Abstract
Magnetosomes are magnetite nanoparticles formed by biomineralization within magnetotactic bacteria. Although there have been numerous genetic and proteomic studies of the magnetosome-formation process, there have been only limited and inconclusive studies of mineral-phase evolution during the formation process, and no real-time studies of such processes have yet been performed. Thus, suggested formation mechanisms still need substantiating with data. Here we report the examination of the magnetosome material throughout the formation process in a real-time in vivo study of Magnetospirillum gryphiswaldense, strain MSR-1. Transmission EM and x-ray absorption spectroscopy studies reveal that full-sized magnetosomes are seen 15 min after formation is initiated. These immature magnetosomes contain a surface layer of the nonmagnetic iron oxide-phase hematite. Mature magnetite is found after another 15 min, concurrent with a dramatic increase in magnetization. This rapid formation result is contrary to previously reported studies and discounts the previously proposed slow, multistep formation mechanisms. Thus, we conclude that the biomineralization of magnetite occurs rapidly in magnetotactic bacteria on a similar time scale to high-temperature chemical precipitation reactions, and we suggest that this finding is caused by a biological catalysis of the process.
Keywords: biomineralization, magnetotactic bacteria, biogenic magnetite, α-hematite precursor
Since the discovery of magnetic bacteria, the nanobiomineral inclusions synthesized within the cell, termed magnetosomes, have enjoyed considerable interest (1). Magnetotactic bacteria are ubiquitous Gram-negative prokaryotes that respond to a magnetic field. They are found in fresh and saltwater sediments. Although some strains can be grown under aerobic conditions, magnetosome synthesis only occurs under microaerobic conditions. Magnetic bacteria biomineralize magnetite (and less commonly greigite) crystals within lipid vesicles arranged in chains along the cell (typically 20–40 particles long), which are responsible for the magnetotaxis. The magnetosomes are highly consistent with respect to size and morphology defined for each strain, which suggests a large degree of genetic control over the synthetic process. The bacteria have a sensitive iron-uptake system that can concentrate large quantities of iron within the cell to biomineralize magnetosomes, and it is this biomineralization process that has attracted the most biological and materials science interest.
The process of biomineralization is important with respect to the lineage of evolution because it is the rapid increase in biomineralization that ultimately saw the evolution of skeletal organisms, which is thought to be responsible for the Cambrian explosion (2). Furthermore, magnetotactic bacteria are the simplest single-cell organisms in which biomineralization occurs and, as such, offer an ideal model system to study the biomineralization mechanism. An improved understanding of biomineralization processes could lead to advances in the inorganic synthesis of similar materials. In particular, knowledge of how magnetite particle formation and morphology are controlled by microorganisms could lead to better control of these parameters for in vitro magnetite formation.
Research efforts have focused on identifying biomineralization proteins. To date, iron transport proteins, scaffold proteins, and vesicle-forming proteins unique to magnetotactic bacteria have been identified (3–6). These findings suggest a mechanism for the process whereby Fe2+ is transported into a preformed empty vesicle within the cell. Fe2+ accumulates and forms amorphous ferrous oxyhydroxide, which is then thought to be partially oxidized by an Fe-oxidase; water is then lost, and magnetite is crystallized (7).
Although much biological research aims to understand the mechanism of magnetosome formation by using proteomic and genetic methods, there is little research on the nature of the actual immature magnetite, and there is no direct evidence to support the proposed mechanism. High-resolution transmission EM (TEM) imaging has shown amorphous material on the surface of particles; however, other than magnetite, no other crystalline phases were observed (8, 9). Mössbauer analysis of magnetosomes has shown the presence of another iron species, as well as magnetite. It was suggested that this material was composed of immature magnetosomes, and it could be seen from the Mössbauer signal that the material's origin was some oxide or oxyhydroxide of iron. Thus, it was speculated that the signal was from either superparamagnetic magnetite particles that had yet to reach the single-domain size threshold or a precursor material such as ferritin or ferrihydrite (10, 11). This material has since been assumed to be ferrihydrite because it is the biomineralization precursor to the magnetite component of Chiton's (marine molluscs) teeth (12–14). Contrary to this notion, lepidocrocite has been suggested as the magnetosome precursor in a proposed biomineralization mechanism, similar to that of an inorganic magnetite-film process (15). However, the actual identity of the magnetite precursors in magnetotactic bacteria still remains unknown.
