Abstract
B7-H3 and B7x are recently discovered members of the B7-CD28 family thought to dampen peripheral immune responses via negative costimulation. We evaluated their potential expression in human prostate cancer using a large cohort of patients with 7 years of follow-up. We identified 823 patients with tissue available treated with radical prostatectomy between 1985 and 2003. Immunohistochemistry was performed on tissue microarray sections using anti-B7-H3 and -B7x. The percentage and intensity of immunoreactivity by tumor cells were blindly evaluated by two urological pathologists, and outcome analyses were conducted. Both B7-H3 and B7x were highly expressed; 93% and 99% of tumors had aberrant expression, respectively. The median percentage of tumor cells staining positive was 80% for each molecule. Strong intensity for B7-H3 and B7x was noted in 212 (26%) and 120 (15%) patients, respectively. Patients with strong intensity for B7-H3 and B7x were significantly more likely to have disease spread at time of surgery (P < 0.001 and P = 0.005, respectively). Additionally, patients with strong intensity for B7-H3 and B7x were at significantly increased risk of clinical cancer recurrence (P < 0.001 and P = 0.005) and cancer-specific death (P = 0.004 and P = 0.04, respectively). To our knowledge, we present the largest investigation of B7 family molecules in a human malignancy and a previously undescribed evaluation of B7x in prostate cancer. B7-H3 and B7x are abundantly expressed in prostate cancer and associated with disease spread and poor outcome. Given the proposed immune-inhibitory mechanisms of B7-H3 and B7x, these molecules represent attractive targets for therapeutic manipulation in prostate cancer.
Keywords: immune tolerance, prostatic neoplasms, treatment outcome, biological tumor markers
Within the last decade, it has become evident that T cell priming, growth, maturation, and tolerance are ultimately governed by costimulatory ligands and receptors expressed by antigen presenting cells and T cells (1, 2). Consistent with this, T cell receptor ligation by an antigenic peptide in the absence of a simultaneous costimulatory signal results in anergy, ignorance, or dysfunction (1, 2). The first costimulatory signal described demonstrated that B7–1 (CD80) engagement with the T cell receptor CD28 enhances T cell proliferation and IL-2 production (1, 2). Subsequently, it became evident that CTLA-4 could similarly interact with B7-1 and -2 (CD 86); however, this interaction delivers a negative or inhibitory signal, perhaps to truncate ongoing T cell responses and abort induction of autoimmunity (2, 3). Thus, CTLA-4 blockade via monoclonal antibody was proposed to stimulate T cell-mediated regression of tumors (4, 5), which has led to numerous ongoing phase II/III clinical trials today. More recently, other B7-CD28 family members have been discovered, including B7-H1 (PD-L1) (6), B7-H3 (7), and B7x (B7-H4, B7S1) (8–10). Numerous human tumors have been reported to aberrantly express B7-H1, and several long-term investigations demonstrate that B7-H1 expression is associated with adverse pathologic features and poor survival (11–14). Thus, B7-H1 has been proposed as an additional negative regulatory pathway in the B7 family, where manipulation via monoclonal antibody, similar in theory to CTLA-4, may provide clinical benefit by fostering unimpeded immune-mediated regression of antigenic tumors (15). Because of their relatively recent discovery, only one study to date has examined the potential clinical impact of both B7-H3 and B7x in a human malignancy (lung cancer) (16).
B7-H3 was discovered in 2001 and serves as an accessory costimulatory molecule after initial antigen priming in cooperation with a putative counterreceptor (7). The precise physiologic role of B7-H3 remains debatable, because both stimulatory properties, including promotion of T cell proliferation and IFN-γ production, along with inhibitory properties, including impairment of type 1 T-helper cell responses and protection from NK cell-mediated lysis, have been described (7, 17–19). B7-H3 protein expression has been described in numerous peripheral organs along with human malignancies of the lung, stomach, and prostate (16, 20, 21). B7x was discovered in 2003, and in contrast to B7-H3, has consistently demonstrated a negative costimulatory mechanism via inhibition of CD4+ and CD8+ T cell proliferation, cell-cycle progression, IL-2 production, and rendering tumor cells refractory to apoptosis (8–10, 22). Similar to B7-H3, B7x is a type I transmembrane protein and has a yet-unidentified counterreceptor. However, B7x protein expression seems to be more restricted compared with B7-H3. Despite mRNA expression in various human tissues, immunohistochemistry (IHC) for B7x fails to reveal detectable protein expression in any healthy human organs (23). In stark contrast, aberrant expression of B7x has been observed in cancers of the breast (22, 24), lung (16), ovary (22, 25), uterus (26), and kidney (27). Thus, B7x, and perhaps B7-H3, have been proposed as potential therapeutic targets in human malignancy. However, the literature is currently devoid of clinical observations of B7x in human prostate cancer.
