Abstract
We explored the impact of task context on subliminal neural priming using functional magnetic resonance imaging. The repetition of words during semantic categorization produced activation reduction in the left middle temporal gyrus previously associated with semantic-level representation and dorsal premotor cortex. By contrast, reading aloud produced repetition enhancement in the left inferior parietal lobe associated with print-to-sound conversion and ventral premotor cortex. Analyses of effective connectivity revealed that the task set for reading generated reciprocal excitatory connections between the left inferior parietal and superior temporal regions, reflecting the audiovisual integration required for vocalization, whereas categorization did not produce such backward projection to posterior regions. Thus, masked repetition priming involves two distinct components in the task-specific neural streams, one in the parietotemporal cortex for task-specific word processing and the other in the premotor cortex for behavioral response preparation. The top-down influence of task sets further changes the directions of the unconscious priming in the entire cerebral circuitry for reading.
Keywords: effective connectivity, masked priming, reading, repetition suppression and enhancement, task set
Unconscious exposure to written words facilitates the subsequent conscious processing of the same stimuli, a behavioral phenomenon known as subliminal priming (1). At the neural level, such response facilitation is mediated in part by the visual word recognition system in the left occipitotemporal cortex. By the use of functional magnetic resonance imaging (fMRI), the hierarchical architecture of this region has been demonstrated as a serial, posterior-to-anterior processing stream for increasingly abstract representations (2–4). For instance, a shape-invariant neural code for letter strings has been found at the left middle fusiform gyrus (FG) showing response attenuation to the repeated presentation of words and word roots, whereas a more anterolateral sector of the posterior temporal cortex exhibits sensitivity to semantic-level representations (5). For nonalphabetic languages, the repetition of logographic Kanji elicits a similar neural adaptation at the left middle temporal gyrus (MTG) associated with semantic knowledge (6).
Importantly, most previous work measured the unconscious neural priming under a single word-recognition task tapping lexicosemantic representation (5–7). Thus, it remains open whether, and to what extent, the adoption of a voluntary task set modulates subliminal priming in the cerebral reading network. In fact, such an influence of intentional behavior on nonconscious cognitive processing has been proposed by neurocognitive models of consciousness (8, 9). Our transcranial magnetic stimulation study has further demonstrated a differential effect of task on subliminal priming (10). However, no previous work has examined the entire extent of the cerebral structures involved in unconscious word processing as a function of task context.
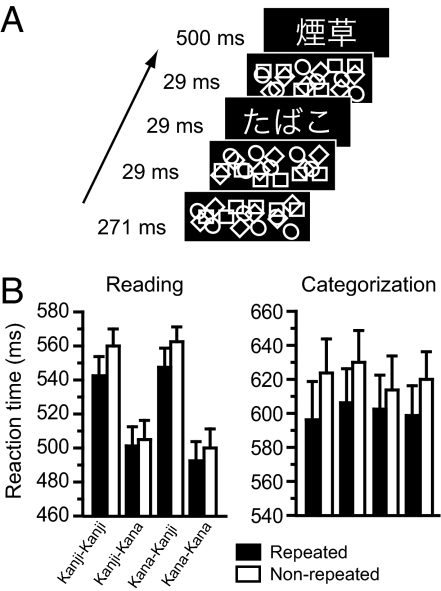
In the present work, we explored how subliminal neural priming changed under two different task instructions, reading aloud and semantic categorization, by using a masked priming method with fMRI. Our behavioral paradigm consisted of a timed sequence of a subliminal prime and a visible target, each written in either logographic Kanji or syllabic Kana (Fig. 1A). For Japanese readers, unconscious priming occurs even when the same word is repeated across Kanji and Kana formats, such as “ ” and “
” and “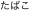 ” (6). Because these logographic and syllabic scripts have no mutual correspondence at the subword level, this “cross-script priming” paradigm targets the cognitive locus of across-script convergence between the two writing systems, allowing us to isolate the neural codes associated with higher-order, abstract-level representation beyond orthography. Crucially, the pattern of the neural priming may change with the task set engaged by participants, because the behavioral response facilitation involves different cognitive components according to the task requirements. That is, masked repetition priming during reading aloud has been attributed to either the orthography-to-phonology computation (11) or further downstream to the motor-articulatory codes (12). By contrast, repetition priming during categorization reflects the unconscious activation of lexicosemantic knowledge (6), and may also involve a motor representations of the manual response (4). We further examined the influence of cognitive sets on the cerebral language network by computing the effective connectivity between the brain structures showing task-specific priming effects.
” (6). Because these logographic and syllabic scripts have no mutual correspondence at the subword level, this “cross-script priming” paradigm targets the cognitive locus of across-script convergence between the two writing systems, allowing us to isolate the neural codes associated with higher-order, abstract-level representation beyond orthography. Crucially, the pattern of the neural priming may change with the task set engaged by participants, because the behavioral response facilitation involves different cognitive components according to the task requirements. That is, masked repetition priming during reading aloud has been attributed to either the orthography-to-phonology computation (11) or further downstream to the motor-articulatory codes (12). By contrast, repetition priming during categorization reflects the unconscious activation of lexicosemantic knowledge (6), and may also involve a motor representations of the manual response (4). We further examined the influence of cognitive sets on the cerebral language network by computing the effective connectivity between the brain structures showing task-specific priming effects.
