Abstract
Current global fisheries production of ≈160 million tons is rising as a result of increases in aquaculture production. A number of climate-related threats to both capture fisheries and aquaculture are identified, but we have low confidence in predictions of future fisheries production because of uncertainty over future global aquatic net primary production and the transfer of this production through the food chain to human consumption. Recent changes in the distribution and productivity of a number of fish species can be ascribed with high confidence to regional climate variability, such as the El Niño–Southern Oscillation. Future production may increase in some high-latitude regions because of warming and decreased ice cover, but the dynamics in low-latitude regions are governed by different processes, and production may decline as a result of reduced vertical mixing of the water column and, hence, reduced recycling of nutrients. There are strong interactions between the effects of fishing and the effects of climate because fishing reduces the age, size, and geographic diversity of populations and the biodiversity of marine ecosystems, making both more sensitive to additional stresses such as climate change. Inland fisheries are additionally threatened by changes in precipitation and water management. The frequency and intensity of extreme climate events is likely to have a major impact on future fisheries production in both inland and marine systems. Reducing fishing mortality in the majority of fisheries, which are currently fully exploited or overexploited, is the principal feasible means of reducing the impacts of climate change.
Keywords: fisheries, net primary production
Terrestrial and aquatic food production systems differ in fundamental ways that affect our ability to study and interpret the impacts of climate and to predict the consequences of future changes. Most terrestrial food production comes from agriculture, in which selected crops are grown under controlled conditions, often with the addition of fertilizer and removal of predators and pests. Selected herbivores are enclosed, or herded, and culled to maximize production or to meet market requirements. Genetic selection (and recently manipulation) of plants and animals has been practiced since agriculture began. Aquatic food production, particularly aquaculture, is adopting some of these characteristics, but capture fisheries continue to harvest wild populations, which often have large ranges and are part of natural ecosystems. Where capture fisheries exert differential selectivity, this may cause adaptive genetic changes in the population. The changes may be undesirable (e.g., selection for small size), but the rate and magnitude of such changes remains uncertain (1). Production of fish in many aquatic ecosystems varies considerably as a result of interannual and decadal variability in their environment, for which the term “climate variability” is used. For example, annual catches of Peruvian anchoveta (Engraulis ringens), the biggest single-species fishery in the world, ranged from 94,000 tons to >13 million tons during the period 1970–2004, with much of the variability resulting from changes in the El Niño–Southern Oscillation (ENSO) (2, 3).
Three premises need to be kept in mind when considering future fisheries production (i.e., the fraction of fish production that is caught) and food security. (i) Fishermen, the fishing industry, and communities dependent on fish supplies have historically had to adapt to variability in the supply of fish. (ii) Our ability to control the variability in supply is limited because (with the exception of aquaculture and some freshwater systems) our only control over the system that produces the fish is by adjusting catching activity. (iii) As fisheries increasingly develop the selective and managed characteristics of terrestrial food production (e.g., by increasing aquaculture production), our ability to predict and adapt to impacts of climate change on fish production may improve. However, this development depends, among other things, on the continuing availability of suitable feed sources for aquaculture, which is by no means assured (4, 5). Note that food security depends on economic ability to purchase, as well as on the supply of food, but this and the role that fish plays in the global protein supply are beyond the scope of this article.
Fish production depends on the amount of net primary production (NPP) and how this production passes through the aquatic food chain and enters the human food chain (6). NPP is transformed as it passes from prey to predator, with a loss of ≈70–90% at each trophic step (7). Fish production is rather weakly related to NPP because of variability in the number of trophic steps and in the transfer efficiency at each step. Species at higher trophic levels are, on average, larger and more valuable per unit weight. When forage fish species are captured and processed as food supply for aquaculture, this increment in value must compensate for the trophic loss. In predicting future food supply from fisheries, quantity, composition, and distribution all need to be considered.
Present Fisheries Production, Trends, and Threats
Production and Trends.
Seventy-seven percent of the 164 million tons total global aquatic production in 2004 was from marine systems, and the remaining 23% was from inland waters. Sixty-eight percent of the total production of fish, crustaceans, and mollusks came from capture fisheries, and the remaining 32% came from aquaculture. Aquaculture production is rising rapidly, and by 2030 it is estimated that aquaculture production will be close to that of capture production (Fig. 1). Seventy-six percent of world fisheries production in 2002 was used for direct human consumption, and the remaining 24% was used for fishmeal and oil, much of it in aquaculture. Unlike terrestrial farming systems, in which the bulk of global production is based on a limited number of animal and plant species, >220 different farmed aquatic animal and plant species were reported in 2002 (8, 9) (Table 1).
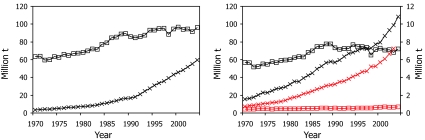
Fig. 1.