Prostate cancer is the most common malignancy in men, with >200,000 cases per year in the United States alone (28). To date, only one study has attempted to correlate ectopic tumor expression of B7 family molecules in human prostate cancer with clinical outcome (21). Roth et al. (21) recently performed IHC on 338 men with prostate cancer, demonstrating that patients with high IHC intensity for B7-H3 were more likely to have adverse pathologic features and cancer recurrence after surgical extirpation (21). In the current study, we investigate the clinical impact of both B7-H3 and B7x in >800 patients treated with radical prostatectomy. With a median follow-up of 7 years, we demonstrate that both B7-H3 and B7x are highly expressed in prostate cancer. Moreover, we observed a significant association between cancer spread, progression-free survival, and cancer-specific survival among patients with strong B7-H3 and B7x intensity. We externally validate the findings from Roth et al. (21) and provide data that B7x may function at the clinical level in prostate cancer, potentially facilitating tumor progression by undermining host immunity.
Results
Comparison of Patients With and Without Interpretable Tissue.
Among 948 patients, 118 did not have tumor present on at least two tissue microarray cores, and seven patients had missing pathologic data. These 125 patients were therefore excluded from analysis, leaving 823 patients for our study cohort. There were no significant differences in biochemical recurrence, clinical recurrence-free survival, or cancer-specific survival between patients with and without interpretable tissue (all P > 0.15).
Clinical and Pathologic Features and Patient Outcome.
The clinical and pathologic features for the 823 patients included in the analyses are detailed in Table 1. At last follow-up, 171 patients developed a biochemical recurrence, 70 developed a clinical recurrence, and 22 died from prostate cancer. The median follow-up for patients who did not reach any of these events was 7.0 years (interquartile range 5.3–8.3). In a multivariable Cox proportional hazards regression model, incorporation of preoperative prostate-specific antigen (PSA), Gleason score, extracapsular extension, seminal vesicle invasion, lymph node involvement, and surgical margin status yielded a concordance index (c-index) of 0.781 for clinical failure.
Table 1.
Clinical and pathologic features for 823 patients with prostate cancer
| Feature | Median (interquartile range) or frequency (%) |
|---|---|
| Age | 62 (56–66) |
| Preoperative serum PSA in ng/ml | 7.80 (5.9–12.7) |
| Gleason score | |
| 5–6 | 285 (35) |
| 7 | 457 (56) |
| 8–10 | 81 (10) |
| Extracapsular extension | 251 (30) |
| Seminal vesicle invasion | 68 (8) |
| Positive surgical margins | 292 (35) |
| Lymph node involvement | 25 (3) |
| Non-organ confined | 276 (34) |
| B7-H3 (n = 803) | |
| Percentage of positive tumor cells | 80 (50–90) |
| Immunostaining intensity none | 57 (7) |
| Immunostaining intensity weak | 218 (27) |
| Immunostaining intensity moderate | 316 (39) |
| Immunostaining intensity strong | 212 (26) |
| B7x (n = 814) | |
| Percentage of positive tumor cells | 80 (70–95) |
| Immunostaining intensity none | 9 (1) |
| Immunostaining intensity weak | 377 (46) |
| Immunostaining intensity moderate | 308 (38) |
| Immunostaining intensity strong | 120 (15) |
Immunohistochemical Analysis of B7-H3.
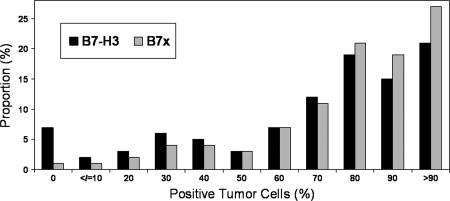
Among the 823 patients under study, 803 had at least two TMA cores that could be interpreted for B7-H3. B7-H3 was highly expressed (Fig. 1) with a median of 80% of tumor cells staining positive (Table 1). Only 57 (7%) specimens did not have evidence of tumor cell expression of B7-H3. For each 10% increase in the percentage of tumor cells expressing B7-H3, there was a significant association with the development of a clinical recurrence [hazard ratio (HR) 1.09 for each 10% increase; 95% confidence interval (C.I.) 1.00–1.19; P = 0.045] and a trend toward death from prostate cancer (HR 1.19 for each 10% increase; 95% C.I. 1.00–1.43; P = 0.054). The percentage of B7-H3-positive tumor cells, evaluated as a continuous variable, was not significantly associated with biochemical recurrence (HR 1.01; 95% C.I. 0.96–1.06; P = 0.7).
