Abstract
Although prostate cancer (CaP) is the most frequently diagnosed malignant tumor and the second leading cause of cancer deaths in American men, the mechanisms explaining the development and progression of CaP remain largely unknown. Recent studies have shown that some aberrantly expressed microRNAs (miRNAs) are involved in tumorigenesis. Although aberrant expression of certain miRNAs has been discovered in CaP, their function in this disease has not yet been defined. In this study, we found differential expression of miR-125b in androgen-dependent and independent CaP cells, as well as in benign and malignant prostate tissues. Furthermore, androgen signaling was able to up-regulate the expression of miR-125b. In addition, transfection of synthetic miR-125b stimulated androgen-independent growth of CaP cells and down-regulated the expression of Bak1. Our results suggest that miR-125b acts as an oncogene, contributing to the pathogenesis of CaP.
Keywords: microRNA, miR-125b, ISH, LNCaP
Prostate cancer (CaP) is the most frequently diagnosed malignant tumor and the second leading cause of cancer deaths in American men (1). One of the most troubling aspects of CaP is that, after androgen ablation therapy, androgen-dependent (AD) CaP inevitably progresses to an androgen independent (AI) state, for which no effective treatment has been developed (2). Although several molecular pathways have been invoked to explain the pathogenesis of this disease (3–6), to date the mechanisms for the development and progression of CaP remain largely unknown.
MicroRNAs (miRNAs) are a class of naturally occurring small noncoding RNAs that control gene expression by the complementarity of their 5′ end nucleotides 1–8 with a binding site in the 3′-untranslated regions (3′ UTRs) of target transcripts, resulting in translational arrest and, in some instances, in transcript degradation (7). To date, >400 human miRNAs have been identified whereas up to ≈1,000 miRNAs have been postulated to exist (8). Bioinformatic analyses have predicted that single miRNAs have multiple targets, and thus miRNAs could mediate the regulation of a great number of protein-coding genes. Recent estimates suggest that one-third of human mRNAs may be regulated by miRNAs (9). Studies have found that miRNA expression patterns are significantly different in normal and neoplastic tissues, suggesting that miRNAs may play a role in tumorigenesis (10). In human cancers, some miRNAs may have oncogenic function due to their overexpression in malignant tissue whereas certain miRNAs may act as tumor suppressor genes because of decreased expression in malignant tissue (11, 12). Differential expression of miRNAs has been reported in CaP (13). To date, however, the importance of miRNAs in CaP has hardly been explored.
To understand the role of miRNAs in CaP, we analyzed the expression of selected human miRNAs in prostate cell lines and found differential expression of some of these miRNAs in benign and malignant cells, in androgen receptor (AR)-positive and -negative cells, and in AD and AI cells. In this report, we focused on miR-125b, a homologue to lin-4 that is the first-identified miRNA (14). In vitro experiment revealed that androgen stimulation increased the cellular abundance of miR-125b. Furthermore, transfection of synthetic miR-125b mimic (miR-125bm) stimulated the AI growth and down-regulated the expression of Bak1. Our results suggest that miR-125b has oncogenic function in CaP.
Results
Differential Expression of miR-125b in LNCaP and Its Sublines.
We examined the expression of 11 miRNAs in 9 prostatic cell lines using Northern blotting. These miRNAs have been reported to be aberrantly regulated in CaP or in other cancer types (13, 15, 16). We found that most CaP lines had increased miRNA expression compared with benign prostate epithelial lines, and AR-positive cell lines expressed increased let-7c and miR-125b compared with AR-negative cell lines [supporting information (SI) Fig. 7]. More interesting, two miRNAs (miR-92 and miR-106a) were down-regulated (Fig. 1A), whereas five miRNAs (miR-125b, miR-16, miR-21, miR-30c, and miR-100) were up-regulated (Fig. 1B), in the AI cds1 and cds2 cell lines compared with their parental AD LNCaP line. The finding raises the possibility that these differentially expressed miRNAs might contribute to the progression of CaP cells. Gel scanning of the miR-125b bands followed by normalization for U6 RNA demonstrated a 5-fold greater expression of miR-125b in cds1 and cds2 cells than in LNCaP cells. In this study, we focused on miR-125b and investigated its involvement in CaP.
Fig. 1.
Northern blot analyses of miRNA expression in LNCaP line and androgen-independent cds lines. Two miRNAs were down-regulated (A) and five were upreregulated (B) in cds lines compared with their parental LNCaP line.
Detection of miR-125b in Clinical CaP Samples.