Fig. 1.
Experimental paradigm and behavioral results. (A) Sequence of events used for the behavioral tasks. Participants either read aloud visible targets or categorized them as representing natural or artificial objects. (B) Mean reaction times (±SEM) during reading and categorization.
Results
Behavioral results.
Mean accuracy was 96.9% for reading aloud and 94.8% for semantic categorization. The prime visibility did not depart from the chance level [51.2% correct, t (15) = 0.56, P > 0.5 for reading; 52.3% correct, t (15) = 0.60, P > 0.5 for categorization], suggesting that participants were unable to see the prime words consciously under both tasks.
The behavioral priming effect for each experiment is illustrated in Fig. 1B. For reading aloud, the global effect of priming (i.e., collapsed across the four types of script change) was highly significant [F (1, 15) = 44.12, P < 0.001]. Participants responded 25 ms faster to Kana-targets than to Kanji-targets [F (1, 15) = 409.51, P < 0.001]. These effects interacted with each other [F (1, 15) = 18.34, P < 0.001]. There was a significant interaction between prime script and target script [F (1, 15) = 12.25, P < 0.005]. No other main effects and interactions were significant. For categorization, the main effect of priming was also significant [F (1, 15) = 44.83, P < 0.001]. Neither prime script nor target script affected the participants' performance [F (1, 15) = 3.37, P > 0.05; F (1, 15) = 1.45, P > 0.2]. No other interactions reached significance.
Planned comparisons confirmed that the repetition of words accelerated the participants' response systematically in each of the four priming conditions, both for reading [F (1, 15) = 38.36, P < 0.001; F (1, 15) = 6.58, P < 0.05; F (1, 15) = 26.67, P < 0.001; F (1, 15) = 5.74, P < 0.05, for the Kanji–Kanji, Kanji–Kana, Kana–Kanji, Kana–Kana trials, respectively] and for categorization [F (1, 15) = 24.54, P < 0.001; F (1, 15) = 37.33, P < 0.001; F (1, 15) = 7.78, P < 0.05; F (1, 15) = 14.54, P < 0.005, each respectively].
Between-task comparison revealed that participants responded 85 ms faster in reading than in categorization [F (1, 30) = 15.05, P < 0.001]. The magnitude of priming was 11 ms greater for categorization relative to reading [F (1, 30) = 8.67, P < 0.01], suggesting that the task context affects the subliminal priming during word recognition. This task-by-priming interaction changed with target script [triple-interaction; F (1, 30) = 9.71, P < 0.01] but not with prime script [F (1, 30) = 2.63, P > 0.1], suggesting greater saving effects for Kana targets during categorization and for Kanji targets during reading. No other interactions with task were significant.
Imaging Results
Repetition Priming Effects.
Both reading aloud and semantic categorization produced widespread activation of the bilateral frontoparietotemporal regions. We searched for the brain regions showing script-specific activation irrespective of the task requirements. In the cerebral network for reading, Kanji targets activated a medial part of the bilateral FG (x = −30, y = −51, z = −18, Z = 3.68, and x = 42, y = −67, z = −12, Z = 3.80) relative to Kana targets [supporting information (SI) Fig. 6]. Conversely, Kana targets activated the bilateral occipital pole (x = −14, y = −97, z = −10, Z = 5.12, and x = 20, y = −97, z = −4, Z = 3.71) relative to Kanji targets. No other region exhibited significant script-specific activation across the two tasks.
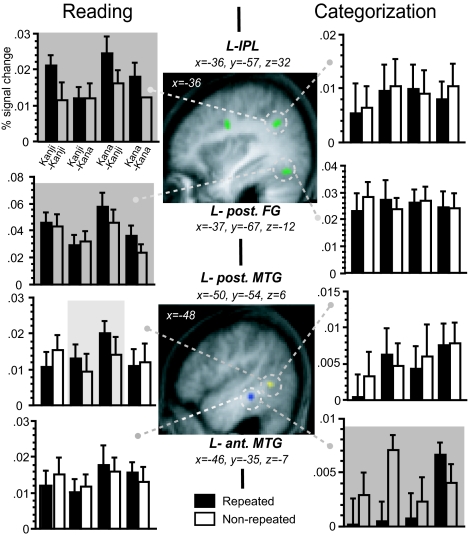
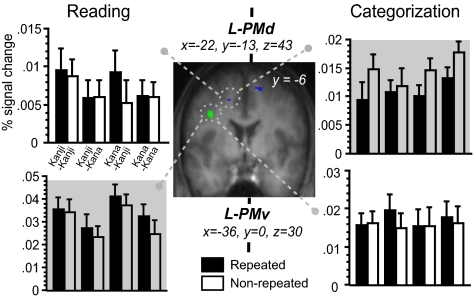
We then examined two forms of neural priming, i.e., repetition suppression and enhancement (13), by searching for the cerebral regions showing the repetition-induced decrease and increase of activation. For reading aloud, the global priming effect was detected as repetition enhancement in the left ventral premotor cortex (PMv), inferior parietal lobe (IPL), and posterior FG (Figs. 2 and 3). Note that the script-dependent, asymmetric pattern of priming at this fusiform cluster came mainly from primes in Kana. Only the left lateral prefrontal cortex and posterior MTG exhibited response enhancement in cross-script trials (i.e., when primes and targets were presented in different scripts). However, no significant repetition suppression was found throughout the entire brain volume.