Fig. 1.
Distribution of B7-H3 and B7x percentage of positive tumor cells.
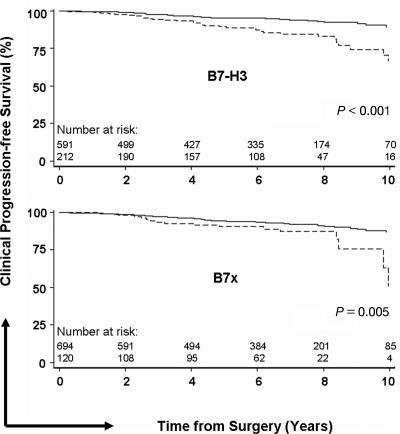
The intensity of B7-H3 staining is outlined in Table 1 and demonstrated in Fig. 2. Strong intensity was noted in 212 (26%) patients. Patients with strong intensity for B7-H3 were significantly more likely to have extracapsular extension (P < 0.001), seminal vesicle invasion (P = 0.019), and nonorgan-confined disease (P < 0.001), compared with patients without strong intensity (Table 2). Patients with strong intensity for B7-H3 were also significantly more likely to develop a biochemical recurrence (HR 1.39; 95% C.I. 1.01–1.92; P = 0.042), as well as a clinical recurrence (HR 2.79; 95% C.I. 1.74–4.46; P < 0.001; probability of failure shown in Fig. 3) and were more likely to subsequently die from prostate cancer (HR 3.48; 95% C.I. 1.50–8.09; P = 0.004; Table 3). Adding B7-H3 intensity to the multivariable model detailed above, B7-H3 intensity was an independent predictor of clinical failure (P = 0.001) but marginally increased the c-index from 0.781 to 0.788.
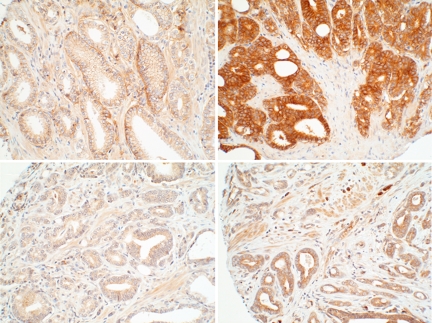
Fig. 2.
Photomicrographs of B7-H3 (Upper) and B7x (Lower) expression in prostate cancer. Weak (Left) and strong (Right) immunostaining intensity is demonstrated. (Magnification: ×20.)
Table 2.
Comparison of clinical and pathologic features by tumor B7-H3 and B7x intensity
| Feature | Tumor B7-H3 intensity |
Tumor B7x intensity |
||||
|---|---|---|---|---|---|---|
| None to moderate, n = 591 | Strong, n = 212 | P value | None to moderate, n = 694 | Strong, n = 120 | P value | |
| Median preoperative serum PSA (interquartile range) | 5.6 (7.8–12.7) | 5.2 (8.0–13.9) | 0.8 | 5.6 (7.9–12.7) | 5.1 (7.2–11.3) | 0.19 |
| Gleason score | 0.2 | 0.08 | ||||
| 5–6 | 215 (36) | 64 (30) | 248 (36) | 34 (28) | ||
| 7 | 317 (54) | 127 (60) | 383 (55) | 68 (57) | ||
| 8–10 | 59 (10) | 21 (10) | 63 (9) | 18 (15) | ||
| Extracapsular extension | 160 (27) | 88 (42) | <0.001 | 200 (29) | 48 (40) | 0.018 |
| Seminal vesicle invasion | 40 (7) | 26 (12) | 0.019 | 46 (7) | 48 (40) | <0.001 |
| Positive surgical margins | 203 (34) | 82 (39) | 0.3 | 248 (36) | 42 (35) | 0.9 |
| Lymph node involvement | 15 (3) | 10 (5) | 0.16 | 18 (3) | 7 (6) | 0.08 |
| Nonorgan confined | 177 (30) | 96 (45) | <0.001 | 219 (32) | 54 (45) | 0.005 |
Data are given as frequency (percentage) unless otherwise noted.