A detailed study of the whole formation and growth process of magnetosomes from a materials perspective would not only complement proteomic and genetic studies, but also would provide valuable insight into the process mechanism and time scale of the biomineralization of the magnetosome. This study would be especially valuable if the material's geometric and magnetic structures could be monitored throughout.
One method of analyzing the structural and magnetic character of materials is x-ray magnetic circular dichroism (XMCD), which probes the magnetic ions to provide information about the site occupancy and oxidization state of these ions. XMCD is the difference of the x-ray absorption spectra (XAS) over the L2,3 absorption edges, recorded with the magnetization parallel and antiparallel to the circular polarization vector of the x-ray beam. The measured XMCD spectrum is the sum of the components from each magnetic ion in the material. By using calculated XMCD spectra determined for each ion, any given spectrum can be modeled to obtain the relative quantities of each ion within the material. This method has been used previously to probe magnetite and other related iron oxide species (16). Magnetite has three distinct iron ion sites: the octahedral Fe2+ site, designated d6oct; and Fe3+ ions in both octahedral and tetrahedral positions, designated d5oct and d5tet, respectively. Stoichiometric magnetite has a site occupancy of d6oct = d5oct = d5tet = 1, whereas maghemite (γ-Fe2O3) has a site occupancy of d6oct = 0, d5oct = d5tet = 1, and so different combinations of magnetic iron oxides can be differentiated in this way.
Here we report an in vivo experiment to track the formation of magnetite magnetosomes in the bacterium Magnetospirillum gryphiswaldense, strain MSR-1. Magnetosome formation was induced in nonmagnetic cells by creating a microaerobic environment and then adding iron. Samples were taken at set time intervals after the induction, and the magnetosome mineral phase was analyzed by TEM and XMCD.
Results
Large-scale fermenter growth of nonmagnetic cells was performed with the omission of iron at an oxygen tension of 5%. Although trace iron was present in the medium, the O2 tension is sufficiently high to prevent magnetosome formation. This finding was confirmed by the lack of a mineral fraction and TEM analysis of these cells (Fig. 1a).
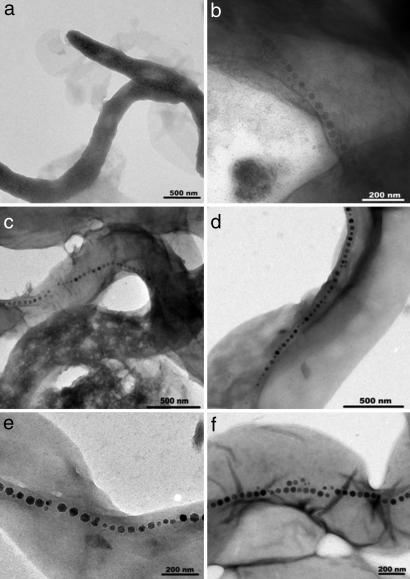
Fig. 1.
TEM images of M. gryphiswaldense at various times after magnetosome induction. (a) Zero minutes (before induction). (b and c) Fifteen minutes after induction. (d) One hour after induction. (e) Three hours after induction. (f) Six hours after induction.
Magnetosome formation was induced by reducing the O2 tension to 0.5–1%, creating a microaerobic environment, and then adding iron. Previous iron-uptake studies have suggested that the time scale for the initiation of magnetosome formation is ≈10 min after induction, and small (5- to 20-nm), immature particles have been observed after 30 min (17). Then continuous growth of the magnetosome particles was seen over the next 20 h (18). Thus, we adopted a similar time scale. The minimum practical time interval to obtain and prepare the samples was 15 min, and so samples were taken every 15 min after magnetosome formation was induced for the first hour, then every 30 min for the next 2 h, and finally hourly until 10 h after formation was induced. A final sample was taken 24 h after induction was initiated. The utmost care was taken to ensure the termination of the magnetosome-forming processes as soon as the sample was removed from the fermenter. In particular, the samples were collected on ice and were immediately treated with chloramphenicol to halt biological process and protein production. TEM grid preparation, cell density measurements, and centrifugation (2–4°C) were performed simultaneously at this stage. The sample pellets containing the bulk mineral phases were resuspended in cell lysis buffer and plunged into liquid N2 for storage. The effectiveness of the sampling collection and subsequent protocols were tested before the experiment by means of several dummy runs. These samples were analyzed by TEM, and it was shown that the pretreatment was effective in halting magnetosome formation.