Because locked nucleic acid (LNA)-modified miRNA probes have been reported to markedly increase hybridization affinity to miRNAs compared with traditional RNA- or DNA-based probes (17), a digoxigenin (DIG)-labeled LNA-miR-125b probe was used to detect miR-125b abundance in human CaP samples using in situ hybridization (ISH). Initially, we examined 10 primary CaP samples with Gleason scores of 6–8 and two benign prostatic tissues purchased from Cybrdi Co. (Chaoying Biotechnology, Xian, China). Compared with the benign prostatic tissues, five CaP samples highly expressed miR-125b, and four exhibited a moderate increase in miR-125b. Fig. 2 depicts representative ISH results showing that two CaP samples with Gleason score 7 (Fig. 2 A and C; H&E staining) expressed markedly increased (Fig. 2B) or moderately increased (Fig. 2D) miR-125b compared with a benign prostatic tissue (Fig. 2E; H&E staining) that weakly expressed miR-125b (Fig. 2F). In this experiment, all prostate samples were also hybridized by using a DIG-labeled LNA-scrambled miRNA negative control with no signal being detected (data not shown). Our ISH results suggest that overexpression of miR-125b may occur in a considerable number of CaPs.
Fig. 2.
In situ hybridization detection of miR-125b abundance in CaP samples. Two human CaP samples (B and D) with Gleason score 7 and one benign prostate tissue (F) were stained by using the DIG-LNA-miR-125b probe. According to the intensity of staining, the miR-125b abundance was categorized as high (B), moderate (D), or low level (F). These tissues were also stained with hematoxylin and eosin (A, C, and E).
Up-Regulation of miR-125b by Androgens.
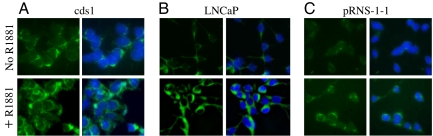
We evaluated the effect of androgens on the expression of miR-125b in LNCaP cells, as well as in cds1 cells that express a much higher level of the AR than LNCaP cells (18). ISH analysis of miR-125b abundance was performed by using a Fitc-LNA-miR-125b probe. AR-null DU145 cells that express undetectable miR-125b as shown in SI Fig. 7 and in a previous study (19) were used as a negative control. In the absence of androgens, more cytoplasmic fluorescent signal was detected in cds1 cells (Fig. 3A Upper) than in LNCaP cells (Fig. 3B Upper). This observation is consistent with the Northern blotting results shown in Fig. 1. Treatment of both LNCaP and cds1 cells using 1.0 nM synthetic androgen R1881 for 24 h markedly enhanced fluorescent intensity (Fig. 3 A and B Lower). No signal was detected in either LNCaP or cds1 cells that were hybridized with the Fitc-LNA-negative control probe (SI Fig. 8 A and B), and in R1881- or mock miRNA-treated DU145 cells that were hybridized with the Fitc-LNA-miR-125b probe (SI Fig. 8 C and D). We also performed Northern blot assay of miR-125b expression in R1881-treated LNCaP and cds1 cells. Quantitative gel scanning revealed 5- and 3-fold increase in miR-125b abundance in R1881-treated LNCaP and cds1 cells, respectively, relative to untreated cells (SI Fig. 9). To determine whether the AR mediates the R1881-stimulated up-regulation of miR-125b, ISH was performed on the pRNS-1-1-ARWT cell line that was established by transfecting AR-negative pRNS-1-1 cells with the wild-type AR (20). Because pRNS-1-1 cells express an undetectable level of miR-125b as shown in SI Fig. 7, as we expected, miR-125b was not detected in pRNS-1-1-ARWT cells in the absence of androgens (Fig. 3C Upper), as well as in pRNS-1-1 cells treated with R1881 or with mock miRNA (SI Fig. 8 E and F). However, treating pRNS-1-1-ARWT cells with 1.0 nM R1881 for 24 h moderately increased the cytoplasmic fluorescent signal (Fig. 3C Lower).
Fig. 3.
In situ hybridization analyses of miR-125b expression in prostatic cell lines. Cds1 (A), LNCaP (B), and pRNS-1-1-ARWT cells (C) were hybridized by using a Fitc-LNA-miR-125b probe in the absence (Upper) or the presence (Lower) of the synthetic androgen R1881. The fluorescent signals are cytoplasmic in location (green). The nuclei were stained by using DAPI (blue). The experiment was performed twice with similar results.
Next, we tested whether the AR is able to load to the 5′ DNA region of the miR-125b locus to serve as a transcriptional factor. There are two predicted miR-125b members in the Sanger miRNA database: miR-125b-1 on chromosome 11 and miR-125b-2 on chromosome 21. We analyzed their 5′ regions up to 4.0 kb in length and identified a typical TATA box in the 5′ region of miR-125b-2, but not in miR-125b-1, which supports a previous observation that in PC3 CaP cells the mature miR-125b is derived mostly from the miR-125b-2 (19). By using the PROMO 3.0 program, 16 potential androgen responsive elements (AREs) that form four ARE clusters illustrated as A–D in Fig. 4A were identified in the 5′ region of miR-125b-2, suggesting the existence of a promoter in the 5′ region. To determine AR loading, chromatin immunoprecipitation (ChIP) was performed, and two primer pairs were used to separately amplify the −558/−368 fragment in cluster A and the −2666/−2388 fragment in cluster C. We selected these two clusters to study because they contain ARE sequences most similar to the consensus ARE (5′-A/GGCTCTnnnA/TGTTCT). It was found that treatment of LNCaP cells with 1.0 nM R1881 induced a 6.5-fold increase in AR loading at Cluster A and a 4.5-fold increase at cluster C (Fig. 4B). Primers outside these ARE-clustering regions failed to amplify the chromatin DNA (data not shown). In addition, treatment with estradiol (E2) failed to increase the loading. Taken together, the results shown in Figs. 3 and 4 suggest that androgen-AR signaling mediates the regulation of miR-125b in CaP cells.