Fig. 3.
Progression-free survival for clinical cancer recurrence by tumor B7-H3 (Upper) and B7x intensity (Lower). The solid line represents weak to moderate intensity, and the dashed line represents strong intensity.
Table 3.
Univariate associations of B7-H3 and B7x intensity with biochemical recurrence, clinical failure, and death from disease after radical prostatectomy
| Hazard ratio (95% C.I.) | P value | |
|---|---|---|
| Biochemical recurrence | ||
| B7-H3 intensity | ||
| None to moderate | 1.0 (reference) | |
| Strong | 1.39 (1.01–1.92) | 0.042 |
| B7x intensity | ||
| None to moderate | 1.0 (reference) | |
| Strong | 1.38 (0.94–2.02) | 0.10 |
| Clinical failure | ||
| B7-H3 intensity | ||
| None to moderate | 1.0 (reference) | |
| Strong | 2.79 (1.74–4.46) | <0.001 |
| B7x intensity | ||
| None to moderate | 1.0 (reference) | |
| Strong | 2.22 (1.27–3.87) | 0.005 |
| Death from disease | ||
| B7-H3 Intensity | ||
| None to moderate | 1.0 (reference) | |
| Strong | 3.48 (1.50–8.09) | 0.004 |
| B7x intensity | ||
| None to moderate | 1.0 (reference) | |
| Strong | 2.71 (1.04–7.02) | 0.040 |
Immunohistochemical Analysis of B7x.
Among the 823 patients under study, 814 had at least two TMA cores that could be interpreted for B7x. Similar to B7-H3, B7x was highly expressed (Fig. 1) with a median of 80% of tumor cells staining positive (Table 1). Only nine (1%) tumors did not have evidence of tumor cell expression of B7x. Evaluated as a continuous variable, a 10% increase in the percentage of tumor cells expressing B7x was not significantly associated with biochemical recurrence (HR 0.99; 95% C.I. 0.93–1.06; P = 0.7), clinical recurrence (HR 1.08; 95% C.I. 0.97–1.21; P = 0.17), or death from prostate cancer (HR 1.07; 95% C.I. 0.88–1.30; P = 0.5).
The intensity of B7x staining is outlined in Table 1 and demonstrated in Fig. 2. Strong intensity was noted in 120 (15%) patients. Similar to B7-H3, patients with strong intensity for B7x were significantly more likely to have extracapsular extension (P = 0.018), seminal vesicle invasion (P < 0.001), and nonorgan-confined disease (P = 0.005) compared with patients without strong intensity (Table 2). Additionally, patients with strong intensity for B7x were more likely to have positive lymph nodes (6% vs. 3%), although this difference did not reach statistical significance (P = 0.08). Patients with strong intensity for B7-H3 were more likely to develop a biochemical recurrence (HR 1.38; 95% C.I. 0.94–2.02; P = 0.10), and were significantly more likely to subsequently develop a clinical recurrence (HR 2.22; 95% C.I. 1.27–3.87; P = 0.005; probability of failure is shown in Fig. 3) and die from prostate cancer (HR 2.71; 95% C.I. 1.04–7.02; P = 0.04; Table 3). Adding B7x intensity to the multivariable model detailed above, B7x intensity was not an independent predictor of clinical failure (P = 0.3).
Discussion
The fundamental regulatory mechanisms governing immune system activation, deactivation, and dysfunction have sparked growing interest within the past decade. Related to this, the B7-CD28 family of costimulatory molecules has been intensely studied for their potential clinical impact in human malignancies, especially with regard to ectopic tumor cell expression of negative costimulating molecules. To our knowledge, we present the largest clinical investigation of the B7-CD28 family in a human malignancy. Our results demonstrate that both B7-H3 and B7x, unlike B7-H1 (21), are highly and aberrantly expressed by most human prostate cancer cells. Patients with strong IHC intensity for either B7-H3 or B7x are significantly more likely to have disease spread at time of surgery (i.e., extraprostatic extension or seminal vesicle invasion) and are at significant risk of metastatic recurrence or subsequent development of hormone-refractory prostate cancer. Further, we observed a significant association between cancer-specific death, which is rare after surgery, and strong IHC intensity for both B7-H3 and B7x independently. The basis for these associations may relate to the recognized ability of B7-H3 and B7x to inhibit T cell-mediated immunity (8–10, 17–19). Consistent with the basic science literature, these results provide further data that B7-H3 and B7x are functioning at the clinical level, perhaps to facilitate cancer progression of otherwise localized tumors through impairment of host T cell-mediated immunity. Thus, therapeutic manipulation of B7-H3 and B7x to preempt impending cancer progression warrants further investigation.