Cells containing full chains of magnetosomes are seen in the first sample 15 min after formation was initiated (Fig. 1 b and c). Long chains of magnetosomes of the same size are seen in every subsequent sample thereafter in ever-increasing numbers of cells (Fig. 1 d–f). TEM analysis of cells collected 15 min after induction reveals that over half of the cells have long chains of full-sized magnetosomes, although they appear paler than in later samples. However, we saw no evidence of small partially formed particles in chains that would resemble the precursors to the fully formed chains reported in ref. 18. A small number of cells were observed, with a few scattered particles similar to those described in the previous literature (17), but these cells were not in a chain formation. This finding suggests that the formation of chains of mature-sized magnetosomes is a rapid step, occurring within 15 min. As time increased, the number of cells containing magnetosome chains increased so that 30–60 min after induction, most cells contained long chains of dark, full-sized mature magnetite (Fig. 1 d–f). There is no apparent correlation between time and particle size or chain length (on this time scale) because long chains and full-size particles are present throughout the process.
Furthermore, it can be seen from Fig. 1 that the chains of magnetosomes throughout the samples are long, contrary to chains seen in M. gryphiswaldense grown under standard conditions, which normally have a chains motif of 20–30 crystals. This finding suggests that the starvation of iron and the subsequent induction of rapid magnetosome synthesis result in longer and more irregular chains.
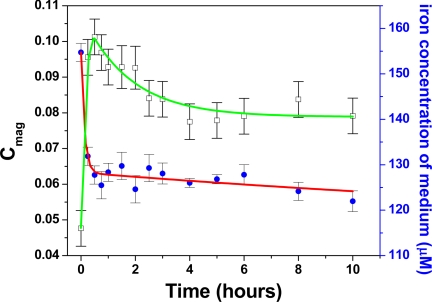
The short time frame for the formation also is reflected in the bacterial iron uptake, which is dramatic over the first few minutes (Fig. 2). Of the total iron taken up by the cells, 75% is consumed in the first 15 min and 86% in the first 30 min. This result is supported by recent iron-uptake gene studies that also show significant cellular iron uptake within 10 min of induction, which is similar to the results presented here (19). The magnetic optical density (Cmag) is a qualitative measure of the cells' magnetization (20). In this study, the cells acquire a significant magnetization in the first 15–30 min and then remain so thereafter (a decrease in magnetism over time in the figure is because of the increase in cell number that increases multiple scattering, which in turn reduces the extent of differential light scattering). This result confirms that the magnetosomes become magnetic within this time (Fig. 2).
Fig. 2.
Iron concentration of the medium (μM), blue circle (red line is a guide for the eye); qualitative measurement of bacterial magnetism (Cmag), open square (green line is a guide for the eye) over the 10-h formation period.