World fisheries production from capture fisheries (open squares) and aquaculture (crosses). (Left) Global totals, including China. (Right) Global totals, excluding China (because of doubts over the reliability of the statistics) and showing the marine (black) and inland (red) production separately. Note that the right-hand scale applies for aquaculture (crosses).
Table 1.
World fisheries production in 2004
| Type | Production, million tons |
||
|---|---|---|---|
| Inland | Marine | Total | |
| Capture production | |||
| Fish, crustaceans, mollusks | 8.8 | 85.8 | 94.6 |
| Aquaculture production | |||
| Fish, crustaceans, mollusks | 27.2 | 18.3 | 45.5 |
| Aquatic plants | 0.0 | 13.9 | 13.9 |
Source: FAO Yearbook of Fisheries Statistics (www.fao.org/fi/statist/statist.asp).
Excluding production by China (9, 10), the capture production of fish, crustaceans, and mollusks has been declining annually by ≈233,000 tons since 1989. At least 70% of world fish stocks are estimated to be fully exploited, overexploited, or recovering from a period of depletion (8).
Threats.
The principal threats to future fisheries production identified here are expected to act progressively (i.e., a linear response) and to interact with each other. However, marine ecosystems can also respond to changes in physical or biological forcing in a nonlinear way (11), e.g., when a threshold value is exceeded and a major change in species composition, production, and dynamics takes place. We know that such nonlinear responses occur (see Regional Climate Variability and Regime Shifts, below) but do not yet understand how or under what conditions. This is a key limitation in our ability to forecast future states of marine ecosystems.
Fishing activity.
Fishing is the greatest threat to future global fish production; however, the impacts of fishing and of climate change interact in a number of ways, and they cannot be treated as separate issues (Fig. 2). Fishing causes changes in the distribution, demography, and stock structure of individual species and direct or indirect changes in fish communities and marine ecosystems. These changes have consequences for other ecosystem services (such as nutrient cycling and recreational use) and for sustainability, resilience and ability to adapt to climate change, and other pressures. Future sustainable fisheries depend on effective management of fishing activity, which in turn requires an understanding of the effects of climate change on the productivity and distribution of exploited stocks. Management must take into account the interactive effects of fishing, climate, and other pressures.
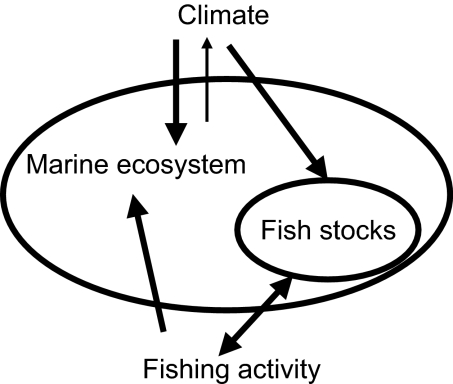
Fig. 2.
Schematic representation of impacts of climate change and fishing activity on the marine ecosystem and its fish component.
Fishing is size-selective and causes changes in the size and age structure of populations, which results in greater variability in annual recruitment in exploited populations (12). The truncation of age structure and loss of geographic substructure within populations makes them more sensitive to climate fluctuations (13, 14). To sustain the resilience of fish populations, in particular when they are confronted by additional pressures such as climate change, their age and geographic structure must be preserved rather than relying only on management of their biomass. We are currently fishing most stocks at levels that expose them to a high risk of collapse, given the trends in climate and the uncertainty over impacts.
Fishing is one of a number of human pressures that have resulted in a global decline in biodiversity (15). This raises concerns over the role biodiversity plays in maintaining ecosystem services and, in particular, resilience to climate change. A recent metaanalysis concluded that the oceans' capacity to provide food, maintain water quality, and recover from perturbation has been impaired through loss of biodiversity (16), but other studies of the relationship between biodiversity and ecosystem functioning and services produce a more nuanced picture (17).
Direct and indirect effects of climate change on distribution, productivity, and extinction.
Climate change has both direct and indirect impacts on fish stocks that are exploited commercially. Direct effects act on physiology and behavior and alter growth, development, reproductive capacity, mortality, and distribution. Indirect effects alter the productivity, structure, and composition of the ecosystems on which fish depend for food and shelter.
The effects of increasing temperature on marine and freshwater ecosystems are already evident, with rapid poleward shifts in distributions of fish and plankton in regions such as the North East Atlantic, where temperature change has been rapid (18–20). Further changes in distribution and productivity are expected due to continuing warming and freshening of the Arctic (21). Some of the changes are expected to have positive consequences for fish production (22), but in other cases reproductive capacity is reduced and stocks become vulnerable to levels of fishing that had previously been sustainable (23). Local extinctions are occurring at the edges of current ranges, particularly in freshwater and diadromous species such as salmon (24) and sturgeon (25).