Fig. 2.
Neural priming in the left parietotemporal cortex. Global priming effects (green blobs) were significant in the left IPL and posterior FG for reading and in the left MTG for categorization (shadowed in dark gray). The left posterior MTG (blue blob) exhibited cross-script repetition enhancement during reading (in light gray). Bars represent the mean percent signal change (±SEM) for each condition relative to the baseline.
Fig. 3.
Neural priming in the premotor cortex. The left PMv (green) exhibited repetition enhancement during reading, whereas the PMd (blue) showed repetition suppression during categorization across the four priming conditions.
For semantic categorization, the main effect of repetition suppression was significant only in the left anterior MTG (see Fig. 2). This region exhibited reduced response in cross-script trials but not in within-script trials. When the search was extended to the brain regions involved in hand response (masked at P < 0.05 for categorization vs. baseline), the main effect of repetition suppression was detected in the bilateral dorsal premotor cortex (PMd; x = −22, y = −13, z = 43, = 3.03, and x = 16, y = 5, z = 53, Z = 2.88). Note that the left PMv, which showed repetition enhancement during reading aloud, now exhibited repetition suppression under categorization for both the within-script (8 voxels, x = −24, y = −12, z = 41, Z = 2.96) and cross-script conditions. For each task, other regions showing priming effects are presented in SI Table 1.
The task-dependent change of priming directions was also found in the left IFG, which showed repetition enhancement during reading but exhibited a nonsignificant trend of repetition suppression during categorization (Z = 2.70). The categorization task produced no significant repetition enhancement in the entire search volume, neither at the global nor at the within-script levels. Only the cross-script repetition produced response enhancement in the left precentral area (x = −61, y = −14, z = 34, Z = 3.90), bilateral basal ganglia (x = 24, y = 15, z = 10, Z = 3.89), and cerebellum (x = −8, y = −67, z = −13, Z = 3.36).
In the joint analysis of reading and categorization, the global repetition enhancement across the two tasks was significant at the left frontoparietal junction and bilateral posterior parietal area and cerebellum. In this neural circuit, the repetition effect interacted with task at the left IPL (x = −38, y = −57, z = 32, Z = 3.48) and precuneus (x = −8, y = −78, z = 43, Z = 3.47). A weaker trend of between-task difference was found for the left PMv (x = −34, y = −2, z = 31, Z = 2.94). In contrast, the global repetition suppression across the two tasks was detected only as a nonsignificant trend in the bilateral medial frontal and posterior temporal cortices and deep nuclei. However, the priming-by-task interaction (corrected at P < 0.05 across the search volume) was significant in the left medial superior temporal gyrus (STG; x = −36, y = −30, z = 25), caudate (x = −26, y = 27, z = 8), MTG (x = −46, y = −35, z = −7), and PMd (x = −20, y = −13, z = 43).
Effective Connectivity Analysis.
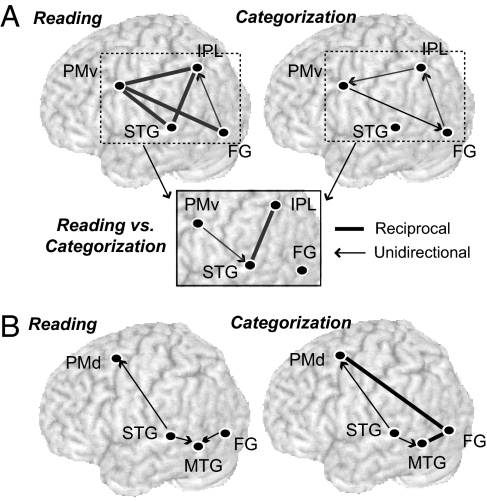
We examined the interregional connectivity between the left premotor and parietotemporal regions showing global priming effects during each task. We focused on the influence of cognitive set over the two task-dependent cerebral networks for reading, each of which was assumed to receive the visual input commonly at the FG during the execution of tasks. Specifically, we constructed a dynamic causal model (DCM), which comprises the IPL and PMv for the “dorsal” stream involved in reading aloud (Fig. 4). For the “ventral” stream involved in categorization, we created another DCM comprising the MTG and PMv. Based on the known role of the left temporal lobe in word comprehension, we additionally included the left STG in both DCMs (see Methods). The stimulus-free contextual effects were then estimated as the latent or intrinsic interregional coupling under a given task (14).
