Abstract
Autosomal dominant familial isolated hypoparathyroidism (AD-FIH) is caused by a Cys → Arg mutation (C18R) in the hydrophobic core of the signal peptide of human preproparathyroid hormone (PPTH). Although this mutation impairs secretion of the hormone, the mechanism by which one mutant allele produces the autosomal-dominant disease is unexplained. Using transfected HEK293 cells, we demonstrate that the expressed mutant hormone is trapped intracellularly, predominantly in the endoplasmic reticulum (ER). This ER retention was found to be toxic for the cells, which underwent apoptosis, as evident from the marked increase in the number of cells staining positive for Annexin V binding and for the TUNEL reaction. The cells producing mutant hormone also had marked up-regulation of the ER stress-responsive proteins, BiP and PERK, as well as the proapoptotic transcription factor, CHOP. Up-regulation of these markers of the unfolded protein response supported a causal link between the ER stress and the cell death cascade. When the C18R PPTH was expressed in the presence of 4-phenylbutyric acid, which is a pharmacological chaperone, intracellular accumulation was reduced and normal secretion was restored. This treatment also produced remarkable reduction of ER stress signals and protection against cell death. These data implicate ER stress-induced cell death as the underlying mechanism for AD-FIH and suggest that the pharmacological manipulation of this pathway by using chemical chaperones offers a therapeutic option for treating this disease.
Keywords: endoplasmic reticulum stress, parathyroid hormone, unfolded protein response, 4-phenylbutyric acid
Familial isolated hypoparathyroidism (FIH) represents a heterogeneous group of genetic disorders characterized by low calcium and high phosphorus levels in the blood serum, where the amount of immunoreactive parathyroid hormone (PTH) is low or undetectable. Clinical symptoms include neuromuscular irritability, tingling of the fingers and toes, and spontaneous or latent tetany (1, 2). These diseases vary in mode of inheritance. Mutations in the preproparathyroid hormone (PPTH) gene are found to be associated with the disease in some pedigrees (3–5).
PPTH is synthesized as a 115-aa precursor in the parathyroid gland and is converted to the pro-PTH upon removal of the signal peptide (by signal peptidase) within the endoplasmic reticulum (ER). After trafficking to the Golgi apparatus, the pro-PTH is cleaved to the 84-aa mature PTH (Fig. 1). Then the mature PTH is packaged into secretory granules and, depending on needs, released into the blood, where it functions as a major regulator of the calcium level. The release of the PTH by the parathyroid gland is strictly calcium-dependent (6).
Fig. 1.
Primary sequence of human PTH and organization of its domains. The pre (signal sequence), pro (black box), and mature regions (boxed) of PTH are labeled. The arrowhead points toward the Cys residue (C18) that is replaced by an Arg (R) in some AD-FIH patients (3).
In 1990, Arnold et al. (3) identified a T to C point mutation in the signal peptide-encoding region of the PPTH gene in a family with autosomal dominant FIH (AD-FIH) (7). This mutation disrupts the hydrophobic core of the signal sequence by changing the codon at position 18 (−8 position with respect to the signal cleavage site) from a highly conserved cysteine to arginine (Fig. 1). In the presence of microsomal membranes, the in vitro-translated nascent C18R PPTH showed impaired translocation and processing to its pro form (8). Transient expression in COS-7 cells confirmed the processing and secretion defect of the mutant hormone. However, when cotransfected with wild-type PPTH cDNA, the C18R mutant failed to demonstrate any apparent interfering effect on the secretion of its wild-type counterpart (8). Therefore, how this mutation in only one allele could mechanistically explain the observed dominant phenotype of the disease remained unclear.
In several genetic disorders, aberrant intracellular accumulation of mutant proteins has been reported to cause cellular toxicity and cell death (9, 10). Instances are known (e.g., in diseases like Alzheimer's, Parkinson's, Huntington's, and type 1 diabetes) where a mutation results in the accumulation of a conformationally defective protein in the ER and activates several signaling pathways, collectively known as the unfolded protein response (UPR), which ultimately contributes to disease progression through apoptosis (11–14). Two recent examples illustrate that a protein with a signal sequence mutation can undergo faulty processing, which may play a critical role in cellular toxicity and thus cause a dominant disease (15, 16). In this study, we used the transiently transfected HEK293 cell culture system to test this model for explaining the pathogenesis of AD-FIH. We provide evidence that the C18R PPTH is accumulated abnormally in the ER and causes cell death by the ER stress-induced apoptotic pathway. In addition, we corrected the intracellular accumulation of the mutant hormone by using a chemical chaperone, 4-phenylbutyric acid (PBA). This correction was accompanied by attenuation of expression of ER stress markers and protection from cell death. These studies provide mechanistic insight into the C18R PPTH-induced AD-FIH and suggest a strategy for therapeutic intervention in this disease.