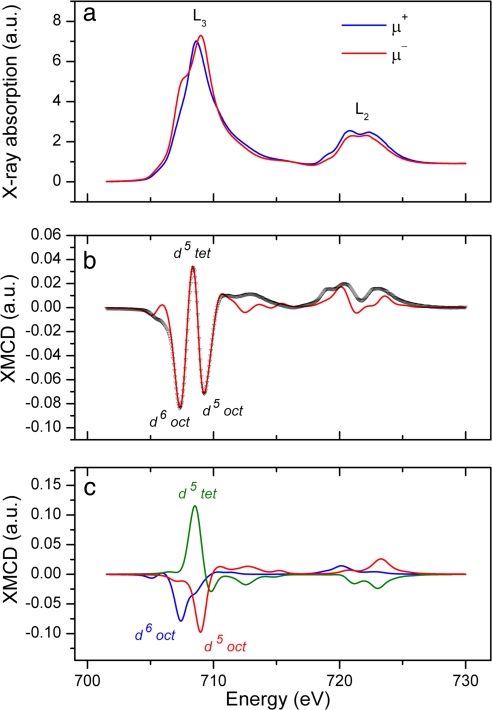
XAS obtained with the magnetic field applied parallel and antiparallel to the x-ray beam, together with the resulting XMCD spectrum, are shown in Fig. 3. These spectra were measured for the final mature sample obtained 24 h after induction. The difference in the two absorption curves can be clearly seen in Fig. 3a and demonstrates the magnetic nature of the sample (a nonmagnetic sample would yield identical absorption spectra). The XMCD spectrum (taken as the difference of the two curves in Fig. 3a) is shown together with a best-fit calculation in Fig. 3b. The differing magnetic alignments of the Fe ions on the three magnetite sites (two octahedral and one tetrahedral) lead to positive and negative peaks in the XMCD, which can be associated with each site, as indicated in Fig. 3b. The calculated XMCD for each site in stoichiometric magnetite is shown in Fig. 3c. By scaling each component and by using the resultant summed spectrum (red line in Fig. 3b) to fit the XMCD data, the site occupancies for a given sample can be determined. The values obtained for the mature (24-h) sample are given in Table 1 and suggest the formation of magnetite in a slightly reduced form.
Fig. 3.
XAS and XMCD spectra obtained from the fully mature magnetosomes 24 h after induction. (a) Spectra shown are the absorption curves obtained with the magnetic field applied parallel (μ+) and antiparallel (μ−) to the x-ray beam. The positions of the L2 and L3 absorption peaks are indicated. (b) Experimental XMCD spectrum (black curve) taken as the difference of the two absorption curves, together with the calculated best fit (red curve). Features in the XMCD corresponding to the different Fe sites in magnetite are labeled. (c) Calculated XMCD components for each Fe site, which were used to obtain the best fit to the experimental XMCD.
Table 1.
Occupation of the three Fe sites in magnetosome magnetite as a function of formation time as determined from the best fit to the XMCD spectra
| Formation time | Site occupation (±0.02) |
||
|---|---|---|---|
| d6oct | d5tet | d5oct | |
| 30 min | 1.07 | 0.98 | 0.95 |
| 45 min | 1.12 | 0.96 | 0.92 |
| 4 h | 1.08 | 0.98 | 0.94 |
| 24 h | 1.08 | 0.96 | 0.96 |
The values shown are relative to stoichiometric magnetite for which d6oct = d5tet = d5oct = 1.
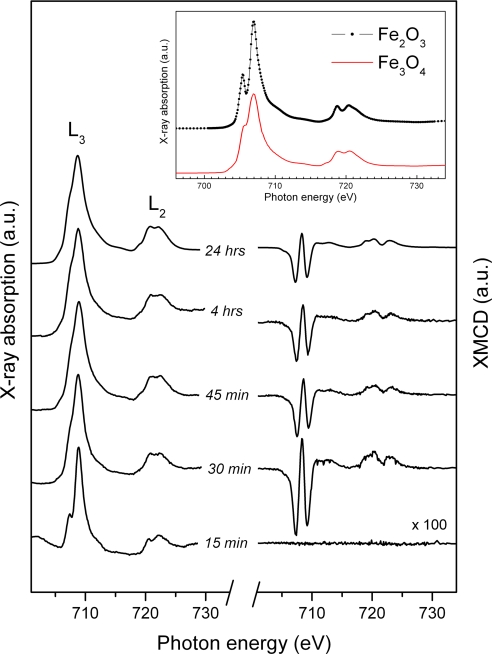
The magnetization-averaged XAS for a series of magnetosomes with increased formation time, together with their associated XMCD curves, are shown in Fig. 4. These magnetization-averaged absorption curves give useful information on the chemical valance of a sample. It can be seen from the figure that the sample obtained 15 min after induction yielded a different XAS, compared with the more mature samples, with a pronounced shoulder feature clearly observed on the left of the main L3 peak. Comparison with the XAS curves shown in Fig. 4 Inset (obtained from mineral samples) would suggest the presence of an Fe2O3 phase in these immature magnetosomes. Further, the absence of an XMCD curve would suggest that this phase is not magnetic, thus differentiating it from the magnetic γ-Fe2O3 (maghemite) phase. However, there is an apparent discrepancy with the data in Fig. 2, which suggests that these immature magnetosomes are magnetic. The explanation for this finding lies in the limited probing depth of the XMCD technique used here, which is only 3–4 nm into the sample surface. Together, all of these factors suggest the presence of a surface layer of the nonmagnetic (strictly speaking antiferromagnetic) α-Fe2O3 (hematite) phase, covering a magnetic phase (presumably magnetite) beneath.
