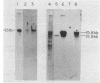
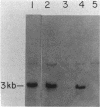
Abstract
Transduction of plasmid pC194 and bacteriophage phi 11de varied inversely with the multiplicity of infection. As the multiplicity of infection decreased from 10(-1) to 10(-5) PFU/CFU, the transduction frequency of pC194 increased 10(4)-fold; the transduction frequency of phi 11de increased 300-fold with a 100-fold decrease in multiplicity of infection. Physical and genetic analysis of the transduced DNA showed that pC194 resided in the phage particle as a random, circularly permuted linear concatemer. In DNA prepared from phage that cotransduced pC194 and phi 11de, pC194 resided in the transducing phage primarily as a linear multimer of 15.8 kilobases, or about 5.4 pC194 monomers. The pC194 multimer was randomly inserted into the phi 11 genome.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W. Transduction of chromosomal genes and episomes in Escherichia coli. Virology. 1960 May;11:273–288. doi: 10.1016/0042-6822(60)90066-0. [DOI] [PubMed] [Google Scholar]
- Bächi B. Physical mapping of the BglI, BglII, PstI and EcoRI restriction fragments of staphylococcal phage phi 11 DNA. Mol Gen Genet. 1980;180(2):391–398. doi: 10.1007/BF00425853. [DOI] [PubMed] [Google Scholar]
- Dyer D. W., Rock M. I., Iandolo J. J. Autogenous transduction of phi 11de in Staphylococcus aureus: transfer and genetic properties. J Bacteriol. 1984 May;158(2):689–695. doi: 10.1128/jb.158.2.689-695.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M., Sakaguchi K. A site-specific recE4-independent intramolecular recombination between Bacillus subtilis and Staphylococcus aureus DNAs in hybrid plasmids. Gene. 1980 Dec;12(1-2):95–102. doi: 10.1016/0378-1119(80)90019-0. [DOI] [PubMed] [Google Scholar]
- Grubb W. B., O'Reilly R. J. Joint transduction of separate extrachromosomal drug resistance determinants in Staphylococcus aureus E169. Biochem Biophys Res Commun. 1971 Feb 5;42(3):377–383. doi: 10.1016/0006-291x(71)90381-0. [DOI] [PubMed] [Google Scholar]
- Grubb W. B., O'Reilly R. J., May J. W. Segregation o co-transduced streptomycin and tetracycline resistance in Staphylococcus aureus. Genet Res. 1972 Aug;20(1):43–50. doi: 10.1017/s0016672300013574. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordănescu S. Recombinant plasmid obtained from two different, compatible staphylococcal plasmids. J Bacteriol. 1975 Nov;124(2):597–601. doi: 10.1128/jb.124.2.597-601.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordănescu S. Relationships between cotransducible plasmids in Staphylococcus aureus. J Bacteriol. 1977 Jan;129(1):71–75. doi: 10.1128/jb.129.1.71-75.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfdahl S., Sjöström J. E., Philipson L. Cloning of restriction fragments of DNA from staphylococcal bacteriophage phi 11. J Virol. 1981 Feb;37(2):795–801. doi: 10.1128/jvi.37.2.795-801.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfdahl S., Zabielski J., Philipson L. Structure and restriction enzyme maps of the circularly permuted DNA of staphylococcal bacteriophage phi 11. J Virol. 1981 Feb;37(2):784–794. doi: 10.1128/jvi.37.2.784-794.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Iordanescu S., Surdeanu M., Edelman I. Transduction-related cointegrate formation between Staphylococcal plasmids: a new type of site-specific recombination. Plasmid. 1981 Sep;6(2):159–172. doi: 10.1016/0147-619x(81)90064-0. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Khan S. A., Murphy E., Iordanescu S., Edelman I., Krolewski J., Rush M. Hitchhiking transposons and other mobile genetic elements and site-specific recombination systems in Staphylococcus aureus. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):67–76. doi: 10.1101/sqb.1981.045.01.013. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Murphy E., Gryczan T. J., Baron E., Edelman I. Penicillinase plasmids of Staphylococcus aureus: restriction-deletion maps. Plasmid. 1979 Jan;2(1):109–129. doi: 10.1016/0147-619x(79)90010-6. [DOI] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Pariza M. W., Iandolo J. J. Base ratio and deoxyribonucleic acid homology studies of six Staphylococcus aureus typing bacteriophages. Appl Microbiol. 1974 Feb;27(2):317–323. doi: 10.1128/am.27.2.317-323.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruhfel R. E., Robillard N. J., Thorne C. B. Interspecies transduction of plasmids among Bacillus anthracis, B. cereus, and B. thuringiensis. J Bacteriol. 1984 Mar;157(3):708–711. doi: 10.1128/jb.157.3.708-711.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaberg D. R., Clewell D. B., Glatzer L. Conjugative transfer of R-plasmids from Streptococcus faecalis to Staphylococcus aureus. Antimicrob Agents Chemother. 1982 Aug;22(2):204–207. doi: 10.1128/aac.22.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Iandolo J. J. Chromosomal locus for staphylococcal enterotoxin B. Infect Immun. 1978 Apr;20(1):273–278. doi: 10.1128/iai.20.1.273-278.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Iandolo J. J. Genetics of staphylococcal enterotoxin B in methicillin-resistant isolates of Staphylococcus aureus. Infect Immun. 1979 Sep;25(3):902–911. doi: 10.1128/iai.25.3.902-911.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Iandolo J. J. Transduction of staphylococcal enterotoxin B synthesis: establishment of the toxin gene in a recombination-deficient mutant. Infect Immun. 1980 Jan;27(1):280–282. doi: 10.1128/iai.27.1.280-282.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Stiffler P. W., Sweeney H. M., Cohen S. Co-transduction of plasmids mediating resistance to tetracycline and chloramphenicol in Staphylococcus aureus. J Bacteriol. 1974 Nov;120(2):934–934. doi: 10.1128/jb.120.2.934-944.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubelaker M. H., Rosenblum E. D. Transduction of plasmid determinants in Staphylococcus aureus and Escherichia coli. J Bacteriol. 1978 Feb;133(2):699–707. doi: 10.1128/jb.133.2.699-707.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman L., Goering R. V., Novick R. P. Genetic control of chromosomal and plasmid recombination in Staphylococcus aureus. Genetics. 1974 Apr;76(4):681–702. doi: 10.1093/genetics/76.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]