In the context of malignancy, the precise physiologic and pathologic role of B7-H3 expression has yet to be fully elucidated. Transfection of B7-H3 into experimental tumor lines (P815 mastocytoma, Colon-26, and EL-4 lymphoma) has been reported to accelerate in vivo tumor rejection, supporting a net positive costimulatory effect (29–31). Additionally, observations of human gastric carcinoma suggest that B7-H3-positive tumor cells are associated with improved patient survival (20). In contrast, tumor cell expression of B7-H3 in human lung cancer was reported to be associated with an increased risk of lymph node metastases (16). More recently, Roth et al. (21) evaluated B7-H3 in human prostate cancer patients who underwent radical prostatectomy is a study design similar to our investigation. Despite unique patient populations, differences in antibodies used, variations in definitions of endpoints (i.e., PSA >0.4 vs. >0.2 ng/ml), and different pathologists evaluating the specimens, we independently obtained remarkably similar results (21). That is, most prostate cancer cells express B7-H3; absolute percentage of expression is not as predictive as intensity; and strong intensity is associated with extracapsular extension, seminal vesicle invasion, and clinical recurrence of cancer (21). Our study was also able to demonstrate an association with cancer-specific survival, although this may relate to our larger sample cohort. External validation studies have recently been highlighted to be of paramount importance for proper translation of biomarkers into the clinical setting. To that end, single studies of a given molecule within one cohort of patients are often inadequate to firmly establish its value for others (32). Thus, we externally and independently validate the findings from the Mayo Clinic, and collectively these studies support, at least in prostate cancer, that B7-H3 functions at the clinical level, precluding immune containment and thereby fostering cancer recurrence.
B7x, discovered in 2003, is the newest member of the B7 family (8–10). Because of its relatively recent identification, clinical observations of B7x in prostate cancer have not been previously reported. However, aberrant expression of B7x has been observed in cancers of the breast (22, 24), lung (16), ovary (22, 25), uterus (26), and kidney (27). Krambeck et al. (27) recently evaluated B7x in 259 patients with renal cell carcinoma, demonstrating that tumor expression of B7x was associated with adverse pathologic features (including advanced tumor size, grade, and stage) and an increased risk of death from disease (27). Interestingly, B7x was found to be preferentially expressed on tumor vasculature, suggesting a potential role for initiating or maintaining tumor angiogenesis (27). Because B7x has consistently demonstrated in vitro and in vivo inhibitory properties (8–10, 22), it was postulated that B7x represents a promising target for antitumor immunotherapy in renal cell carcinoma (27). Consistent with this, we demonstrate that nearly all (99%) patients with prostate cancer have aberrant or ectopic expression of B7x. Similar to B7-H3, strong B7x intensity is associated with cancer spread along with clinical cancer recurrence and subsequent death. These findings will need external validation for confirmation. However, B7x currently represents a potential therapeutic target in human prostate cancer, whereas blockade may provide appropriate protection for effector lymphocytes.
We recognize the limitations of this study. Evaluation was performed in a retrospective fashion. However, the tissue and data were collected and assimilated into a prospective database for future use. Although by nature this study is retrospective, it would take the span of one's career to duplicate this study in a prospective fashion. Additionally, the percentage of tumor cells that stained positive was not consistently associated with outcome as compared with staining intensity. The percentage of tumor cells staining positive was analyzed as a continuous variable; however, we were reluctant to perform post-hoc analyses in an attempt to find a certain percentage where statistical significance is achieved. These findings are often fraught with problems and invite significant interobserver variability, especially with the relatively crude estimation of IHC (i.e., what is the difference between 80% and 90% expression?). Additionally, we did not observe robust associations between B7-H3 and B7x expression and biochemical recurrence (in contrast to clinical recurrence and cancer-specific mortality). This phenomenon may relate to the fact that many patients, perhaps because of scant remaining benign prostate tissue after surgery, develop a PSA >0.2 ng/ml yet fail to recur clinically or even develop a rising PSA (33). Last, we do not believe our findings support the routine use of B7-H3 or B7x for independent prognostication in prostate cancer; however, longer follow-up is warranted, because clinical progression and cancer-specific death are uncommon after surgery for prostate cancer, and follow-up in this study was relatively limited at a median of 7 years.