Fig. 4.
XAS and XMCD spectra measured from magnetosomes at specified time intervals after induction. The curves were shifted vertically for clarity. (Inset) XAS measured from mineral iron oxide samples (30).
Samples prepared with formation times of ≥30 min were found to give XAS curves characteristic of magnetite, as can be seen by comparison with the reference curve in Fig. 4 Inset. In addition, the onset of a strong XMCD was observed after only 30 min, with the characteristic features of magnetite shown in Fig. 3b. By fitting each XMCD curve by using the procedure outline earlier, the site occupancies for each sample were determined (Table 1). From these values, it appears that the site occupancy remains fixed as soon as magnetite formation is observed, 30 min after induction, and is unchanged after 24 h. Only one sample (45 min) was found to show a slight variation from the final mature magnetosome values.
The intensity of the measured XMCD signal is proportional to the degree of magnetization of the sample. Thus, the reduced XMCD seen for samples with formation times of >30 min is likely to be caused by incomplete magnetic saturation of these samples in the magnetic field applied in this experiment (0.6 T). The magnetic field required for complete saturation is a function of the size of the magnetite and will decrease as the particles become small enough to approach the so-called superparamagnetic limit. Thus, the change in the overall intensity of the XMCD, seen in Fig. 4, is probably a result of the slight enlargement of magnetosomes as their formation progresses.
Thus, it can be concluded that immature magnetosomes are formed within 15 min, which appear to be magnetite particles coated with a hematite precursor layer, and fully mature magnetite is formed within 30 min and remains unchanged thereafter. This rapid formation is consistent with the magnetic and microscopic analyses. The slightly reduced composition of magnetite is the same as that identified for several bacterial biogenic magnetite samples, synthesized both inter- (21) and extracellularly (22), with near identical stoichiometry as seen here (22).
Discussion
It is clear that the time scale of this reaction is far shorter than previously anticipated and is contrary to the Komeili et al. (18) study, which claims magnetosomes take ≤21 h to reach their full size. It is possible that the surface layer of α-Fe2O3 (hematite) found in this study could be the remains of a precursor phase used in the magnetite-formation process. Previous authors have highlighted the possibility of an Fe2O3 phase being present in magnetosomes (10, 11). However, it also could be the result of some enhanced surface oxidation that occurs because of the greater surface area in the immature magnetosomes. It would be surprising that such a pure phase should be obtained under such uncontrolled oxidation conditions. In any case, it is clear that this surface layer has completely disappeared between 15 and 30 min after induction. Thus, we can set an upper limit of 30 min for the lifetime of this α-Fe2O3 phase.
The discrepancy between the results presented here and those of Komeili for M. magneticum AMB1 is mainly because of the growth conditions used, rather than the difference in bacterial species. Study of magnetosome formation in the same bacterium, M. magneticum AMB1, by Suzuki et al. (19) showed that iron uptake was extremely rapid, with 60% of total uptake occurring within 10 min, with an intracellular concentration of iron >3,000 nmol/109 cells. By contrast, in the work by Komeili, the first rapid phase of iron uptake was only completed after 5 h. In both this and related work, including that of Komeili (17–19), magnetosome formation occurs concomitantly with iron uptake, and there is no evidence for a prior iron storage stage. This finding suggests that iron uptake is a limiting step in magnetosome biosynthesis. Rapid magnetosome formation appears correlated with respiratory growth under microaerobic conditions at low pO2; the slower magnetosome formation reported by Komeili was for bacteria grown anaerobically with nitrate as the terminal electron acceptor, and hence energy production would be lower. However, the secondary phase of maturation from small, immature magnetite crystals of 5–20 nm into perfect cubooctahedral particles, previously observed for both M. gryphiswaldense MSR-1 and M. magneticum AMB1 (17, 19), appears much shorter in our studies.