Fig. 4.
ChIP analysis of the AR loading to the 5′ DNA region of miR-125b. (A) Schematic representation of miR-125b-2 and its 5′ DNA region. A TATA box (TATAAA) is located at −172 upstream of miR-125b-2. ●, Potential ARE clusters (labeled A–D). The arrows indicate the locations of primers used to amplify the −558/−368 fragment in cluster A and the −2666/−2388 fragment in cluster C. (B) PCR amplification of the 5′ DNA of miR-125b-2. IgG- or anti-AR-precipitated chromatin DNA from PC3, DU145, and R1881- or estradiol (E2)-treated LNCaP cells was amplified by using the primers indicated in Fig. 4A. Input is the control reaction of genomic DNA. The numbers under the gels are the fold changes of the −558/−368 fragment (Top) and the −2666/−2388 fragment (Middle Upper) relative to untreated LNCaP cells (Untreat.). Fold changes were calculated by scanning the bands of PCR products and normalizing for input.
MiR-125b Stimulates AI Growth of LNCaP Cells.
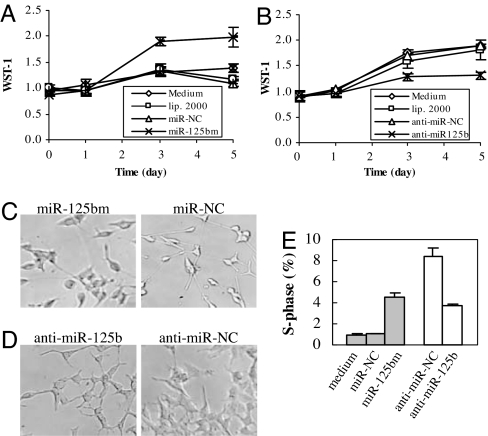
To test the hypothesis that miR-125b contributes to the progression of CaP, LNCaP cells cultured in androgen-depleted medium were transfected with synthetic miR-125b (miR-125bm), a short double-stranded RNA that has been chemically modified to encourage correct strand uptake. Similarly, cds1 cells were transfected with synthetic anti-miR-125b, a single-stranded RNA that has also been chemically modified to prolong their inhibition effect. Cell growth was assessed by using the WST-1 cell proliferation assay. Treatment with miR-125bm significantly stimulated the growth of LNCaP cells (Fig. 5A), cds1 cells, and another AD CaP line PC-346C (21) (SI Fig. 10 A and B), whereas the miRNA negative control (miR-NC), no treatment, or transfection reagent failed to affect cell growth. Anti-miR-125b, but not the anti-miRNA negative control (anti-miR-NC) or other controls, significantly inhibited the growth of cds1 cells (Fig. 5B). In the absence of androgens, however, transfection of LNCaP cells with anti-miR-125b did not significantly increase the growth arrest caused by androgen deprivation (SI Fig. 10C). Additionally, miR-125b-negative DU145 cells did not respond to transfection with miR-125bm or anti-miR-125b (SI Fig. 10D). Light microscopic analysis demonstrated that the miR-125bm-transfected LNCaP cells retained typical morphological features when grown for five days in the absence of androgens whereas those treated with miR-NC acquired a neuroendocrine (NE) phenotype, characterized by rounding of the cell bodies and extension of neuritic processes (Fig. 5C). Similarly, inhibition of miR-125b activity with anti-miR-125b in cds1 cells mediated a reduction in cell size and increased NE features compared with anti-miR-NC-treated cells (Fig. 5D). Because the S-phase fraction is a parameter reflecting cellular proliferation, we also determined the influence of miR-125bm on the S-phase fraction. After being transfected and grown for five days in the absence of androgens, cell cycling was assayed by DNA flow cytometry. In LNCaP cells transfection with 50 nM miR-125bm induced a 3-fold increase in the S-phase fraction compared with transfection with miR-NC (P = 0.005), whereas treatment of cds1 cells with 100 nM anti-miR-125 resulted in a 54% reduction of the S-phase fraction relative to anti-miR-NC (P = 0.02, Fig. 5E). To determine whether inhibiting miR-125b activity induces apoptosis, cds1 cells cultured in androgen-deprived medium were treated with 100 nM anti-miR-125b for five days and sub-G1 cells were determined by flow cytometry. This treatment resulted in a significant increase of sub-G1 cells compared with the anti-miR-NC (P = 0.01, SI Fig. 11), suggesting that anti-miR-125b transfection facilitates apoptosis in cds1 cells. Taken together, these results demonstrated that miR-125b is associated with the AI growth of CaP cells.