Fig. 4.
Contextual effects on the task-specific neural pathways. (A) The influence of task set over the neural circuitry involved in reading aloud. Spoken word production relies on distributed forward and backward connections in the left hemisphere, whereas categorization partially recruits the same network (Upper). The task-context exerts a differential impact at the reciprocal STG-IPL connections and the PMv-STG projection (Lower). (B) The influence of task set over the neural circuitry involved in categorization. The left FG was involved in reciprocal couplings with PMd and MTG during categorization. These two structures received excitatory input from STG during both reading and categorization. However, none of these connections differed in coupling strength between the two tasks.
Mean intrinsic connection parameters for each task are presented in SI Table 2. For the dorsal network, there were significant excitatory inputs from STG to PMv and IPL during reading aloud, whereas these connections were not significant under categorization (Fig. 4A). No other within-task connections exceeded zero, neither for reading nor for categorization (all ts < 2.1). Between-task comparisons confirmed that the task set for reading increased the reciprocal intrinsic coupling between STG and IPL relative to the task set for categorization (t = 2.98, P = 0.006 for STG-IPL; t = 3.02, P = 0.005 for IPL-STG). The between-task difference was marginally significant for the PMv-STG projection (t = 1.95, P = 0.06). No other intrinsic connections differed between the two tasks (all ts < 1.3).
For the ventral stream, there were significant excitatory projections from STG to PMd and MTG during categorization (Fig. 4B). However, these connections were also significant during reading aloud and did not differ between the two tasks (both ts < 1). The categorization task produced reciprocal excitatory inputs from FG to MTG and PMd, but neither of these connections differed from those under reading aloud (all ts < 1.2). The intrinsic coupling from PMd to MTG was marginally significant during categorization, but did not survive the between-task comparison (t < 0.1). No other intrinsic connections differed between the two tasks (all ts < 1).
For each task, we further estimated the modulatory effects of repetition (across the four priming conditions) on the task-relevant regions. The repetition of words induced an increase of connectivity from PMv to IPL for reading aloud [mean connection strength: +0.021/−0.007 for repeated/unrepeated trials, respectively; F (1, 15) = 4.45, P = 0.05] but not for categorization [+0.0006/+0.0007; F (1, 15) < 0.1]. In between-task comparisons, this modulatory influence interacted with task set at a trend level [F (1, 30) = 2.80, P = 0.10]. For categorization, the repetition of words produced a marginally significant change of connectivity in two forward projections, i.e., increase at the FG-PMd [+0.0040/−0.0015; F (1, 15) = 3.73, P = 0.07] and decrease at the FG-STG [−0.0004/+0.0002; F (1, 15) = 3.71, P = 0.07] connections, but it was not the case for reading aloud, neither for FG-PMd [+0.0038/−0.0018; F (1, 15) = 3.08, P = 0.1] nor for FG-STG [+0.0002/−0.0007; F (1, 15) = 0.36]. The modulatory effect at the FG-PMd projection was significant across two tasks [F (1, 30) = 6.73, P < 0.02] but did not differ between the two tasks [F (1, 30) <1]. No other projections exhibited significant modulatory effects, neither for within-task nor for across-task analyses.
Discussion
We performed the two subliminal priming experiments to investigate the influence of task instructions over the neural network involved in unconscious word processing. Behaviorally, significant priming effect was obtained across the four script conditions, both for reading aloud and semantic categorization. These two types of behavioral priming each should have at least two distinct components, one from linguistic and the other from motoric levels (4). For semantic categorization, motor-response priming could occur because the unrepeated trials consisted of primes and targets that belonged to opposite categories (e.g., prime natural/target artifact). Thus, the unconscious processing of primes would lead to covert preparation of an appropriate motor response, which needs to be overcome by the overt response on targets. Indeed, we previously showed that such response bias contributes to the generation of behavioral priming under the same design (6). The difference in motoric congruity between repeated and unrepeated trials would yield a similar response bias in the reading-aloud task involving an articulatory output. At the neural level, such motor priming has been associated with downstream regions beyond the classical language regions, including the primary motor cortex (15), premotor area (16) and basal ganglia (17).
Task-Specific Neural Connections in Unconscious Priming.
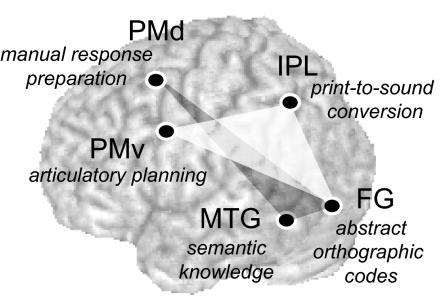
We found two task-specific neural connections operating for unconscious word processing. Namely, the main effect of priming was found as repetition suppression at the MTG-PMd during categorization but was detected as repetition enhancement at the IPL-PMv and posterior FG during reading aloud (Fig. 5). These findings overall suggest that the task instructions engaged by participants induce a biasing influence over the task-relevant regions even for unconsciously perceived stimuli.