The findings presented here demand a rethinking of this biomineralization process with respect to time scale and mechanism. Thus, analysis of magnetosome material in the seconds or minutes that follow induction is needed, although the logistics of such a study are daunting.
Although fast precipitation of magnetite is not uncommon in chemical synthesis, previously suggested biomineralization mechanisms have proposed longer multistep synthesis owing to the well defined high quality of the crystals and a lack of structural defects synonymous with slower growing crystals. An earlier presumption that crystals grow bilaterally and sequentially from the center of the chain out was thought likely because it accounts for smaller crystals at the ends, as well as being a similar method to the biomineralization of Chitons teeth. However, this theory was recently disproved by Komeili et al. (18) and is reinforced in this study, where crystals are seen to grow simultaneously within a chain, and full long chains are seen rapidly. Crystals appear to be of a mature size and well defined morphology in the first sample, taken just 15 min after induction.
Chemical precipitation of highly crystalline particulate magnetite can be a spontaneous reaction (23). However, such chemical reactions are carried out at high temperatures, with ambient temperature reactions being slower and generally producing poor quality crystals (24). The results presented here suggest a similar rapid magnetite formation within magnetic bacteria, but without the use of high temperatures. It is well known that biological systems catalyze reactions at physiological temperatures that would require high temperatures for purely chemical conversion to overcome large activation barriers. The classical case is the conversion of nitrogen to ammonia by prokaryotic nitrogenase complexes (25). Although the role of all of the magnetosome-associated proteins and the exact formation process have not been fully identified, it is clear that magnetite formation is highly regulated by the organism with regard to iron transport, nucleation, crystal growth, morphology, and chain formation. We now suggest that there is a biologically catalyzed magnetosome synthesis that allows rapid precipitation of magnetite (normally seen at high temperature) at room temperature.
Methods
Fermenter Cultivation.
A nonmagnetic inoculum of M. gryphiswaldense, strain MSR-1 (DSMZ 6361), was prepared in screw-capped 10-ml volumes of a modified activated charcoal medium (26), with the omission of agar and iron. This medium contained (per liter of deionizer water) 2.38 g/liter Hepes, 3.00 g/liter sodium pyruvate, 0.10 g/liter yeast extract, 3.00 g/liter soybean peptone, 0.34 g/liter NaNO3, 0.10 g/liter KH2PO4, 0.15 g/liter MgSO4·7H2O, and 3.00 g/liter 20–40 mesh-activated charcoal. The medium was autoclaved after adjustment of the pH to 7.0. Then 1 mM sterile 1,4-DTT and 20 μM ferric-quinate were added aseptically after autoclaving. The cultures were grown overnight at 28°C at 150 rpm shaking. The fermenter inoculum of 1.5 liters of nonmagnetic cells was cultured at 28°C in a loose-capped bottle in a microaerobic cabinet (1% O2).
Fermenter growth was carried out in a 15-liter fermenter (Applikon) in the same medium as earlier, but with the omission of DTT, Hepes, activated charcoal, and any iron, at an O2 tension of 3–5% at 28°C, with medium and protocol followed as in Heyen et al. (27). Dissolved O2 tension was controlled by measuring the dissolved oxygen tension with an oxygen electrode (Oxyprobe) and use of a PID control loop on the AD1030 biocontroller. Once OD600 = 0.315 was reached, the O2 tension was reduced to 0.5–1% before adding ferric quinate to a final concentration of 150 μM. Thereafter, the set point for dO2 control was 0.5%. Sampling was performed at set time intervals of 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 7, 8, and 10 h after induction, as well as samples taken before induction and at the end of the experiment 24 h after induction.
Sampling and Analysis.