Results
Signal Sequence Mutation in PPTH Impairs Hormone Secretion, Resulting in Intracellular Accumulation.
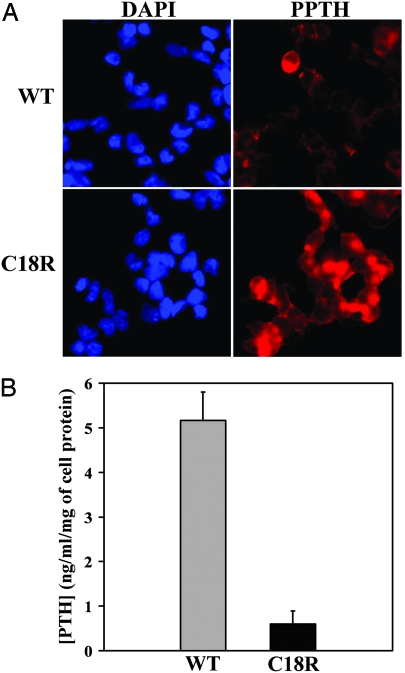
To analyze the intracellular expression and secretion of the mature hormone in the culture media, the wild-type and C18R PPTH cDNAs were transfected into HEK293 cells. Immunohistochemical analysis of the transfected cells demonstrated increased intracellular accumulation of the PTH-species in C18R PPTH-transfected cells, compared with the wild-type (Fig. 2A). That this discrepancy is not because of differential transcription was confirmed by quantitative RT-PCR, which showed comparable levels of expression of wild-type and mutant mRNAs (data not shown). Culture media collected from the wild-type and C18R PPTH-transfected cells were compared for the presence of immunoreactive hormone (Fig. 2B). The concentration of PTH in the media from C18R PPTH-transfected cells was only ≈10% (0.6 ng·ml−1 per milligram of cell protein) of that from cells transfected with the wild type (5.16 ng·ml−1 per milligram of cell protein). These data suggest that the mutation directly interferes with the processing and secretion of the hormone, rather than affecting gene expression. These results are consistent with earlier studies that monitored the gene product by metabolic labeling (8).
Fig. 2.
C18R PPTH-transfected cells exhibit abnormal intracellular accumulation and reduced secretion of PTH. (A) HEK293 cells were transfected with wild-type or C18R PPTH-expressing cDNAs. Seventy-two hours after transfection, the cells were fixed and immunostained with polyclonal antibody against PTH, followed by TRITC-conjugated (red) secondary antibody and visualized by fluorescence microscopy. Nuclear staining with DAPI (blue) accounted for the total number of cells in the field. (B) Concentration of the secreted PTH in the media from the wild-type or C18R PPTH-transfected cells were measured by competitive ELISA using standard PTH peptide provided with the kit. The actual values were normalized with respect to total cell protein, and the data are presented as mean ± SD of three independent media samples.
Induction of Apoptosis in the Cells Producing the C18R Mutant Hormone.
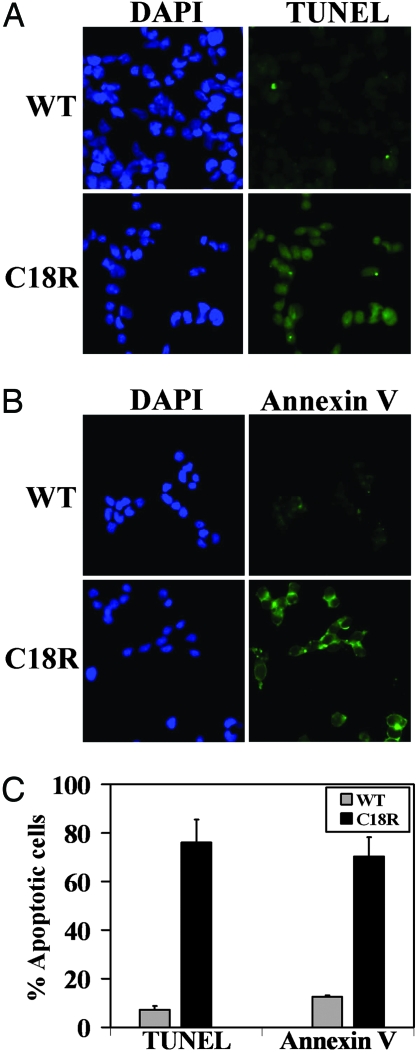
We next asked whether the intracellular accumulation of C18R PPTH can induce cell death. To address this question, we studied apoptosis in transfected cells by TUNEL assay and Annexin V-FITC labeling. Expression of C18R PPTH promoted apoptosis as evidenced by >75% TUNEL-positive cells (indicative of nuclear DNA fragmentation). In contrast, only 7% of the wild-type PPTH-transfected cells were apoptotic (P < 10−6) (Fig. 3 A and C). By Annexin V-FITC labeling, we compared the number of cells with surface-exposed phosphatidylserine (PS), which allows Annexin V binding to the cell surface, another hallmark of cells that have entered the apoptotic pathway. Annexin V binding was observed in >70% of cells expressing the mutant hormone, compared with only 12% of the cells transfected with wild-type PPTH (P < 0.002) (Fig. 3 B and C). These studies demonstrate the ability of the signal sequence mutation to induce apoptosis in hormone-producing cells.