Fig. 5.
Effects of miR-125b on the growth of LNCaP and cds1 cells. (A) WST-1 analysis of the growth of LNCaP cells that were transfected with synthetic premiR-125b (miR-125bm) or miR-NC. (B) WST-1 analysis of the growth of cds1 cells that were transfected with synthetic anti-miR-125b or anti-miR-NC. In both A and B, the experiments were repeated at least three times with similar results obtained each time. The representative results are shown as M ± SD (n = 3). No transfected cells (medium) and transfection reagent-treated cell (lip. 2000) are additional controls. The bars represent SDs. (C) Morphology of LNCaP cells after transfection with miR-125bm or miR-NC. (D) Morphology of cds1 cells after transfection with anti-miR-125b or anti-miR-NC. (E) Flow cytometry assay of S-phase fraction in miR-125bm-treated LNCaP cells (filled) and anti-miR-125b-treated cds1 cells (open). The assay was repeated three times, and similar results were obtained. The representative results are shown as M ± SD (n = 3).
MiR-125b Down-Regulates Bak1.
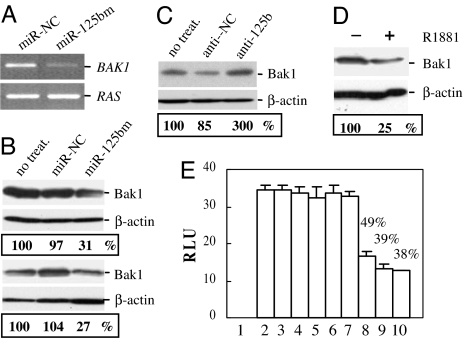
To identify the target molecules of miR-125b, microarray assay of global gene expression (SI Materials and Methods) was performed in LNCaP cells that were cultured in androgen-depleted medium for 2 days and then transfected with miR-125bm or miR-NC. DNA-Chip Analyzer software (22) was used to identify differentially expressed genes in three independent miR-125bm-treated cultures compared with controls. By using a 2.0-fold difference as the cut-off, there were 60 differentially expressed genes: 13 were up-regulated and 47 were down-regulated (SI Tables 1 and 2). Analyses of these down-regulated genes were performed by using the Sanger miRNA database target search program. The 3′ UTR of BAK1 carries a binding site for miR-125b (SI Fig. 12), suggesting that BAK1 mRNA might be a direct target of miR-125b. We therefore evaluated the effect of miR-125b on the expression of this gene. RT-PCR analysis of RNA extracted from miR-125bm-treated LNCaP cells exhibited a marked decrease in the expression of BAK1 (Fig. 6A), thus validating the microarray results. Next, we examined the level of Bak1 protein in miR-125bm- and anti-miR-125b-treated LNCaP and/or cds1 cells. Transfection with miR-125bm led to ≈70% down-regulation of Bak1 in both cell lines (Fig. 6B), whereas treatment with anti-miR-125b induced 200% increase in the Bak1 in LNCaP cells (Fig. 6C). Because R1881 stimulates miR-125b expression as shown in Fig. 3, we analyzed the Bak1 in LNCaP cells that had been treated with 1 nM R1881 for 48 h. Compared with untreated LNCaP cells, R1881 treatment induced a 75% reduction of Bak1 (Fig. 6D).
Fig. 6.
MiR-125b regulates Bak1. (A) RT-PCR analysis of BAK1 mRNA in miR-125bm-treated LNCaP cells. RAS was used as a loading control. (B) Western blot analysis of Bak1 expression in miR-125bm-treated LNCaP cells (Upper) and cds1 cells (Lower). (C) Western blot analysis of Bak1 expression in LNCaP cells that had been transfected with anti-miR-125b (anti-125b). (D) Western blot analysis of Bak1 in LNCaP cells that were treated with 1.0 nM R1881 for 48 h. In B, C, and D, β-actin is a loading control, and the numbers are percentages relative to untransfected cells (B and C, no treat.), calculated by scanning the Bak1 bands followed by normalization for β-actin. (E) Luciferase analysis in DU145 cells. 1, DU145 cell; 2, luciferase vector (LUC-V); 3, LUC-V plus 50 nM miR-125bm; 4, BAK1 3′ UTR antisense LUC-V (LUC-V-AS); 5, LUC-V-AS plus 50 nM miR-125bm; 6, BAK1 3′ UTR sense LUC-V (LUC-V-S); 7, LUC-V-S plus 50 nM miR-NC; 8, LUC-V-S plus 25 nM miR-125bm; 9, LUC-V-S plus 50 nM miR-125bm; 10, LUC-V-S plus 100 nM miR-125bm. The percentages represent enzyme activity in miR-125bm-treated LUC-V-S transfectants relative to that in untreated LUC-V-S transfectants. The assay was repeated three times with each assay being performed in four wells, and similar results were obtained each time. The representative results are shown as M ± SD (n = 4). RLU, relative luciferase unit.