We and others have now observed aberrant expression of B7-H1, B7-H3, and B7x in numerous human malignancies and proposed that therapeutic manipulation of these immune regulatory proteins represents one of the most promising targets in cancer therapy today (1, 2, 11–14, 16, 21–27). A compelling precedent for abrogating inhibitory T cell signaling to enhance antitumor immunity has been established with the experience of CTLA-4 blockade (34–37). CTLA-4 blockade has also recently been investigated in patients with hormone-refractory prostate cancer (38). One distinct difference between CTLA-4 and other B7-CD28 members is that CTLA-4 ligand expression is limited to “professional” antigen presenting cells (dentritic cells, macrophages, etc.) in lymphoid tissues. In contrast, B7-H3 and B7x are aberrantly expressed by tumor cells. Thus CTLA-4 is predominantly functioning early in the immune activation process in lymphoid tissues, whereas B7-H3 and B7x are functional more downstream in the immune response, where effector T cells are in the vicinity of the peripheral tumor (1, 3). Nevertheless, effective immune modulation for clinical efficacy may ultimately require manipulation at multiple sites. Based on evidence to date, we surmise that blockade of B7x may provide a more tumor-specific effect compared with B7-H3 for prostate cancer patients. This hypothesis is based on the observations that B7x protein expression seems to be more restricted compared with B7-H3 (23), and B7x has more consistently demonstrated inhibitory properties in laboratory investigation. Nevertheless, our data strongly suggest that B7-H3 and B7x represent promising therapeutic targets to preempt cancer progression for numerous human malignancies, including prostate cancer.
Conclusion
We performed the largest evaluation of B7 family molecules in a human cancer. B7-H3 and B7x are highly expressed in prostate cancer and associated with extracapsular extension, seminal vesicle invasion, development of metastatic or hormone-refractory cancer, and cancer-specific death. Given the proposed immune inhibitory mechanisms of B7-H3 and B7x, these molecules represent attractive targets for therapeutic manipulation in the multimodal management of prostate cancer.
Materials and Methods
Patient Identification.
After Institutional Board Review approval, we identified 948 patients with tissue available in paraffin-embedded blocks who were treated with a pelvic lymph node dissection and radical retropubic prostatectomy at Memorial Sloan–Kettering Cancer Center for localized prostate cancer between 1985 and 2003. Patients who received androgen-deprivation therapy or radiation before surgery or in an adjuvant setting were excluded.
Clinical and Pathologic Features.
Clinical and pathologic features, along with follow-up information, were obtained from the Memorial Sloan–Kettering Cancer Center prostate cancer database. This database contains >150 unique variables for each patient with data collected in a prospective fashion. The clinical features studied included age at surgery and preoperative serum PSA. The pathologic features studied included Gleason score, extracapsular extension, seminal vesicle invasion, surgical margin status, and lymph node involvement. Nonorgan-confined disease was defined as the presence of extracapsular extension, seminal vesicle invasion, or positive lymph nodes.
Biochemical recurrence was defined as a PSA >0.2 ng/ml with a confirmatory level (39). Clinical failure was defined as the development of metastases or hormone–refractory prostate cancer. Patient vital status was also recorded, and death from causes other than prostate cancer was censored in the analyses.
Creation of Tissue Microarrays.
H&E slides of prostatectomy specimens were reviewed by two urological pathologists (H.A.A.-A. and V.E.R.) and slides containing tumor were marked and matched with corresponding paraffin blocks. Tissue cores of 0.6 mm were then punched out in triplicate from locations randomly selected within the marked tumor areas and then mounted in blank recipient blocks using an automated tissue microarrayer (Beecher Instruments).
Antibodies and Immunohistochemistry.