Competitors and pathogens.
Climate change can enable both competitive species, such as the Pacific oyster (Crassostrea gigas) (26), and pathogenic species to spread to new areas. Climate change has been implicated in mass mortalities of many aquatic species, including plants, fish, corals, and mammals, although lack of adequate data makes it difficult to attribute causes (27). A combination of field observation, experiments, and coupled physical–biological modeling has been used to study the northward spread of two protozoan parasites (Perkinsus marinus and Haplosporidium nelsoni) from the Gulf of Mexico to Delaware Bay and further north, where they have caused mass mortalities in Eastern oysters (Crassostrea virginica). Winter temperatures consistently <3°C limit the development of the MSX disease caused by Perkinsus (28), and the poleward spread of this and other pathogens can be expected to continue as such winter temperatures become rarer.
Threats to inland fisheries and aquaculture.
Many inland fisheries are threatened by alterations to water regimes that, in extreme cases, cause whole lakes [e.g., Lake Chad (29)] and waterways to disappear. Climate change has direct effects, through reduced precipitation and greater evaporation, and indirect effects when more water is used for irrigation to offset reduced precipitation. Threats to aquaculture arise from (i) stress due to increased temperature and oxygen demand and decreased pH, (ii) uncertain future water supply, (iii) extreme weather events, (iv) increased frequency of diseases and toxic events, (v) sea level rise and conflict of interest with coastal defenses, and (vi) an uncertain future supply of fishmeal and oils from capture fisheries (4, 30). Aquaculture poses some additional threats to capture fisheries, and the development of aquaculture could affect the resilience of capture fisheries in the face of climate change (5). There will also be some positive effects due to increased growth rates and food conversion efficiencies, longer growing season, range expansion, and the use of new areas as a result of decrease in ice cover.
Loss of structures that support fisheries.
Coral reefs have begun to suffer bleaching and mortality as a result of exceptionally warm periods (31). So far, events such as the 1998 mass coral bleaching in the Indian Ocean have not provided evidence of negative short-term bioeconomic impacts for coastal reef fisheries (32, 33). However, in the longer term, fisheries production is likely to be affected by the loss or reduced structural complexity of coral communities, which result in reduced fish species richness, local extinctions, and loss of species within key functional groups of reef fish (34, 35). A projected decline in pH will also adversely affect coral growth and hence, probably, associated fisheries.
Autonomous adaptive capacity.
Climate change and climate variability have occurred throughout history; natural systems, and the fisheries based on them, have developed a capacity to adapt that will help them mitigate the impact of future changes. However, three factors will limit this adaptive capacity in the future: (i) the rate of future climate change is predicted to be more rapid than previous natural changes; (ii) the resilience of species and systems is being compromised by concurrent pressures, including fishing, loss of genetic diversity, habitat destruction, pollution, introduced and invasive species, and pathogens; and (iii) rising CO2 levels are lowering the pH of the oceans, with consequences that are largely unknown (36).
Interaction of threats in a major fish-producing region.
The lower Mekong River basin illustrates the interaction of many threats to future fish production. Recent estimates of the catch in the Mekong basin from capture fisheries alone exceed 2.5 million tons (37), and two thirds of the 60 million people living there are in some way active in fisheries. It is likely that the impacts of human population growth, flood mitigation, increased water abstraction, changes in land use, and overfishing on the fisheries will be greater than the effects of climate, but the pressures are strongly interrelated (38). Direct effects of climate will occur as a result of changing patterns of precipitation, snow melt, and rising sea level, which will affect hydrology and water quality. Indirect effects will result from changing vegetation patterns that may alter the food chain and increase soil erosion.
Economic impacts.
A key factor concerning future economic impacts is the need to identify which countries and regions are most vulnerable. Modeling studies have assessed country vulnerability on the basis of exposure of its fisheries to climate change, high dependence on fisheries production, and low capacity to respond. The studies show that climate will have the greatest economic impact on the fisheries sectors of central and northern Asian countries, the Western Sahel, and coastal tropical regions of South America (38), as well as on some small and medium-sized island states (39). Indirect economic impacts will depend on the extent to which local economies are able to adapt to new conditions in terms of labor and capital mobility. Change in natural fisheries production is often compounded by decreased harvest capacity and reduced access to markets (38). Global fish production is forecast to increase more slowly than demand to 2020, and the proportion of production coming from aquaculture is forecast to increase (40). Therefore, zero growth in capture fisheries production will not threaten total supply unduly, but a decline could affect global fish consumption (41).
Evidence of Climate Impacts
Climate change affects the survival, growth, reproduction, and distribution of individuals within a species, but impacts can also be shown at the level of populations, communities, or entire ecosystems. The following examples of observed climate impacts are intended to illustrate some of the main processes involved, their complexity, and their interactions. The climate-related drivers include temperature, salinity, windfields, oxygen, pH, and the density structure of the water column. The examples range in scale from experimental studies on individual fish, through a combination of experimental and field studies, to modeling and observation of whole ecosystems and large sea areas.
