses an exchange transport system for AMP. Chromatographic analysis of the rickettsiae demonstrated that transported AMP appeared intracellularly as AMP, ADP, and ATP, and no hydrolytic products appeared in either the intracellular or extracellular compartments. The phosphorylation of AMP to ADP and ATP was prevented by pretreatment of the cells with 1 mM N-ethylmaleimide without inhibiting the transport of AMP. Although no efflux was demonstrable in the absence of nucleotide in the medium, the intracellular adenine nucleotide pool could be exchanged with external unlabeled adenine nucleotides. Both ADP and ATP were as effective as AMP at inhibiting the uptake of [3H]AMP. Although this transport system was inhibited by low temperature (0 degrees C) and partially inhibited by the protonophore carbonyl cyanide-m-chlorophenyl hydrazone (1 mM), it was relatively insensitive to KCN (1 mM). The uptake of AMP at 34 degrees C had an apparent Kt for influx of 0.4 mM and a Vmax of 354 pmol min-1 per mg. At 0 degrees C there was a very rapid and unsaturable association of AMP with these organisms. Correction of the uptake data at 34 degrees C for the 0 degrees C component lowered the apparent Kt to 0.15 mM. Both magnesium and phosphate ions are required for optimal transport activity. Chemical measurements of the total intracellular nucleotide pools demonstrated that this system was not a net adenine nucleotide transport system, but that uptake of AMP was the result of an exchange with internal adenine nucleotides.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- Hochstadt-Ozer J. The regulation of purine utilization in bacteria. IV. Roles of membrane-localized and pericytoplasmic enzymes in the mechanism of purine nucleoside transport across isolated Escherichia coli membranes. J Biol Chem. 1972 Apr 25;247(8):2419–2426. [PubMed] [Google Scholar]
- Khym J. X. An analytical system for rapid separation of tissue nucleotides at low pressures on conventional anion exchangers. Clin Chem. 1975 Aug;21(9):1245–1252. [PubMed] [Google Scholar]
- Klingenberg M. Metabolite transport in mitochondria: an example for intracellular membrane function. Essays Biochem. 1970;6:119–159. [PubMed] [Google Scholar]
- SNYDER J. C., BOVARNICK M. R., MILLER J. C., CHANG R. S. M. Observations on the hemolytic properties of typhus rickettsiae. J Bacteriol. 1954 Jun;67(6):724–730. doi: 10.1128/jb.67.6.724-730.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsberg P. D., Loo T. L. Simultaneous analysis of ATP, ADP, AMP, and other purines in human erythrocytes by high-performance liquid chromatography. J Chromatogr. 1980 Jan 11;181(1):103–107. doi: 10.1016/s0378-4347(00)81276-1. [DOI] [PubMed] [Google Scholar]
- Smith D. K., Winkler H. H. Characterization of a lysine-specific active transport system in Rickettsia prowazeki. J Bacteriol. 1977 Mar;129(3):1349–1355. doi: 10.1128/jb.129.3.1349-1355.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takadera T., Mohri T. Effect of N-ethylmaleimide on leucine transport in the Chang liver cell. II. Effect on the kinetics of Na+-independent transport. Biochim Biophys Acta. 1983 Nov 9;735(2):197–202. doi: 10.1016/0005-2736(83)90293-6. [DOI] [PubMed] [Google Scholar]
- WISSEMAN C. L., Jr, JACKSON E. B., HAHN F. E., LEY A. C., SMADEL J. E. Metabolic studies of rickettsiae. I. The effects of antimicrobial substances and enzyme inhibitors on the oxidation of glutamate by purified rickettsiae. J Immunol. 1951 Aug;67(2):123–136. [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. Growth and physiology of rickettsiae. Bacteriol Rev. 1973 Sep;37(3):259–283. doi: 10.1128/br.37.3.259-283.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C. Adenine nucleotide degradation by the obligate intracellular bacterium Rickettsia typhi. Infect Immun. 1980 Apr;28(1):74–81. doi: 10.1128/iai.28.1.74-81.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Peterson J. C. Enzymatic activities leading to pyrimidine nucleotide biosynthesis from cell-free extracts of Rickettsia typhi. Infect Immun. 1976 Aug;14(2):439–448. doi: 10.1128/iai.14.2.439-448.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Weiss E. Energy metabolism of Rickettsia typhi: pools of adenine nucleotides and energy charge in the presence and absence of glutamate. J Bacteriol. 1978 Jun;134(3):884–892. doi: 10.1128/jb.134.3.884-892.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Rickettsial permeability. An ADP-ATP transport system. J Biol Chem. 1976 Jan 25;251(2):389–396. [PubMed] [Google Scholar]
- Yagil E., Beacham I. R. Uptake of adenosine 5'-monophosphate by Escherichia coli. J Bacteriol. 1975 Feb;121(2):401–405. doi: 10.1128/jb.121.2.401-405.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