Antibodies used in this study included goat anti-B7-H3 (catalog no. BAF1027), goat anti-B7x (catalog no. AF2154), and isotype control antibodies (R&D Systems). Positive and negative controls, as determined by flow cytometry, were cell lines MDA-MB-468 (B7-H3 negative and B7x positive), MDA-MB-231 (B7-H3 negative, B7x negative, and B7-H1 positive), and B7-H3/BWZ (B7-H3 positive and B7x negative), which was transfected to express B7-H3. Immunohistochemical staining of B7-H3 and B7x was done using the EnVision Peroxidase Detection System and DAKO TechMate 500/1000 staining machine (BioTek Solutions). Four-micrometer sections were mounted on Superfrost plus slides, deparaffinized, rehydrated, incubated with Dako Target Retrieval Solution (pH = 9), and heated at 900 W for 2 min. The sections were incubated with anti-B7-H3 (5 μg/ml) and anti-B7x (10 μg/ml) and further processed using the EnVision Peroxidase Detection System with biotinylated secondary antibodies. The sections were counterstained with Mayer's hematoxylin solution, and coverslips were applied. Irrelevant isotype-matched antibodies were used to control for nonspecific staining.
The IHC stains were evaluated by two urological pathologists (H.A.A.-A. and V.E.R.) without knowledge of patient outcome. The percentage of tumor cells staining positive for B7-H3 and B7x was recorded in 10% intervals, and the level of IHC intensity was recorded as none, weak, moderate, or strong. Rarely, only 5% of tumor cells demonstrated expression of B7-H3 or B7x, and these tumors were considered positive. Because B7-H3 staining showed distinct membranous accentuation by tumor cells, the intensity was considered weak when either cytoplasmic expression or rare membranous condensation was present, moderate when incomplete and discontinuous moderate membranous expression was present, and strong when complete membranous expression of the molecule was present. When no tumor was present on any core, this was recorded as “unable to interpret.”
Statistical Analysis.
The percentage of positive tumor cells and IHC intensity was defined as the maximum value of the three cores; patients with only zero or one core scored were treated as missing for that costimulatory molecule. Looking at the distribution of maximum percentage of positive tumor cells without reference to outcome (Fig. 1), both B7-H3 and B7x appeared to have a continuous distribution. Accordingly, the percentage of positive tumor cells was evaluated as a continuous variable. Immunostaining intensity was evaluated as a categorized variable comparing strong vs. none to moderate intensity. Immunostaining intensity was also evaluated as a categorized variable comparing strong vs. moderate vs. none or weak intensity. Because the P values obtained from classifying patients into two groups were similar to classification into three groups (for example, the global P value for a difference in clinical failure by B7-H3 and B7x intensity was <0.001 and <0.02 for both classifications), HR and 95% C.I. are reported only for classification into two groups (i.e., strong vs. none or moderate intensity).
Comparisons between B7-H3/B7x expression and pathologic features were evaluated by using Fisher's exact test and with preoperative PSA using the Mann–Whitney test. Cancer progression after surgery was estimated by using the Kaplan–Meier method. The duration of follow-up was calculated from the date of surgery to the date of cancer progression, last follow-up, or last postoperative serum PSA. Associations of B7-H3 and B7x with cancer progression and cancer-specific survival were evaluated by using Cox models. Predictive accuracy was defined in terms of the c-index. In brief, the c-index is comparable to the area under the receiver-operating curve and can be used to quantify discrimination for survival time data in single-variable and multivariable models. All c-indices were bootstrap-corrected with 200 replications. Statistical analyses were conducted by using Stata 9.0 (StataCorp LP). All tests were two-sided, and P values <0.05 were considered statistically significant.
Acknowledgments
We are indebted to Stephen P. Hanson, who sponsored the urologic oncology fellowship for R.H.T. This project was supported in part by generous gifts from the David H. Koch Foundation and the Prostate Cancer Foundation. X.Z. was supported by a fellowship from the Cancer Research Institute.
Footnotes
The authors declare no conflict of interest.