Experimental Studies.
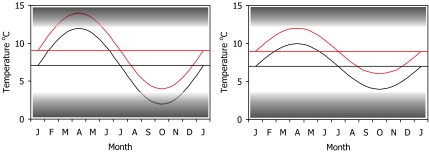
Rainbow trout (Oncorhyncus mykiss) were used in one of the very few experimental studies designed to show the effects of increased temperature (42). A 2°C temperature increase produced positive effects on their appetite, growth, protein synthesis, and oxygen consumption in winter, but the same increase in summer produced negative effects. Because of these seasonal differences, it is difficult to generalize about the balance of effects. Information about the amplitude of seasonal temperature variability is needed, as well as information about the annual mean (Fig. 3). Temperature also interacts with declining pH and increasing nitrogen and ammonia to increase metabolic costs.
Fig. 3.
Schematic effect of a 2°C increase in temperature. The shading represents temperature regions with progressively more adverse effects. The red lines show seasonal temperatures that are 2°C above the black lines. (Left) The black seasonal temperature pattern enters the adverse region in winter but not in summer. The red pattern escapes from adverse winter temperature but enters the adverse region in summer. The mean temperatures are the same in both panels, but seasonal amplitude is reduced in Right, and neither pattern enters the adverse region. Climate change may, of course, affect the amplitude of such seasonal cycles, as well as the mean.
Metabolic Stress and Its Effects.
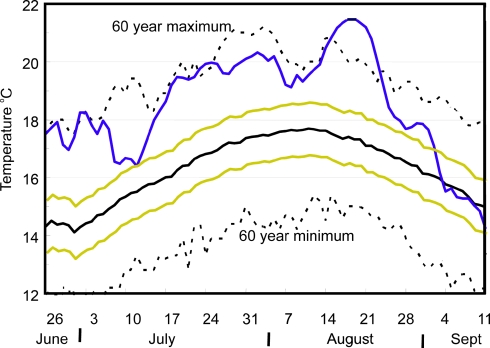
Changes in the distribution of common eelpout (Zoarces viviparous) in the southern North Sea have been related to thermally limited oxygen delivery during summer hot spells, using a combination of experimental and field work to identify the physiological effects and consequences for mortality (43). Salmon in the Fraser River, Canada, suffered enhanced mortality when summer temperatures exceeded the levels previously recorded in a 60-year time series over a period of weeks in the summer of 2004 (Fig. 4). These examples show that the impacts of climate change can occur during short periods within a year and should, therefore, be ascribed to changes in the frequency and intensity of extreme events (floods, droughts, heat waves, hurricanes), as well as to changes in the mean values.
Fig. 4.
Temperature profile at Hell's Gate (Fraser River, BC, Canada) in 2004 (blue line), also showing the 60-year mean (black solid line), ±1 standard deviation (yellow lines), and 60-year minimum and maximums (black dashed lines). For several days in mid-August, Fraser River water temperatures, as measured at Hell's Gate, were the highest ever recorded (from Canadian Standing Committee on Fisheries and Oceans, 2005, www-comm.pac.dfo-mpo.gc.ca/publications/2004psr/Williams5_e.htm).
Species and Ecosystems Close to Their Physiological Tolerance Limits.
The Baltic Sea is almost totally enclosed, and its salinity and oxygen regime depend on intermittent inflows of saline and oxygenated water from the Skagerrak. Atlantic cod (Gadus morhua) is the principal demersal fish species in the Baltic, producing annual catches of up to 400,000 tons during the 1980s; however, it is close to the extremes of its physiological tolerance in this area. Mild winters with strong westerly winds, above-normal rainfall, and reduced frequency of inflows have prevailed since the mid-1980s, probably because of climate-related changes in atmospheric pressure fields (44) and have resulted in freshening of the Baltic (45). Experiments show that at salinities <11, cod spermatozoa cease to be active and the eggs sink because their density is greater than that of the water (46). The eggs cannot survive anoxic conditions in the deeper layers. Biological interactions with other fish and with planktonic prey also influence the dynamics of cod in the Baltic, but the system is ultimately governed by changes in large-scale climatic conditions (47).
Past Warming Periods as Analogues for Climate Change.