To verify that the putative miR-125b binding site in the 3′ UTR of BAK1 is responsible for regulation by miR-125b, we cloned this 3′ UTR into the pRIM Reporter-luciferase vector. Examination of the antisense sequences of this 3′ UTR did not identify a miR-125b binding site, and thus the antisense-inserted vector was used as a negative control. The sense- or antisense-inserted reporter vector was cotransfected with miR-125bm into miR-125b-negative DU145 cells. Luciferase activity was measured two days after transfection. As shown in Fig. 6E, miR-125bm transfection did not alter luciferase activity in DU145 cells cotransfected with either pMIR-REPORT Luciferase vector or BAK1 3′ UTR antisense-inserted vector. However, transfection of miR-125bm at concentrations of 25–100 nM resulted in ≈50–60% reduction of the reporter in the sense vector-transfected DU145 cells. These results indicate that the 3′ UTR of BAK1 transcript is a miR-125b target.
Discussion
In this study, we found that miR-125b is overexpressed in most clinical CaP samples tested and in many cultured CaP cell lines. Moreover, transfection of synthetic miR-125bm stimulated the growth of LNCaP cells in the absence of androgens whereas anti-miR-125b inhibited the growth of the AI cds1 cells. Our findings are consistent with a previous observation that down-regulation of miR-125b induced profound growth inhibition of PC3 cells that express a moderate level of miR-125b, whereas cotransfection of miR-125b into these PC3 cells led to cell growth (19). High expression of miR-125b has been observed in pancreatic cancer (23) and oligodendroglial tumors (24). Furthermore, miR-125b may play a role in the development of precursor B-cell acute lymphoblastic leukemia (25). These reports suggested that miR-125b might be associated with tumorigenesis of other types of human cancer. Consistent with these published findings, our results support the concept that overexpressed miR-125b may act as an oncogene in CaP.
Recently, miR-125b was reported to be a tumor suppressor gene in human breast cancer (13). Functional analysis in breast cancer cells revealed that this miRNA suppressed the expression of both ERBB2 (HER2) and ERBB3 (HER3) by annealing to their 3′ UTRs (26). Increased expression of HER2 has been reported in CaP and considered to be a mechanism related to androgen independence (27). We previously observed that LNCaP and its cds sublines express HER2, particularly in the cds sublines that express increased HER2 (18). In the present study, we also analyzed the expression of HER2 and HER3 in miR-125bm-transfected LNCaP and cds1 cells and did not observe a miR-125b-mediated down-regulation of HER2 and HER3 (SI Fig. 13). Thus, it seems that in CaP cells miR-125b does not target HER2 and HER3. At present, we do not know why the same miRNA behaves very differently in different cancer types. The expression data of miR-125b, however, are consistent with its function as an oncogene or a tumor suppressor. A previous microarray analysis of miRNA expression in human solid tumors showed that miR-125b was markedly down-regulated in breast cancer whereas high level of miR-125b-2 was detected in CaP, as well as in benign prostate tissue (13). Here, we provide evidence showing that high expression of miR-125b occurs in clinical CaP samples and in CaP cell lines relative to benign prostate tissue and cell lines. Therefore, in combination with its occurrence in other cancer types including pancreatic cancer and oligodendroglial tumors (23, 24), it seems reasonable to believe that miR-125 plays an oncogenic role in certain types of cancer.
We asked which factor affects or regulates the cellular abundance of miR-125b in CaP cells. In the biogenesis of miRNAs, pri-miRNAs are transcribed by RNA polymerase II (16, 28) or RNA polymerase III (29) and then are posttranscriptionally processed by two RNases (Drosha and Dicer) to generate mature miRNAs (30, 31). These enzymes are general factors that nonspecifically regulate the generation of miRNAs. It is possible, therefore, that other factors may be involved in the regulation of miRNA expression. In the present study, we found that androgen stimulation induced an enhanced abundance of miR-125b in AR-positive LNCaP and cds1 cells, as well as in AR-transfected pRNS-1-1-ARWT cells. Furthermore, we observed that the AR is recruited to the 5′ DNA region of miR-125b-2 in the presence of R1881. Because estradiol does not induce this AR loading, ligand-specific AR loading may provide an explanation for the functional difference of miR-125b in breast cancer and CaP. The findings that R1881 stimulates the expression of miR-125b and AR loading suggest that androgen-AR signaling may mediate the regulation of miR-125b in CaP cells. In addition to androgen regulation, we observed a marked elevation of miR-125b in the AI cds1/cdcs2 sublines relative to their parental AD LNCaP line, suggesting an AI regulation of miR-125b. As shown in SI Fig. 13 and in our previous study (18), the AI cds sublines overexpress HER2. This epidermal growth factor receptor activates the AR pathway in the absence of androgens (27). The HER2-AR signal pathway might therefore be involved in the up-regulation of miR-125b. Further characterization of AD and AI regulations of miRNA expression is currently in progress in our group.