References
- 1.Chen L. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 2.Zang X, Allison JP. Clin Cancer Res. 2007;13:5271–5279. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 3.Chambers CA, Kuhns MS, Egen JG, Allison JP. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 4.Townsend SE, Allison JP. Science. 1993;259:368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- 5.Leach DR, Krummel MF, Allison JP. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 6.Dong H, Zhu G, Tamada K, Chen L. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 7.Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, et al. Nat Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 8.Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. Proc Natl Acad Sci USA. 2003;100:10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. Immunity. 2003;18:849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 10.Prasad DV, Richards S, Mai XM, Dong C. Immunity. 2003;18:863–873. doi: 10.1016/s1074-7613(03)00147-x. [DOI] [PubMed] [Google Scholar]
- 11.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L, et al. Proc Natl Acad Sci USA. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, et al. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 13.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, et al. Proc Natl Acad Sci USA. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, et al. Clin Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 15.Thompson RH, Dong H, Kwon ED. Clin Cancer Res. 2007;13:S709–S715. doi: 10.1158/1078-0432.CCR-06-1868. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, Wang X. Lung Cancer. 2006;53:143–151. doi: 10.1016/j.lungcan.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, et al. Nat Immunol. 2003;4:899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 18.Castriconi R, Dondero A, Augugliaro R, Cantoni C, Carnemolla B, Sementa AR, Negri F, Conte R, Corrias MV, Moretta L, et al. Proc Natl Acad Sci USA. 2004;101:12640–12645. doi: 10.1073/pnas.0405025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prasad DV, Nguyen T, Li Z, Yang Y, Duong J, Wang Y, Dong C. J Immunol. 2004;173:2500–2506. doi: 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- 20.Wu CP, Jiang JT, Tan M, Zhu YB, Ji M, Xu KF, Zhao JM, Zhang GB, Zhang XG. World J Gastroenterol. 2006;12:457–459. doi: 10.3748/wjg.v12.i3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, Krambeck AE, McKenney ME, Karnes RJ, Blute ML, et al. Cancer Res. 2007;67:7893–7900. doi: 10.1158/0008-5472.CAN-07-1068. [DOI] [PubMed] [Google Scholar]
- 22.Salceda S, Tang T, Kmet M, Munteanu A, Ghosh M, Macina R, Liu W, Pilkington G, Papkoff J. Exp Cell Res. 2005;306:128–141. doi: 10.1016/j.yexcr.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Choi IH, Zhu G, Sica GL, Strome SE, Cheville JC, Lau JS, Zhu Y, Flies DB, Tamada K, Chen L. J Immunol. 2003;171:4650–4654. doi: 10.4049/jimmunol.171.9.4650. [DOI] [PubMed] [Google Scholar]
- 24.Tringler B, Zhuo S, Pilkington G, Torkko KC, Singh M, Lucia MS, Heinz DE, Papkoff J, Shroyer KR. Clin Cancer Res. 2005;11:1842–1848. doi: 10.1158/1078-0432.CCR-04-1658. [DOI] [PubMed] [Google Scholar]
- 25.Tringler B, Liu W, Corral L, Torkko KC, Enomoto T, Davidson S, Lucia MS, Heinz DE, Papkoff J, Shroyer KR. Gynecol Oncol. 2006;100:44–52. doi: 10.1016/j.ygyno.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 26.Miyatake T, Tringler B, Liu W, Liu SH, Papkoff J, Enomoto T, Torkko KC, Dehn DL, Swisher A, Shroyer KR. Gynecol Oncol. 2007;106:119–127. doi: 10.1016/j.ygyno.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 27.Krambeck AE, Thompson RH, Dong H, Lohse CM, Park ES, Kuntz SM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. Proc Natl Acad Sci USA. 2006;103:10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 29.Luo L, Chapoval AI, Flies DB, Zhu G, Hirano F, Wang S, Lau JS, Dong H, Tamada K, Flies AS, et al. J Immunol. 2004;173:5445–5450. doi: 10.4049/jimmunol.173.9.5445. [DOI] [PubMed] [Google Scholar]
- 30.Lupu CM, Eisenbach C, Kuefner MA, Schmidt J, Lupu AD, Stremmel W, Encke J. J Gastrointest Surg. 2006;10:635–645. doi: 10.1007/BF03239969. [DOI] [PubMed] [Google Scholar]
- 31.Sun X, Vale M, Leung E, Kanwar JR, Gupta R, Krissansen GW. Gene Ther. 2003;10:1728–1734. doi: 10.1038/sj.gt.3302070. [DOI] [PubMed] [Google Scholar]
- 32.Altman DG, Royston P. Stat Med. 2000;19:453–473. doi: 10.1002/(sici)1097-0258(20000229)19:4<453::aid-sim350>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 33.Amling CL, Bergstralh EJ, Blute ML, Slezak JM, Zincke H. J Urol. 2001;165:1146–1151. [PubMed] [Google Scholar]
- 34.Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A, et al. Proc Natl Acad Sci USA. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, et al. Proc Natl Acad Sci USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, et al. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korman AJ, Peggs KS, Allison JP. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. Clin Cancer Res. 2007;13:1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 39.Cookson MS, Aus G, Burnett AL, Canby-Hagino ED, D'Amico AV, Dmochowski RR, Eton DT, Forman JD, Goldenberg SL, Hernandez J, et al. J Urol. 2007;177:540–545. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]