Some of the most striking examples of the impacts of climate change on fisheries come from the prolonged warming period that affected many parts of the North Atlantic from the mid-1920s until the 1960s. This large-scale event provides an analogue for the current warming period, and many of the changes in distribution of fish and other marine life that took place, particularly at Greenland from 1925 to 1935, are being repeated now. Species were caught that had previously been either present only in restricted coastal fiords, such as Atlantic cod, or entirely absent, such as haddock (Melanogrammus aeglefinus) and herring (Clupea harengus). Cod rapidly increased on the West Greenland shelf, extending their range northward by >1,000 km in <20 years and giving rise to an international fishery of >400,000 tons annually at its peak (48). Benthic species and marine mammals also extended their ranges, showing how rapidly distributions can change in the sea. From a fisheries perspective, the warming and consequent changes in distribution can be regarded as having a positive effect on fisheries production. The subsequent cooling period, which probably accelerated the decline in cod stock due to overfishing, resulted in another ecosystem switch and an increase in the fishery for shrimps, which largely replaced the revenue generated by the cod fishery. Thus, the consequences of climate change may in some cases be positive from a fisheries yield perspective, and replacement of species or whole ecosystems can take place quite rapidly (49).
Regional Climate Variability and Regime Shifts.
Most of the large global marine capture fisheries are affected by regional climate variability. North Pacific ecosystems are characterized by “regime shifts”—fairly abrupt changes in both physics and biology that persist for periods of at least a decade. These changes have major consequences for the productivity and species composition of fisheries resources in the region (50). ENSO events, which are defined by the appearance and persistence of anomalously warm water in the coastal and equatorial ocean off Peru and Ecuador for periods of 6–18 months, have adverse effects on Peruvian anchovy production in the eastern Pacific (2). However, longer term, decadal anomalies appear to have greater long-term consequences for the foodweb than the short periods of nutrient depletion during ENSO events (3).
Modeling Regional Climate Variability and Its Effects on Production, from Phytoplankton to Fish.
Recruitment of the two tropical species of tuna [skipjack (Katsuwonus pelamis) and yellowfin (Thunnus albacares)] and the subtropical albacore (Thunnus alalunga) in the Pacific is related to regimes in the major climate indices, ENSO, and the Pacific Decadal Oscillation (51). Large-scale distribution of skipjack tuna in the western equatorial Pacific warm pool can also be predicted from a model linked to changes in ENSO (52). These tuna models are notable because they simulate NPP, driven by ocean biogeochemistry, and the pelagic fish ecosystem in two trophic levels, thus explicitly relating fish production to primary production. An inland example of the consequences of a change in NPP comes from Lake Tanganyika, where the decline in pelagic fish catches since the late 1970s has been ascribed to a climate-induced increase in the vertical stability of the water column, resulting in reduced availability of nutrients (53).
Future Fish Production
The quantity of future fish production depends on changes in NPP and on what proportion is transferred through the marine ecosystem to human consumption. Because there are considerable uncertainties about both of these factors, very low confidence can be placed in current predictions of future fish production. Regional and local forecasts may be more reliable than the global forecast because of special factors (such as loss of ice cover in high latitudes, which will allow greater light penetration). Some recent observation-based studies (introduced briefly below) found that NPP has been declining, particularly in low latitudes, because of increased warming of the surface layers, which increases stratification and reduces nutrient mixing from depth. The scientific base is improving rapidly, as is evident from the very recent dating of key publications cited here, but we are some way from achieving a reliable consensus.
The examples of observed climate impacts cited above show changes in distribution and abundance of particular species, but because species are often replaced by functionally similar species, the net effect on trophic structure and fish production may be small. It is generally difficult to predict the changes in trophic structure and composition of ecosystems, therefore one simplifying assumption is that such functional replacement always occurs and that fish production is proportional to NPP. A second possible approach is to study the impacts of climate on fish communities (e.g., the pelagic fish community) rather than at the individual-species level (54). The rising proportion of aquaculture in global fisheries production will increasingly determine the trophic structure of fisheries; however, aquaculture is likely to remain dependent on capture fisheries for its food supply.
Predicted changes in NPP may be either positive or negative, and the aggregate impact at the global level is unknown. NPP depends on the availability of light and nutrients, which in turn are governed by runoff, atmospheric dust deposition, ocean mixing processes, cloud cover, and the solar cycle. Satellite measurements of ocean color over the past two decades show changes in global NPP but with large regional differences that can be related to changes in upper-ocean temperature gradients, wind stress, and atmospheric iron deposition (51, 55). An annual reduction in NPP of ≈1% occurred between 1994 and 2004 (56). There is also evidence from both the Pacific and Atlantic that nutrient supply to the upper productive layer of the ocean is declining because of reduced meridional overturning circulation, increased thermal stratification, and changes in windborne nutrients (57, 58). Paleological evidence and simulation modeling show the North Atlantic plankton biomass declining by 50% over a long time scale during periods of reduced meridional overturning circulation (59).
Coupled simulations compared predictions of NPP from six different models, from the beginning of the industrial revolution to 2050 (60). The simulations show global increases in primary production of 0.7–8.1% over this period but with large regional differences. Although such studies are speculative, they are an essential step in gaining better understanding. On the other hand, the observations and model evidence cited above provide grounds for concern that aquatic production, including fisheries production, will suffer regional, and possibly global, decline, and that this decline has already begun.