Fig. 5.
Neurocognitive components of unconscious repetition priming. The left FG receiving the visual input is associated with abstract, shape-invariant representations of pronounceable letter-strings (3). The ventral stream from the FG, comprising the IPL for print-to-sound translation and PMv for speech planning (dark gray), operates in the generation of subliminal priming during reading aloud. In contrast, the dorsal stream from the left FG, comprising the MTG for semantic representations and PMd for manual response preparation (light gray), is involved in repetition priming during categorization.
For categorization, the observed activation reduction in the PMd is in accord with a recent fMRI study showing that stimulus–response congruency induces response attenuation in the same area (18). This region is involved in the learning of arbitrary associations between visual cues and hand response, in both primates (19) and humans (20). Hence, the observed priming in PMd can be attributed to the accumulated response bias for generating the manual response required for the categorization task.
Consistent with our previous work (6), the left MTG exhibited global repetition suppression during categorization. Neuropsychological (21, 22) and functional brain imaging (23, 24) studies converge to suggest that this region is associated with amodal, conceptual-level representations. Our finding thus suggests the existence of another distinct component of cross-script priming that is mediated by the activation of semantic knowledge.
The motor priming during reading aloud was mirrored by response enhancement in the PMv previously associated with articulatory planning (25), supporting the proposal that the masked priming in word naming occurs in the speech production process (12). However, another neural locus of this behavioral priming was found at the left IPL, a region known to play a role in translating written words into spoken forms (26). Indeed, our transcranial magnetic stimulation study (10) has shown that this dorsal pathway from FG to IPL is involved in masked priming during reading aloud, for both words and nonwords. These results thus point to the existence of two separate neurocognitive components of the dorsal processing stream for reading, one in the IPL associated with print-to-sound conversion and the other in the PMv associated with speech planning.
Shifting of Neural Priming Directions.
Repetition priming is thought to reflect a neural adaptation mechanism operating for visual object recognition and mediating more efficient processing of stimuli (13). Combined with psychological priming, the repetition-suppression phenomenon gives access to the neural coding mechanisms associated with a specific cognitive function beyond the limits of conventional brain-imaging paradigms (27). However, the opposite pattern of neural priming, i.e., repetition enhancement, may occur depending on the psychophysiological features of stimuli, such as familiarity (28), perceptual difficulty (29), and prime-target semantic relationship (24). It is an unresolved question as to how the directions of neural priming changes with other experimental factors.
We found that unconscious word perception elicits response enhancement in the task-relevant processing stream only under the reading task. Our results also revealed that reading, but not categorization, relies on excitatory connections within the STG-IPL-PMv stream in the left hemisphere. Moreover, the repetition of written words and their spoken output during reading produced a modulatory backward projection between these structures, whereas unconsciously perceived words during categorization primed only the bottom-up projections from FG to the downstream regions. These findings suggest that the direction of priming changes with additional inputs from other components of the neural circuitry involved in the same task.
In fact, spoken word production may require such reciprocal interactions in the task-relevant network, because the auditory perception of speech sounds is known to activate the PMv automatically (30). Another recent study has further shown a functional coupling between auditory and motor areas during vocalization (31). The premotor and inferior parietal cortices, also involved in speech production, exhibit increased connectivity during speech perception (32). These observations have been taken as reflecting the self-monitoring and feedback regulation whereby speech perception engages the motor system to access a phonetic code with motoric properties. Thus, the STG-IPL-PMv circuitry might be recruited twice, i.e., first by the visual prime-target pairs and then by the auditory monitoring of one's own voice. The observed shift in neural priming would then occur because these additional cognitive processes operate on the target stimuli, resulting in repetition enhancement, rather than suppression (13).
Alternatively, the backward projections during reading may be attributed to an attentional modification of the task-relevant network, because repetition enhancement has been observed under conditions that may increase attentional demands (e.g., visually unfamiliar or degraded stimuli). Indeed, a recent behavioral study has proposed that reading aloud is not fully automatic but may require central attention to maintain the functional architecture of the reading system (33). This account is supported by neurophysiological evidence showing that the repetition of visual stimuli produces enhanced response in the inferotemporal cortex under a top-down attentional modulation by the prefrontal cortex (34). Raposo et al. (24) reported that the semantic overlap between prime and target produced enhanced response during concrete-abstract judgment about consciously perceived words. This observation may also reflect the strategic allocation of attention, because the task demand could interact with the semantic relatedness of stimuli [see Neely (35) for a review].
It is interesting to note that such temporal shifting of fMRI priming after the backward or top-down modification may correspond to the reversal of priming directions observed in magnetoencephalography studies (36, 37). Namely, the left occipitotemporal cortex exhibits increased response to the repeated presentation of words at ≈230 ms after the stimulus onset. By contrast, a distributed left hemisphere network, extending from the parietotemporal to lateral prefrontal regions, exhibits attenuated response in a later period (≈400 ms), whereas this late neural priming is more pronounced in the frontal and anterior temporal regions.