Some aberrantly expressed miRNAs have been shown to contribute to the pathogenesis of human cancer by targeting Bcl2 family members. For example, miR-15 and miR-16, which are down-regulated in chronic lymphocytic leukemia have been reported to inhibit Bcl2 expression, whereas miR-29b, down-regulated in cholangiocarcinoma cells, targets MCL-1, another anti-apoptotic Bcl2 family member (32). In this study, we identified Bak1 as a target of miR-125b that is up-regulated in CaP cells. Three lines of evidence support this finding. First, there is a miR-125b binding site in the 3′ UTR of BAK1. Second, transfection of miR-125bm reduced BAK1 mRNA and protein levels. Third, BAK1 3′ UTR-mediated luciferase activity is specifically responsive to transfected miR-125bm. Bak1 is a Bcl2 protein family member, and functions as a proapoptotic regulator. In previous studies, Bak1 and Bax were reported to regulate apoptotic signaling in CaP synergistically. Knockdown of Bak1 and Bax completely prevented the apoptosis induced by a variety of biological molecules and chemical compounds (33–36). Bak1 expression has also been reported to be associated with the progression of CaP. In a clinical study, Bak1 was detected in 77.5% primary and untreated localized CaP, but in only 33% hormone-refractory CaPs (37). Therefore, down-regulation of Bak1 by miR-125b may contribute to disease progression and resistance to treatment in CaP. However, whereas Bak1 is a direct target of miR-125b, knockdown of Bak1 expression using specific siRNA did not result in significantly increased proliferation of LNCaP and cds1 cells (data not shown). This phenomenon suggests that down-regulating only Bak1 by miR-125b probably cannot induce an AI growth, and a combination of Bak1 with other miR-125b targets may contribute to the cell phenotype. Indeed, a search of the Sanger miRNA target database reveals 414 putative target candidates for miR-125. It is very likely that only a small fraction of predicted targets may be true targets in human cancers (38). Furthermore, validation of those true targets in CaP would be a daunting task. But even so, it would be worthwhile to identify and characterize them. By using microarray analysis of global gene differential expression, we have identified another potential miR-125b target, SGPL1, that may be involved in tumor suppressor and cancer-surveillance pathways (39). We are currently determining the involvement of this gene in CaP. With identification of more miR-125b targets in CaP, we believe that this study will lead to a better understanding of the mechanisms involved in miR-125b-mediated development and progression of this disease.
Materials and Methods
Reagents.
Unlabeled and Fitc-labeled LNA-miR-125b probe and the miRNA negative control were purchased from Exiqon Inc. Four synthetic, chemically modified short single- or double-stranded RNA oligonucleotides: premiR-125b molecule (miR-125bm), premiRNA negative control (miR-NC), anti-miR-125b and anti-miRNA negative control (anti-miR-NC), as well as the pMIR-REPORT Luciferase vector, were purchased from Ambion. The miR-125bm mimics the product of Dicer cleavage and increases the activity of miR-125b. The anti-miR-125b was designed to specifically bind to endogenous miR-125b, inhibiting its activity but not down-regulating its abundance.
Cell Lines.
Two AD human CaP cell lines (PC-346C and LNCaP), five AI CaP cell lines (DU145, PC3, CWR22R, cds1, and cds2), and two immortalized benign prostatic epithelial cell lines (pRNS-1-1 and RWPE-1) were used in this study. The PC-346C line was kindly provided by W. M. van Weerden (Erasmus University, Rotterdam, The Netherlands), the pRNS-1-1 cell line was provided by J. S. Rhim (University of the Health Sciences, Bethesda, MD), and the RWPE-1 was provided by M. M. Webber (Michigan State University, East Lansing, MI). Both cds1 and cds2 cell lines are derivatives of LNCaP (18).
Northern Blot Analysis.
Total RNA was isolated from cultured cells with TRIzol reagent (Invitrogen). Northern blotting was performed as described in ref. 40. Briefly, ≈15 μg of RNA per sample was resolved on a 15% Urea-PAGE gel, and transferred to a nylon membrane. After being UV-cross-linked and baked at 80°C for 45 min, the membrane was prehybridized at 42°C for 45 min and then hybridized with 32P-labeled probes at 35°C overnight. After being washed, the membrane was exposed to x-ray film. The signal was quantified by gel scanning. The membrane was stripped and rehybridized twice.
In Situ Hybridization.