Conclusions and Management Implications
Reducing fishing mortality in the majority of fisheries, which are currently fully exploited or overexploited, is the principal feasible means of reducing the impacts of climate change.
Fishing and climate change are strongly interrelated pressures on fish production and must be addressed jointly. Loss of biodiversity and reductions in demographic and geographic structure due to fishing result in greater sensitivity of fish stocks and marine ecosystems to climate change. Conversely, climate change can reduce (or in some cases enhance) the productivity of stocks through effects on NPP, reproductive output, growth, and survival. Sustainable levels of fishing (often expressed as reference levels for biomass and fishing mortality) must therefore be adjusted to take such climate-induced changes in productivity into account.
Fish are a component of marine ecosystems, and the continuing development of a precautionary, ecosystem-based approach that goes beyond the assessment and management of just a few commercially important species provides a better basis for incorporating climate-induced changes. The possibility of nonlinear, abrupt changes in productivity and species composition also points to the need for a precautionary approach to fisheries management. Because nonlinear changes may be hard to predict, the management system must be able to respond quickly.
Given the complexity and regional variability of marine ecosystems and their responses to climate change, it is difficult to provide detailed management and adaptation strategies for fisheries management. However, it is possible to suggest attributes of management that are likely to be helpful. These include flexibility, adaptability to new information about the marine ecosystem, reflexivity (i.e., continuous evaluation of the consequences of management in relation to targets), and transparency in the use of information and in governance.
Fisheries have always been subjected to large natural variability, and fishing communities have in most, but not all, cases been able to adapt to these changes. Management measures should seek to accommodate such autonomous adaptation by retaining flexibility in transitions between alternative livelihoods. Management measures should also avoid historically based schemes (e.g., catch quota allocations) that cease to correspond to changing distributions and population levels. Objectives for sustainable management of fisheries should build-in expected climate change.
Changes in the amplitude of climate variability are very likely to have greater consequences than changes in mean values. Extreme climate events have significant consequences for fisheries production in both marine and inland systems.
Our present ability to predict regional and global fish production is poor and requires improvement in a number of areas, including the following:
Models that relate interannual variability, decadal (regional) variability, and global climate change, to make better use of information on climate change in planning management adaptations.
Observations and models of regional and global NPP. Development of new models for predicting how changes in NPP will pass through the aquatic food chain to fisheries resources.
Acknowledgment of the consequences of changes in biodiversity for the stability, resilience, and productivity of aquatic systems.
Greater understanding of the consequences of the trend toward increasing aquaculture for future aquatic production.
Acknowledgments
This work is a contribution to the International Council for the Exploration of the Sea/Global Ocean Ecosystem Dynamics (ICES/GLOBEC) Cod and Climate Change Program, which is funded by the U.K. Department of the Environment, Food, and Rural Affairs; the Norwegian Research Council; the Danish Institute for Fisheries Research; and the French Research Institute for Exploitation of the Sea.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission. W.E. is a guest editor invited by the Editorial Board.
References
- 1.Marshall CT, Browman HI. Mar Ecol Prog Ser. 2007;335:249–310. [Google Scholar]