Fig. 3.
Induction of apoptosis in cells expressing C18R PPTH. (A and B) TUNEL staining (A) or Annexin V labeling (B) of the cells transfected with either wild-type or C18R PPTH cDNA, indicating increased apoptosis in cells expressing the mutant hormone. (C) Percentage of TUNEL- or Annexin V-stained cells were calculated with respect to the total number of DAPI (blue) stained cells in a field. Gray and black bars represent cells transfected with wild-type or C18R PPTH cDNAs, respectively. Data are presented as mean ± SD of three fields from different experimental setups.
Evidence for ER Stress and UPR Activation in C18R PPTH-Expressing Cells.
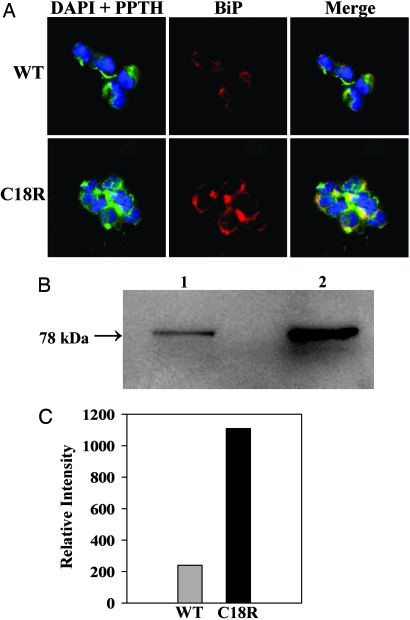
To determine whether apoptotic death of the cells expressing the mutant hormone is correlated with ER stress, we performed immunohistochemical staining with antibodies against PPTH and Grp-78/BiP, an ER-resident chaperone that is elevated during ER stress (17). We observed that the PTH species in C18R PPTH- transfected cells is predominantly distributed in the perinuclear region with punctate staining that is colocalized (as yellow spots) with BiP, confirming that C18R PPTH is indeed retained in the ER (Fig. 4A). Furthermore, our results demonstrate that the cells transfected with the C18R PPTH cDNA are more intensely stained for BiP expression than those transfected with the wild-type cDNA (Fig. 4A). Additional evidence for the up-regulation of BiP was provided by Western blot analysis showing ≈5-fold enhancement in BiP expression in the C18R PPTH-transfected cells, compared with the wild type (Fig. 4 B and C).
Fig. 4.
Colocalization of the retained C18R PPTH and the up-regulated BiP. (A) The cells transfected with either wild-type or C18R PPTH cDNA were coimmunostained with anti-PTH and anti-BiP primary antibodies, followed by secondary antibodies conjugated with Alexa Fluor 488 (green) or Alexa Fluor 546 (red), respectively. (Left and Center) The slides were visualized for DAPI (blue) and PPTH (green) and BiP (red). (Right) A merged image is shown. In C18R PPTH-transfected cells, note the more intense staining of BiP and its colocalization with the hormone (yellow spots). (B) Lysate prepared from wild-type (lane 1) or C18R PPTH (lane 2) cDNA-transfected cells were subjected to Western blot analysis (20 μg protein per lane) with anti-BiP primary and HRP-conjugated secondary antibody. (C) Densitometry analysis of the blot showing 5-fold up-regulation of BiP in C18R PPTH-expressing cells.