Conclusion
Our results suggest that unconscious word perception primes the entire task-relevant neural circuit for processing visible targets, setting up more efficient functional coupling between the left parietotemporal regions associated with word processing and premotor regions associated with behavioral response. The voluntary task control guides even invisible words to the task-relevant processing streams in the left hemisphere, whereas the top-down influence of task sets affects the neural priming direction within the task-relevant language network.
Methods
Participants.
Two groups of sixteen right-handed, native Japanese speakers (age range 25–38 years) were recruited separately for the reading aloud and semantic categorization experiments. The behavioral and brain imaging results for categorization were reported previously (6). All participants gave written informed consent before the imaging experiment. The protocol of this study was approved by the regional ethical committee.
Materials and Procedures.
The visual stimuli consisted of 40 Japanese nouns written commonly both in Kanji and Kana. These words were composed of 1–2 characters when written in Kanji and 2–3 characters when written in Kana. Half of them represented natural objects and the other half artifacts. Each trial consisted of a precisely timed sequence of a masked prime and a visible target (Fig. 1A). In the reading-aloud task, participants named the visible targets as quickly as possible, whereas in the categorization task, they responded by key-press to classify the targets as natural or artificial as quickly and as accurately as possible.
Each fMRI experiment was arranged in a 2 × 2 × 2 factorial design in which the main effects of interest were repetition (identity or control), prime script (Kanji or Kana) and target script (Kanji or Kana). A ninth type of trials comprising the same sequence of masks without prime and target words served as a baseline to measure the event-related activation by words. In each experiment, participants received four scanning sessions, each comprising five initial training trials followed by 200 trials (20 trials for each event type except for the “word-absent” baseline with 40 trials, all in random order).
Immediately after the imaging sessions, participants performed a forced-choice test for prime visibility (64 trials). Each trial comprised the same sequence of masks and words as in the activation task, followed by a pair of choices (a same word as the prime and a distractor) presented left and right of the fixation. Participants determined which of the two items corresponded to the prime word in the preceding event sequence.
fMRI Procedures.
Both experiments were conducted by using a 3-Tesla whole-body system (Bruker) with a standard head coil optimized for a gradient echo-echo planar imaging sequence (26 contiguous axial slices, thickness 4.5 mm with 0.5 mm gap, echo time = 40 ms, flip angle = 90°, field-of-view = 192 × 256 mm2, 64 × 64 pixels). The repetition time was 2,400 ms for both tasks but included a 1,000-ms silence period for reading aloud (thus acquisition time was 2,400 ms for categorization and 1,400 ms for reading). Participants received four scanning sessions each of which lasted ≈8 min, giving 207 volumes for reading and 205 volumes for categorization.
Data Analysis.
After image reconstruction, the functional images were processed by using the SPM2 software (Wellcome Department of Cognitive Neurology, London, U.K.). Five initial images were discarded to eliminate nonequilibrium effects of magnetization. Images were corrected for head motion, resampled every 2 mm by using trilinear interpolation and normalized to the standard brain space, and spatially smoothed with an isotropic Gaussian filter (5 mm full-width at half-maximum). These images were high-pass filtered at 120 s and smoothed with a 4-s Gaussian kernel.
For each participant, a weighted-mean image for each contrast was computed by fitting each voxel time series with the known time series of the nine event types convolved with a canonical hemodynamic response function with time and dispersion derivatives. Group-based statistical inference was made by using random-effect analysis. We first identified the brain regions active during reading aloud by pooling the eight word-present conditions relative to the baseline (voxel-level P < 0.05). The resulting SPM served as a masking image for searching the priming effects in the cerebral network involved in reading. For each task, mean percent signal change was calculated for the eight word-present conditions by using the most significant voxel for each participant.
The effects of repetition suppression and enhancement each were calculated as the decrease and increase of activation in repeated trials relative to nonrepeated trials (13). We examined the neural priming effects associated with the “within-script priming” (including prime–Kanji/target–Kanji and prime–Kana/target–Kana trials) and those associated with “cross-script priming” (including Kanji–Kana and Kana–Kanji trials) corresponding to more abstract, script-independent representations beyond orthographic processing. For each task, global priming effects were computed by collapsing the four priming conditions.
The neural priming effects were compared between reading and categorization by entering the two sets of 16 individual contrast images into one-way ANOVA, treating the task as a between-group factor. We examined the priming-by-task interaction by selecting the voxels showing either global repetition suppression or enhancement across the two tasks (inclusive masking at P < 0.05). Unless stated otherwise, all effects were tested at voxel-level P < 0.005 (spatial extent > 15 contiguous voxels).