- 2.Jacobson LD, De Oliveira JAA, Barange M, Cisneros-Mata MA, Félix-Uraga R, Hunter JR, Kim JY, Matsuura Y, Niquen M, Porteiro C, et al. Can J Fish Aquat Sci. 2001;58:1891–1903. [Google Scholar]
- 3.Barber R. In: Encyclopedia of Ocean Sciences. Steele JH, Thorpe SA, Turekian KK, editors. London: Academic; 2001. pp. 3128–3135. [Google Scholar]
- 4.Naylor RL, Goldburg RJ, Primavera JH, Kautsky N, Beveridge MCM, Clay J, Folke C, Lubchenco J, Mooney H, Troell M. Nature. 2000;405:1017–1024. doi: 10.1038/35016500. [DOI] [PubMed] [Google Scholar]
- 5.Goldburg RJ, Elliot MS, Naylor RL. Marine Aquaculture in the United States: Environmental Impacts and Policy Options. Arlington, VA: Pew Oceans Commission; 2001. [Google Scholar]
- 6.Iverson RL. Limnol Oceanogr. 1990;35:1593–1604. [Google Scholar]
- 7.Pauly D, Christensen V. Nature. 1995;374:255–257. [Google Scholar]
- 8.Food Agriculture Organization of the United Nations, Fisheries Department. The State of World Fisheries and Aquaculture. Rome: FAO; 2004. [Google Scholar]
- 9.Food Agriculture Organization of the United Nations, Fisheries Department. The State of World Fisheries and Aquaculture. Rome: FAO; 2007. [Google Scholar]
- 10.Food Agriculture Organization of the United Nations, Fisheries Department. The State of World Fisheries and Aquaculture. Rome: FAO; 2002. [Google Scholar]
- 11.Hsieh C, Glaser SM, Lucas AJ, Sugihara G. Nature. 2005;435:336–340. doi: 10.1038/nature03553. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh C, Reiss CS, Hunter JR, Beddington JR, May RM, Suguhara G. Nature. 2006;443:859–862. doi: 10.1038/nature05232. [DOI] [PubMed] [Google Scholar]
- 13.Berkeley SA, Hixon MA, Larson RJ, Love MS. Fisheries. 2004;29:23–32. [Google Scholar]
- 14.Ottersen G, Hjermann DØ, Stenset NC. Fish Oceanogr. 2006;15:230–243. [Google Scholar]
- 15.Reynolds J, Dulvy N, Goodwin N, Hutchings J. Proc R Soc London Ser B. 2005;272:2337–2344. doi: 10.1098/rspb.2005.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Worm B, Barbier EB, Beaumont N, Duffy JE, Folke C, Halpern BS, Jackson JBC, Lotze HK, Micheli F, Palumbi SR, et al. Science. 2006;314:787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 17.Balvanera P, Pfisterer AB, Buchmann N, He JS, Nakashizuka T, Raffaelli D, Schmid B. Ecol Lett. 2006;9:1146–1156. doi: 10.1111/j.1461-0248.2006.00963.x. [DOI] [PubMed] [Google Scholar]
- 18.Brander KM, Blom G, Borges MF, Erzini K, Henderson G, MacKenzie BR, Mendes H, Ribeiro J, Santos AMP, Toresen R. In: Hydrobiological Variability in the ICES Area, 1990–1999. Turrell W, Lavin A, Drinkwater KF, St John MA, Watson J, editors. Copenhagen: Intl Council for the Exploration of the Sea; 2003. pp. 261–270. [Google Scholar]
- 19.Beaugrand G, Reid PC, Ibanez F, Lindley JA, Edwards M. Science. 2002;296:1692–1694. doi: 10.1126/science.1071329. [DOI] [PubMed] [Google Scholar]
- 20.Hughes SL, Holliday NP, editors. International Council for the Exploration of the Sea. ICES Report on Ocean Climate 2005. Copenhagen: Intl Council for the Exploration of the Sea; 2006. ICES Cooperative Research Report No 280. [Google Scholar]
- 21.Drinkwater KF. ICES J Mar Sci. 2005;62:1327–1337. [Google Scholar]
- 22.Arctic Council. Arctic Climate Impact Assessment. Cambridge, UK: Cambridge Univ Press; 2005. [Google Scholar]
- 23.Brander KM, Mohn RK. Can J Fish Aquat Sci. 2004;61:1558–1564. [Google Scholar]
- 24.Friedland KD, Reddin DG, McMenemy JR, Drinkwater KF. Can J Fish Aquat Sci. 2003;60:563–583. [Google Scholar]
- 25.Reynolds JD, Webb TJ, Hawkins LA. Can J Fish Aquat Sci. 2005;62:854–862. [Google Scholar]
- 26.Diederich S, Nehls G, van Beusekom JEE, Reise K. Helgoland Mar Res. 2005;59:97–106. [Google Scholar]
- 27.Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJ, Hofmann EE, Lipp EK, Osterhaus AD, Overstreet RM, et al. Science. 1999;285:1505–1510. doi: 10.1126/science.285.5433.1505. [DOI] [PubMed] [Google Scholar]
- 28.Hofmann E, Ford S, Powell E, Klinck J. Hydrobiologia. 2001;460:195–212. [Google Scholar]
- 29.Coe MT, Foley JA. J Plankton Res. 2001;106:3349–3356. [Google Scholar]