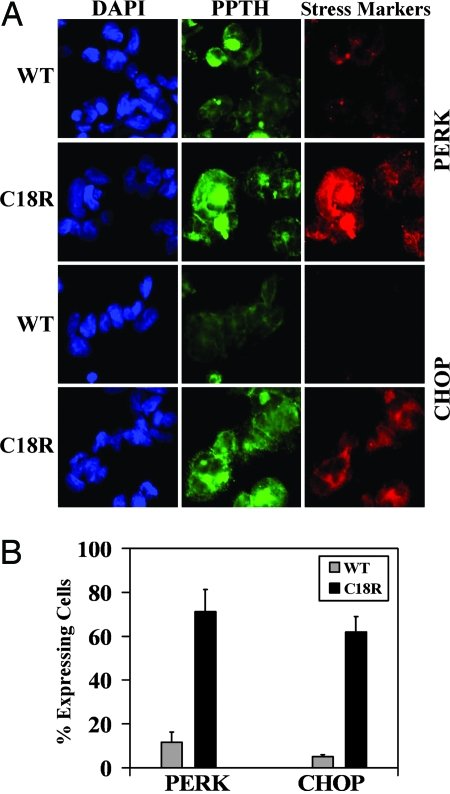
Under sustained ER stress, eukaryotic cells trigger multiple signaling pathways, collectively known as the UPR, to maintain homeostasis (18). Fig. 5 shows that two critical markers of UPR, PERK and CHOP, are robustly up-regulated in cells expressing the mutant hormone (in 71% and 62% cells, respectively). In contrast, the number of wild-type cells expressing immunodetectable levels of these markers is very low (11% and 5% cells, respectively) (P < 0.05 for both). These data suggest that ER accumulation of the mutant hormone results in the activation of the UPR-related genes that are indicative of ER stress.
Fig. 5.
Up-regulation of the UPR markers (PERK and CHOP) in cells expressing C18R PPTH. (A) In a separate set of experiments, the status of PERK and CHOP were analyzed immunohistochemically in cells transfected with wild-type or C18R PPTH cDNA by using respective primary and appropriately labeled secondary antibodies. The slides were visualized for DAPI (blue), PPTH (green), and PERK/CHOP (red). Up-regulation of both the markers is clearly visible in C18R PPTH-expressing cells. (B) Quantification of the results by counting cells positive for PERK or CHOP from three fields of different experimental setups. Values expressed as percentage of cells positive for the presence of the hormone (gray bar, wild-type; black bar, C18R PPTH).
PBA Corrects the Secretion Defect of C18R PPTH.
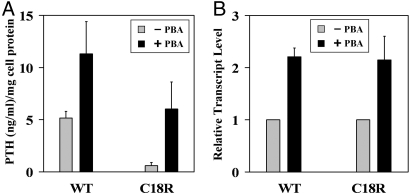
PBA has been shown to rescue several misfolded, trafficking-defective secretory proteins (19, 20). Accordingly, we explored whether this chemical chaperone might be effective in correcting the secretory defect and/or cytotoxicity of the mutant PPTH. Upon treatment of the transfected cells with 2 mM PBA, we observed increased secretion of PTH into the media, both in wild-type and C18R PPTH-transfected cells (2- and 10-fold, respectively) (Fig. 6A). Actually, PBA restored secretion of the hormone by C18R PPTH-expressing cells almost to the level that is normal for the wildtype. This increased secretion in the presence of PBA was accompanied by a significant reduction in intracellular immunoreactive PTH species, as determined by immunohistochemical analysis (data not shown). Because PBA has been reported to be a regulator of transcription for many genes (21), we used quantitative RT-PCR to test whether the increased secretion was because of enhanced transcription. Results presented in Fig. 6B demonstrate that PBA treatment produced a 2-fold increase in transcript levels for both wild-type and mutant hormones. These data suggest that increased transcription in the presence of PBA could account for most of the increase in secretion of the wild-type hormone. However, the ≈10-fold enhancement in secretion by cells expressing the mutant hormone suggests that enhanced posttranslational processing accounts for a larger fraction of the enhanced secretion by cells transfected with C18R PPTH than does the increased transcription.
Fig. 6.
Effect of PBA on expression and secretion of hormone in transfected cells. (A) PTH concentrations in the media collected from cells transfected with wild-type or C18R PPTH cDNA in the presence (black bar) or absence (gray bar) of 2 mM PBA were measured by competitive ELISA. The actual concentrations were normalized with respect to total cell protein, and the data are presented as mean ± SD of three independent media samples. (B) RNA isolated from cells transfected with wild-type or C18R PPTH cDNA in the presence (black bar) or absence (gray bar) of 2 mM PBA were subjected to qualitative RT-PCR. Human β-actin was used as a reference to normalize the relative transcript levels. Reactions were done in triplicate, and data are presented as mean ± SD (see Materials and Methods for details).
PBA Protects the C18R PPTH-Expressing Cells from ER Stress and Apoptosis.