We used dynamic causal modeling to estimate the effective connectivity between the brain regions involved in task-specific priming effects. In brief, DCM treats the brain as a nonlinear dynamic system that is subject to inputs and produces outputs. By using a bilinear approximation to the dynamics of interactions among states, the parameters of the implicit causal model reduces to three sets, i.e., (i) parameters of the extrinsic input to the system, (ii) parameters representing the intrinsic or latent connections that couple responses in one area to the state of others, and (iii) the bilinear parameters representing the changes in the intrinsic coupling induced by inputs (14). The connectivity parameters are estimated in the Bayesian framework whereby inferences about particular connections are made by using the posterior or conditional density. Based on the group results from random-effect analyses, we constructed two different DCMs with four interconnected regions, each of which was seeded with the left FG receiving the visual input. One is the cerebral network including the left PMv and IPL that exhibited neural priming during reading aloud(see SI Table 1 for their respective coordinates). The other was involved in the repetition priming during categorization and comprised the left PMd and MTG. The two fully interconnected models were used based on the known anatomical connections in the frontoparietotemporal cortex (38) and previous functional brain imaging data (39). The left FG was identified as a common activation site across reading and categorization by using conjunction analysis (x = −42, y = −71, z = −12, Z = 5.07, relative to the baseline). Both models also included the left posterior STG that survived the voxel-level threshold at P < 10−6 during reading aloud relative to the baseline (x = −63, y = −19, z = 5, Z = 5.19). This region, previously associated with spoken-word comprehension (26), was active during categorization relative to the baseline (Z = 2.41) but responded more strongly during reading relative to categorization (Z = 4.21).
For each model, we computed the connectivity parameters representing the intrinsic strength of interregional connections during each task. Regional responses per session per participant were extracted by calculating the principal eigenvariate across all voxels within a 6-mm sphere centered at the most significant voxel in the group analyses for each task. For each model, we then computed the contextual effects on the interregional connectivity per session per participant. The design matrix included the eight word-present conditions as direct input to the FG. Because our main interest was the impact of task set on the neural circuits for reading, the intrinsic-connection parameters from four sessions were collapsed per participant and submitted to a second-level analysis by using the classical t-statistics. For each of the interregional coupling, we performed a one-sample t test to examine whether the across-participant mean differed from zero for each task. A lenient statistical threshold (P < 0.05, two-tailed) was used to determine the significant neural connections during each task. We selected the connections that reached the significance in either of reading and categorization and then compared the coupling strength between the two tasks using unpaired t test. All across-task comparisons of connection parameters were made for each DCM, and not between different models. The modulatory effects of repetition were further examined within and across tasks by submitting the bilinear coupling parameters to repeated-measures ANOVA treating the main effect of repetition as a within-participant factor and the task set as an additional between-group factor (the latter only for between-task comparisons).
Supplementary Material
Acknowledgments
This work was supported by Institut National de la Santé et de la Recherche Médicale, Commissariat à l'Énergie Atomique, a centennial fellowship of the McDonnell foundation (to S.D.), a postdoctoral fellowship from the Fondation de la Recherche Médicale, and Grants-in-Aid from the Japan Ministry of Education, Culture, Sports, Science and Technology (15700252) and the Japan Society for the Promotion of Science (19500264) (to K.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704487104/DC1.
References
- 1.Forster KI, Mohan K, Hector J. In: Masked Priming: State of the Art. Kinoshita K, Lupker SJ, editors. Hove, UK: Psychology Press; 2003. pp. 3–37. [Google Scholar]
- 2.Dehaene S, Cohen L, Sigman M, Vinckier F. Trends Cognit Sci. 2005;9:335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, Cohen L. Neuron. 2007;55:143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 4.Dehaene S. In: Functional Neuroimaging of Visual Cognition (Attention and Performance Series, 20) Kanwisher N, Duncan J, editors. New York: Oxford Univ Press; 2003. [Google Scholar]