Five hundred-milliliter culture samples were harvested at set time intervals on ice and treated with 30 μg/ml chloramphenicol. Then 2 × 1-ml aliquots were used for optical and magnetic optical density reading and to prepare TEM grids. The bulk samples were centrifuged at 10,000 × g for 10 min at 4°C, and the cell pellets were resuspended in 7 ml of cell lysis buffer [50 mM Tris and 50 mM EDTA (pH 8.0)] and plunged into liquid N2. Supernatants were filtered, acidified, and then subjected to elemental analysis for iron (Optima 5300 DV ICP-OES; PerkinElmer). Magnetic optical density measurements were performed as described in ref. 20. The optical density of the samples was measured at 600 nm (Ultrospec III spectrophotometer; Amersham Pharmacia). A bar magnet (neodymium boron) was then placed next to the sample holder parallel and perpendicular to the sample to create magnetic fields parallel and perpendicular to the incident beam. The ratio of the two values gives Cmag = OD‖/OD⊥ − 1, providing a qualitative estimation of the magnetization of the samples (20, 28). Thus, Cmag = 0 for nonmagnetic cells and increases with increasing magnetization. TEM samples were washed with water three times, mounted onto a 300 mesh Cu Formvar/carbon-coated grid (Agar Scientific), and stained with uranyl acetate. TEM microscopy was performed on an FEI Tecnai T20 transmission electron microscope operated at 200 kV and equipped with an LaB6 filament (Department of Physics and Astronomy, University of Glasgow, Glasgow, Scotland).
Magnetosome extraction was modified from a method reported in ref. 29. Cells were lysed (Constant Systems Z Plus cell disrupter 20,000 psi), and magnetosomes were collected (magnetic separator rack; Invitrogen), washed [10 mM Hepes (pH 7.4), 20 mM NaCl, and then 10 mM Hepes], and purified by ultracentrifugation at 154,000 × g for 3 h (OTD55B with TH641 swinging bucket rotor; Sorvall) on a sucrose cushion [50% (wt/wt) sucrose in 10 mM Hepes]. All mineral fractions were recovered and washed in PBS. The lipid vesicle was removed at the Synchrotron Radiation Source (SRS; Daresbury, U.K.) in an oxygen-free cabinet sonicated in acetone. XAS measurements were performed on beamline 5U.1 at the SRS by using circularly polarized radiation. The samples were mounted and transferred to the beamline anaerobically and immediately loaded into the vacuum chamber. XAS measurements were obtained by using the total electron yield method of detection, and the XMCD was recorded by reversing the sample magnetization at each point in the scans by using an eight-pole vector magnet. To perform this process, a magnetic field of ±0.6 T was applied along the x-ray beam direction. The measured XMCD was fitted by using a nonlinear least squares method. The calculated XMCD curves for each Fe site that was utilized in the fitting were first convoluted by a Gaussian to account for experimental broadening. In all cases, the number of vacancies was assumed to be zero.
Acknowledgments
We thank Colin How for performing the TEM microscopy; Vicky Coker for valuable discussions and advice on the XMCD experiment and data; the staff at Daresbury Laboratory; Stephen Brotton for supplying data; and Pam Beattie for assistance and support. This work was supported by Engineering and Physical Sciences Research Council Grants EP/C53204X/1 and EP/D057310/1 and the Synchrotron Radiation Source.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Lang C, Schüler D. J Phys Condens Matter. 2006;18:S2815–S2828. [Google Scholar]
- 2.Knoll AH. Rev Mineral Geochem. 2003;54:329–356. [Google Scholar]