For cell lines, cells were grown on sterile slides in 100-mm Petri dishes for 48 h. If androgen stimulation was required, overnight-cultured cells were treated by using 1.0 nM R1881 for an additional 24–48 h. These cells were then stained by using Fitc-labeled LNA-miR-125b probe following the manufacturer's protocol (www.exiqon.com). Nuclei were routinely stained by using DAPI. For formalin-fixed, paraffin-embedded prostate tissues, a 5-μm section was first stained with hematoxylin and eosin (H&E) to identify the tumor areas. The tumor section was deparaffinized and hydrated, followed by treatment with proteinase K and refixation in 4% paraformaldehyde. After PBS-washing and air-drying, the section was hybridized with 10 nM DIG-labeled LNA-miR-125b probe that was prepared by using a DIG-3′-end labeling kit (Roche). The section was then incubated overnight at 4°C in buffer containing anti-DIG-Ab Fab′ fragment (Roche), followed by staining with NBT and BCIP (Promega). The reaction was stopped with PBS and mounted for imaging. The abundance of miR-125b was categorized as high, moderate, or low levels based on the intensity of staining.
ChIP Assay.
ChIP assays were performed as described in ref. 41. Briefly, chromatin DNA was precleaned by using normal rabbit serum (IgG) and protein G-Sepharose beads, and then immunoprecipitated with rabbit anti-AR antibody (PG-21, Upstate). The primers of 5′-GCTGTCTGAATAGTTTGGAG (forward) and 5′-CCCATTACACTGCTATACAG (reverse) were used for amplifying the −558/-368 fragment in ARE cluster A, and 5′-CGCATATAGTAGGTTGTCTC (forward) and 5′-TTGTAAGACCTGCCAGGATC (reverse) for the −2666/-2388 fragment in ARE cluster C.
Cell Proliferation Assay.
Cells (3.5 × 103 per well) were plated in 96-well plates in androgen-depleted medium. After being cultured for 1 or 2 days, cells were transfected with miR-125bm, miR-NC, anti-miR-125b, or anti-miR-NC, at a dose of 50 or 100 nM using lipofectamine 2000 (Invitrigen). The transfection was optimized by using a fluorescent pEGFP-N1 vector (Clontech), and our optimized protocol resulted in the transfection of >90% of LNCaP and cds1 cells. Tetrazolium salt (WST-1) cell proliferation assay was then carried out at various days after transfection.
Luciferase Assay.
To construct a reporter plasmid containing the 3′ UTR of BAK1, a 1.1-kb DNA fragment of the 3′ UTR of BAK1 was amplified from LNCaP genomic DNA and cloned into the pMIR-REPORT Luciferase vector downstream of the Luciferase gene in a sense or antisense direction. All PCR products cloned into the plasmid and the cloning directions were verified by DNA sequencing. For the luciferase assay, DU145 cells (4 × 104 per well) were seeded into 24-well plates and cultured for 24 h. The cells were then fed with fresh medium and cotransfected with pMIR-REPORT luciferase reporters, miR-125bm or miR-NC. Two days later, cells were harvested and lysed with passive lysis buffer (Promega). Luciferase activity was measured by using a dual luciferase reporter assay (Promega). The pRL-SV40 Renilla luciferase plasmid (Promega) was used as an internal control. The results were expressed as relative luciferase activity (LUC/Renilla LUC).
Supplementary Material
Acknowledgments
We thank Dr. Arline D. Deitch for editorial assistance and Dr. Regina Gandour-Edwards for reviewing the clinical prostate samples. This work was supported by National Cancer Institute Grant CA92069-NCI.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706641104/DC1.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Santos AF, Huang H, Tindall DJ. Steroids. 2004;69:79–85. doi: 10.1016/j.steroids.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Abdulkadir SA. Ann NY Acad Sci. 2005;1059:33–40. doi: 10.1196/annals.1339.018. [DOI] [PubMed] [Google Scholar]
- 4.Downing SR, Russell PJ, Jackson P. Can J Urol. 2003;10:1924–1933. [PubMed] [Google Scholar]
- 5.Sharifi N, Farrar WL. Am J Ther. 2006;13:166–170. doi: 10.1097/00045391-200603000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Shand RL, Gelmann EP. Curr Opin Urol. 2006;16:123–131. doi: 10.1097/01.mou.0000193384.39351.64. [DOI] [PubMed] [Google Scholar]
- 7.Lai EC. Nat Genet. 2002;30:363–364. doi: 10.1038/ng865. [DOI] [PubMed] [Google Scholar]