- 5.Devlin JT, Jamison HL, Matthews PM, Gonnerman LM. Proc Natl Acad Sci USA. 2004;101:14984–14988. doi: 10.1073/pnas.0403766101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura K, Dehaene S, Jobert A, Le Bihan D, Kouider S. J Cognit Neurosci. 2005;17:954–968. doi: 10.1162/0898929054021166. [DOI] [PubMed] [Google Scholar]
- 7.Dehaene S, Naccache L, Cohen L, Bihan DL, Mangin JF, Poline JB, Riviere D. Nat Neurosci. 2001;4:752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- 8.Dehaene S, Naccache L. Cognition. 2001;79:1–37. doi: 10.1016/s0010-0277(00)00123-2. [DOI] [PubMed] [Google Scholar]
- 9.Kouider S, Dehaene S. Philos Trans R Soc London Ser B. 2007;362:857–875. doi: 10.1098/rstb.2007.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura K, Hara N, Kouider S, Takayama Y, Hanajima R, Sakai K, Ugawa Y. Neuron. 2006;52:557–564. doi: 10.1016/j.neuron.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 11.Forster KI, Davis C. J Mem Lang. 1991;30:1–25. [Google Scholar]
- 12.Grainger J, Ferrand L. J Mem Lang. 1996;35:623–647. [Google Scholar]
- 13.Henson RN. Prog Neurobiol. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 14.Friston KJ, Harrison L, Penny W. NeuroImage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- 15.Dehaene S, Naccache L, Le Clec HG, Koechlin E, Mueller M, Dehaene-Lambertz G, van de Moortele PF, Le Bihan D. Nature. 1998;395:597–600. doi: 10.1038/26967. [DOI] [PubMed] [Google Scholar]
- 16.Wolbers T, Schoell ED, Verleger R, Kraft S, McNamara A, Jaskowski P, Buchel C. Cereb Cortex. 2006;16:857–864. doi: 10.1093/cercor/bhj029. [DOI] [PubMed] [Google Scholar]
- 17.Aron AR, Schlaghecken F, Fletcher PC, Bullmore ET, Eimer M, Barker R, Sahakian BJ, Robbins TW. Brain. 2003;126:713–723. doi: 10.1093/brain/awg067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanley J, Miall RC. NeuroImage. 2006;34:290–299. doi: 10.1016/j.neuroimage.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 19.Kurata K, Wise SP. Exp Brain Res. 1988;69:327–343. doi: 10.1007/BF00247578. [DOI] [PubMed] [Google Scholar]
- 20.Chouinard PA, Leonard G, Paus T. J Neurosci. 2005;25:2277–2284. doi: 10.1523/JNEUROSCI.4649-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chertkow H, Bub D, Deaudon C, Whitehead V. Brain Lang. 1997;58:203–232. doi: 10.1006/brln.1997.1771. [DOI] [PubMed] [Google Scholar]
- 22.Vandenbulcke M, Peeters R, Dupont P, Van Hecke P, Vandenberghe R. Cereb Cortex. 2007;17:542–551. doi: 10.1093/cercor/bhj179. [DOI] [PubMed] [Google Scholar]
- 23.Tyler LK, Bright P, Dick E, Tavares P, Pilgrim L, Fletcher P, Greer M, Moss H. Cognit Neuropsychol. 2003;20:541–559. doi: 10.1080/02643290244000211. [DOI] [PubMed] [Google Scholar]
- 24.Raposo A, Moss HE, Stamatakis EA, Tyler LK. Neuropsychologia. 2006;44:2284–2295. doi: 10.1016/j.neuropsychologia.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 25.Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, Hauser MF, Witte RJ, Boeve BF, Knopman DS, et al. Brain. 2006;129:1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price CJ. J Anat. 2000;197(Pt 3):335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naccache L, Dehaene S. Cereb Cortex. 2001;11:966–974. doi: 10.1093/cercor/11.10.966. [DOI] [PubMed] [Google Scholar]
- 28.Henson RN, Shallice T, Dolan R. Science. 2000;287:1269–1272. doi: 10.1126/science.287.5456.1269. [DOI] [PubMed] [Google Scholar]
- 29.Turk-Browne NB, Yi DJ, Leber AB, Chun MM. Cereb Cortex. 2006 doi: 10.1093/cercor/bhj159. [DOI] [PubMed] [Google Scholar]
- 30.Wilson SM, Saygin AP, Sereno MI, Iacoboni M. Nat Neurosci. 2004;7:701–702. doi: 10.1038/nn1263. [DOI] [PubMed] [Google Scholar]
- 31.Schulz GM, Varga M, Jeffires K, Ludlow CL, Braun AR. Cereb Cortex. 2005;15:1835–1847. doi: 10.1093/cercor/bhi061. [DOI] [PubMed] [Google Scholar]
- 32.Watkins K, Paus T. J Cognit Neurosci. 2004;16:978–987. doi: 10.1162/0898929041502616. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds M, Besner D. J Exp Psychol Hum Percept Perform. 2006;32:1303–1323. doi: 10.1037/0096-1523.32.6.1303. [DOI] [PubMed] [Google Scholar]
- 34.Desimone R. Proc Natl Acad Sci USA. 1996;93:13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neely JH. In: Basic Processes in Reading: Visual Word Recognition. Besner D, Humphreys GW, editors. Hillsdale, NJ: Erlbaum; 1991. pp. 264–336. [Google Scholar]
- 36.Dhond RP, Buckner RL, Dale AM, Marinkovic K, Halgren E. J Neurosci. 2001;21:3564–3571. doi: 10.1523/JNEUROSCI.21-10-03564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marinkovic K, Dhond RP, Dale AM, Glessner M, Carr V, Halgren E. Neuron. 2003;38:487–497. doi: 10.1016/s0896-6273(03)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wise SP, Boussaoud D, Johnson PB, Caminiti R. Annu Rev Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
- 39.Mechelli A, Crinion JT, Long S, Friston KJ, Lambon Ralph MA, Patterson K, McClelland JL, Price CJ. J Cognit Neurosci. 2005;17:1753–1765. doi: 10.1162/089892905774589190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.