- 30.Intergovernmental Panel on Climate Change. Climate Change 2001: Impacts, Adaptation, and Vulnerability Contribution of Working Group II to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge Univ Press; 2001. [Google Scholar]
- 31.Sheppard CRC. Nature. 2003;425:294–297. doi: 10.1038/nature01987. [DOI] [PubMed] [Google Scholar]
- 32.Grandcourt EM, Cesar HSJ. Fish Res. 2003;60:539–550. [Google Scholar]
- 33.Spalding MD, Jarvis GE. Mar Pollut Bull. 2002;44:309–321. doi: 10.1016/s0025-326x(01)00281-8. [DOI] [PubMed] [Google Scholar]
- 34.Sano M. Fish Sci. 2004;70:41–46. [Google Scholar]
- 35.Graham NAJ, Wilson SK, Jennings S, Polunin NVC, Bijoux JP, Robinson J. Proc Natl Acad Sci USA. 2006;103:8425–8429. doi: 10.1073/pnas.0600693103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, Fabry VJ, Millero FJ. Science. 2004;305:362–366. doi: 10.1126/science.1097329. [DOI] [PubMed] [Google Scholar]
- 37.Hortle K, Bush S. In: New Approaches for the Improvement of Inland Capture Fishery Statistics in the Mekong Basin. Clayton T, editor. Rome: FAO; 2003. pp. 76–88. [Google Scholar]
- 38.Food and Agriculture Organization of the United Nations. Building Adaptive Capacity to Climate Change: Policies to Sustain Livelihoods and Fisheries. No 8. Rome: FAO; 2006. New Directions in Fisheries, A Series of Policy Briefs on Development Issues. [Google Scholar]
- 39.Aaheim HA, Sygna L. Economic Impacts of Climate Change on Tuna Fisheries in Fiji Islands and Kiribati (Cent for Intl Clim Environ Res, Oslo). Rept. 2000;2000:4. [Google Scholar]
- 40.Delgado CL, Wada N, Rosegrant MW, Meijer S, Ahmed M. Fish to 2020: Supply and Demand in Changing Global Markets. Washington, DC, Penang, Malaysia: Intl Food Policy Res Inst, WorldFish Center; 2003. WorldFish Center Tech Rept No 62. [Google Scholar]
- 41.Briones M, Dey MM, Ahmed M. NAGA: WorldFish Cent Q. 2004;27:48–50. [Google Scholar]
- 42.Morgan I, McDonald DG, Wood CM. Global Change Biol. 2001;7:345–355. [Google Scholar]
- 43.Pörtner H-O, Knust R. Science. 2007;315:95–97. doi: 10.1126/science.1135471. [DOI] [PubMed] [Google Scholar]
- 44.Schrum C. Clim Res. 2001;18:31–37. [Google Scholar]
- 45.Meier HEM, Feistel R, Piechura J, Arneborg L, Burchard H, Fiekas V, Golenko N, Kuzmina N, Mohrholz V, Nohr C, et al. Oceanologia. 2006;48:133–164. [Google Scholar]
- 46.Nissling A, Larsson R, Vallin L, Frohlund K. Fish Res. 1998;38(2):169–186. [Google Scholar]
- 47.Koster FW, Mollmann C, Hinrichsen HH, Wieland K, Tomkiewicz J, Kraus G, Voss R, Makarchouk A, MacKenzie BR, John MA. ICES J Mar Sci. 2005;62:1408–1425. [Google Scholar]
- 48.Jensen AS. Biol Med. 1939;XIV:1–75. [Google Scholar]
- 49.Frank KT, Petrie BD, Choi JS, Leggett WC. Science. 2005;308:1621–1623. doi: 10.1126/science.1113075. [DOI] [PubMed] [Google Scholar]
- 50.King JR. Report of the Study Group on Fisheries and Ecosystem Responses to Recent Regime Shifts. Sidney, BC, Canada: N Pac Mar Sci Org/Inst Ocean Sci; 2005. PICES Scientific Report No 28. [Google Scholar]
- 51.Lehodey P, Chai F, Hampton J. Fish Oceanogr. 2003;12:483–494. [Google Scholar]
- 52.Lehodey P. Prog Oceanogr. 2001;49:439–469. [Google Scholar]
- 53.O'Reilly CM, Alin SR, Plisnier PD, Cohen AS, McKee BA. Nature. 2004;424:766–768. doi: 10.1038/nature01833. [DOI] [PubMed] [Google Scholar]
- 54.Jennings S, Brander K. J Mar Syst. 2007 in press. [Google Scholar]
- 55.Gregg WW, Conkright ME, Ginoux P, O'Reilly JE, Casey NW. Geophys Res Lett. 2003;30:1809. [Google Scholar]
- 56.Behrenfeld MJ, O'Malley RTO, Siegel DA, McClain CR, Sarmiento JL, Feldman GC, Milligan AJ, Falkowski PG, Letelier RM, Boss ES. Nature. 2006;444:752–755. doi: 10.1038/nature05317. [DOI] [PubMed] [Google Scholar]
- 57.McPhaden MJ, Zhang D. Nature. 2002:603–608. doi: 10.1038/415603a. [DOI] [PubMed] [Google Scholar]
- 58.Curry R, Mauritzen C. Science. 2005;308:1772–1774. doi: 10.1126/science.1109477. [DOI] [PubMed] [Google Scholar]
- 59.Schmittner A. Nature. 2005;434:628–633. doi: 10.1038/nature03476. [DOI] [PubMed] [Google Scholar]
- 60.Sarmiento JL, Slater R, Barber R, Bopp L, Doney SC, Hirst AC, Kleypas J, Matear R, Mikolajewicz U, Monfray P, et al. Global Biogeochem Cycles. 2005;18:GB3003. [Google Scholar]