- 3.Matsunaga T, Okamura Y. Trends Microbiol. 2003;11:536–541. doi: 10.1016/j.tim.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Grunberg K, Muller E-C, Otto A, Reszka R, Linder D, Kube M, Reinhardt R, Schüler D. Appl Environ Microbiol. 2004;70:1040–1050. doi: 10.1128/AEM.70.2.1040-1050.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schueler D, Bäeuerlein E. In: Transition Metals in Microbial Metabolism. Winkelmann G, Carrano CJ, editors. Amsterdam: Harwood Academic; 1997. pp. 159–185. [Google Scholar]
- 6.Tanaka M, Okamura Y, Arakaki A, Tanaka T, Takeyama H, Matsunaga T. Proteomics. 2006;6:5234–5247. doi: 10.1002/pmic.200500887. [DOI] [PubMed] [Google Scholar]
- 7.Mann S, Webb J, Williams RJP. Biomineralization: Chemical and Biochemical Perspectives. New York: VCH Weinheim; 1989. [Google Scholar]
- 8.Mann S, Frankel RB, Blakemore RP. Nature. 1984;310:405–407. [Google Scholar]
- 9.Mann S, Sparks NHC, Blakemore RP. Proc R Soc London Ser B. 1987;231:477–487. [Google Scholar]
- 10.Frankel RB, Papaefthymiou GC, Blakemore RP, O'Brien W. Biochim Biophys Acta. 1983;763:147–159. [Google Scholar]
- 11.Frankel RB, Blakemore RP, Wolfe RS. Science. 1979;203:1355–1356. doi: 10.1126/science.203.4387.1355. [DOI] [PubMed] [Google Scholar]
- 12.Kirschvink JL, Lowenstam HA. Earth Planet Sci Lett. 1979;44:193–204. [Google Scholar]
- 13.Numako C. Kobutsugaku Zasshi. 1997;26:55–58. [Google Scholar]
- 14.Numako C, Nakai I, Ishii T, Takano B. Bunseki Kagaku. 1995;44:821–827. [Google Scholar]
- 15.Abe M, Ishihara T, Kitamoto Y. J Appl Phys. 1999;85:5705–5707. [Google Scholar]
- 16.Pearce CI, Henderson CMB, Pattrick RAD, van der Laan G, Vaughan DJ. Am Mineral. 2006;91:880–893. [Google Scholar]
- 17.Schüler D, Baeuerlein E. J Bacteriol. 1998;180:159–162. doi: 10.1128/jb.180.1.159-162.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komeili A, Vali H, Beveridge TJ, Newman DK. Proc Natl Acad Sci USA. 2004;101:3839–3844. doi: 10.1073/pnas.0400391101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki T, Okamura Y, Calugay RJ, Takeyama H, Matsunaga T. J Bacteriol. 2006;188:2275–2279. doi: 10.1128/JB.188.6.2275-2279.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schüler D, Uhl R, Baeuerlein E. FEMS Microbiol Lett. 1995;132:139–145. [Google Scholar]
- 21.Weiss BP, Kim SS, Kirschvink JL, Kopp RE, Sankaran M, Kobayashi A, Komeili A. Earth Planet Sci Lett. 2004;224:73–89. [Google Scholar]
- 22.Coker VS, Gault AG, Pearce CI, van der Laan G, Telling ND, Charnock JM, Polya DA, Lloyd JR. Environ Sci Tech. 2006;40:7745–7750. doi: 10.1021/es060990+. [DOI] [PubMed] [Google Scholar]
- 23.Sidhu PS, Gilkes RJ, Posner AM. J Inorg Nucl Chem. 1978;40:429–435. [Google Scholar]
- 24.Cornell RM, Schertmann U. The Iron Oxides: Structure, Properties, Reactions, Occurrence and Uses. New York: VCH; 1996. [Google Scholar]
- 25.Postgate JR. Nitrogen Fixation. Cambridge, UK: Cambridge Univ Press; 1998. [Google Scholar]
- 26.Schultheiss D, Schüler D. Arch Microbiol. 2003;179:89–94. doi: 10.1007/s00203-002-0498-z. [DOI] [PubMed] [Google Scholar]
- 27.Heyen U, Schüler D. Appl Microbiol Biotechnol. 2003;61:536–544. doi: 10.1007/s00253-002-1219-x. [DOI] [PubMed] [Google Scholar]
- 28.Schuler D, Baeuerlein E. In: Transition Metals in Microbial Metabolism. Winkelmann G, Carrano CJ, editors. London: Harwood; 1997. pp. 159–185. [Google Scholar]
- 29.Grunberg K, Wawer C, Tebo BM, Schüler D. Appl Environ Microbiol. 2001;67:4573–4582. doi: 10.1128/AEM.67.10.4573-4582.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brotton SJ, Shapiro R, van der Laan G, Guo J, Glans PA, Ajello JM. J Geophys Res. 2007;112 G03004. [Google Scholar]