- 8.Lewis BP, Burge CB, Bartel DP. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 9.Xie X, Lu J, Kulbokas EJ, Golub TR, Mootha V, Lindblad-Toh K, Lander ES, Kellis M. Nature. 2005;434:338–345. doi: 10.1038/nature03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calin GA, Croce CM. Nat Rev. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 11.Hernando E. Clin Transl Oncol. 2007;9:155–160. doi: 10.1007/s12094-007-0029-0. [DOI] [PubMed] [Google Scholar]
- 12.Chen CZ. N Engl J Med. 2005;353:1768–1771. doi: 10.1056/NEJMp058190. [DOI] [PubMed] [Google Scholar]
- 13.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et al. Proc Natl Acad Sci USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, et al. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 16.Jiang J, Lee EJ, Gusev Y, Schmittgen TD. Nucleic Acids Res. 2005;33:5394–5403. doi: 10.1093/nar/gki863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vester B, Wengel J. Biochemistry. 2004;43:13233–13241. doi: 10.1021/bi0485732. [DOI] [PubMed] [Google Scholar]
- 18.Shi XB, Ma AH, Tepper CG, Xia L, Gregg JP, Gandour-Edwards R, Mack PC, Kung HJ, DeVere White RW. Prostate. 2004;60:257–271. doi: 10.1002/pros.20039. [DOI] [PubMed] [Google Scholar]
- 19.Lee YS, Kim HK, Chung S, Kim KS, Dutta A. J Biol Chem. 2005;280:16635–16641. doi: 10.1074/jbc.M412247200. [DOI] [PubMed] [Google Scholar]
- 20.Shi XB, Xue L, Tepper CG, Gandour-Edwards R, Ghosh P, Kung HJ, DeVere White RW. Prostate. 2007;67:591–602. doi: 10.1002/pros.20544. [DOI] [PubMed] [Google Scholar]
- 21.van Bokhoven A, Varella-Garcia M, Korch C, Johannes WU, Smith EE, Miller HL, Nordeen SK, Miller GJ, Lucia MS. Prostate. 2003;57:205–225. doi: 10.1002/pros.10290. [DOI] [PubMed] [Google Scholar]
- 22.Li C, Wong WH. Proc Natl Acad Sci USA. 2001;98:31–36. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP, Liu CG, Bhatt D, Taccioli C, Croce CM. J Am Med Assoc. 2007;297:1901–1908. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 24.Nelson PT, Baldwin DA, Kloosterman WP, Kauppinen S, Plasterk RH, Mourelatos Z. RNA. 2006;12:187–191. doi: 10.1261/rna.2258506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonoki T, Iwanaga E, Mitsuya H, Asou N. Leukemia. 2005;19:2009–2010. doi: 10.1038/sj.leu.2403938. [DOI] [PubMed] [Google Scholar]
- 26.Scott GK, Goga A, Bhaumik D, Berger CE, Sullivan CS, Benz CC. J Biol Chem. 2007;282:1479–1486. doi: 10.1074/jbc.M609383200. [DOI] [PubMed] [Google Scholar]
- 27.Craft N, Shostak Y, Carey M, Sawyers CL. Nat Med. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 28.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borchert GM, Lanier W, Davidson BL. Nat Struct Mol Biol. 2006;13:1097–1101. doi: 10.1038/nsmb1167. [DOI] [PubMed] [Google Scholar]
- 30.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, et al. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 31.Lee Y, Jeon K, Lee JT, Kim S, Kim VN. EMBO J. 2002;21:4663–4670. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mott JL, Kobayashi S, Bronk SF, Gores GJ. Oncogene. 2007;26:6133–6140. doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Deng H, Basu I, Zhu L. Cancer Res. 2004;64:1377–1385. doi: 10.1158/0008-5472.can-03-2428. [DOI] [PubMed] [Google Scholar]
- 34.Castilla C, Congregado B, Chinchon D, Torrubia FJ, Japon MA, Saez C. Endocrinology. 2006;147:4960–4967. doi: 10.1210/en.2006-0502. [DOI] [PubMed] [Google Scholar]
- 35.Kim YA, Xiao D, Xiao H, Powolny AA, Lew KL, Reilly ML, Zeng Y, Wang Z, Singh SV. Mol Cancer Ther. 2007;6:1599–1609. doi: 10.1158/1535-7163.MCT-06-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao D, Lew KL, Kim YA, Zeng Y, Hahm ER, Dhir R, Singh SV. Clin Cancer Res. 2006;12:6836–6843. doi: 10.1158/1078-0432.CCR-06-1273. [DOI] [PubMed] [Google Scholar]
- 37.Yoshino T, Shiina H, Urakami S, Kikuno N, Yoneda T, Shigeno K, Igawa M. Clin Cancer Res. 2006;12:6116–6124. doi: 10.1158/1078-0432.CCR-06-0147. [DOI] [PubMed] [Google Scholar]
- 38.Zhu S, Si ML, Wu H, Mo YY. J Biol Chem. 2007;282:14328–14336. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 39.Bandhuvula P, Saba JD. Trends Mol Med. 2007;13:210–217. doi: 10.1016/j.molmed.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al. Proc Natl Acad Sci USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Louie MC, Yang HQ, Ma AH, Xu W, Zou JX, Kung HJ, Chen HW. Proc Natl Acad Sci USA. 2003;100:2226–2230. doi: 10.1073/pnas.0437824100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.