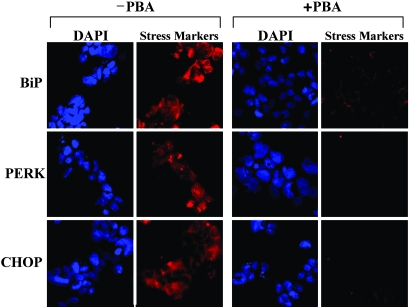
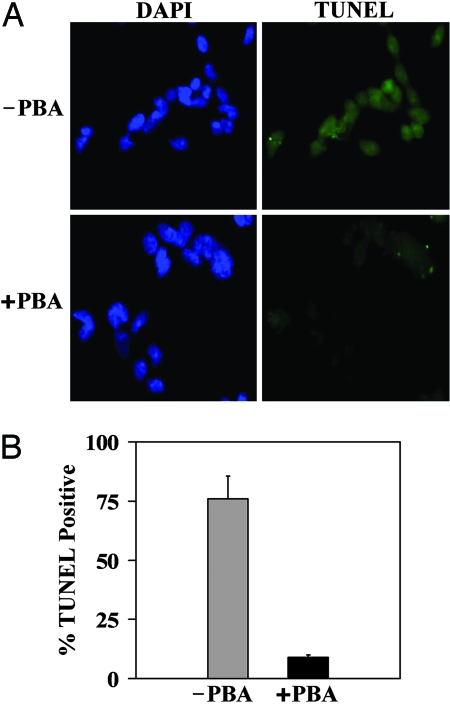
Because PBA enhanced secretion and reduced the intracellular accumulation of misfolded hormone, we hypothesized that PBA would reduce ER stress and apoptosis. To test this hypothesis, we compared markers of ER stress and apoptosis in C18R PPTH-transfected cells in the presence and absence of 2 mM PBA by immunohistochemistry with the respective antibodies (Fig. 7). Dramatic reduction of all ER stress markers (BiP, PERK, and CHOP) was observed in the PBA-treated cells when compared with untreated controls. Similarly, upon treatment with PBA, we found that there was a drastic reduction (from 75% to <10%) (P < 0.002) in the number of C18R PPTH-producing, TUNEL-positive cells (P < 0.002) (Fig. 8). Thus, PBA prevented cells expressing the mutant hormone from entering the apoptotic pathway by reducing the accumulation of unfolded mutant hormone.
Fig. 7.
PBA attenuates ER stress in C18R PPTH-producing cells. ER stress/UPR markers (BiP, PERK, and CHOP) were analyzed immunohistochemically in cells transfected with C18R PPTH cDNA in the presence and absence of 2 mM PBA by using the respective primary antibodies and appropriately labeled secondary antibodies. The slides were visualized for DAPI-stained nucleus (blue) and BiP/PERK/CHOP (red).
Fig. 8.
PBA protects C18R PPTH-expressing cells from apoptosis. (A) Apoptosis was compared by TUNEL staining in cells transfected with C18R PPTH cDNA in the presence or absence of PBA. (B) Percentages of apoptotic cells with respect to total DAPI (blue)-stained cells were calculated from three fields from different transfections and values expressed as mean ± SD.
Discussion
The primary objective of this study was to clarify the pathogenic mechanism of AD-FIH caused by the signal sequence mutation (C18R) in PPTH. Here we confirm evidence that this processing-defective PPTH accumulates intracellularly, predominantly within the ER of transfected cells, and we show further that this accumulation results in their apoptotic death from chronic ER stress. Furthermore, we found that PBA, a chemical chaperone, could substantially correct the abnormal intracellular accumulation of the mutant hormone and the toxicity associated with it. Our findings suggest that the dominant phenotype of the disease can be attributed to the loss of mutant hormone producing cells through apoptosis.
Mutations in the signal sequence of the PPTH gene have been reported in two other families with FIH (4, 5). One involved a splice site mutation, which deleted exon 2 encoding the entire signal sequence (4). The other involved a Ser–Pro change at the −3 position relative to the signal sequence cleavage site. Both mutations produced a recessive form of the disease that was thought to be a consequence of insufficient secretion of the circulating hormone in homozygous individuals. However, this mechanism is inapplicable for the C18RPPTH-induced FIH, where only one mutant allele can cause the disorder even in the presence of the normal counterpart. Extensive in vitro translation studies (8) provided evidence that this mutation induces abnormalities in multiple steps of PTH processing and maturation. It was initially speculated that the mutant PPTH is not only processing-defective, but also interferes with the processing of the wild-type hormone and other secretory proteins. Such a dominant-negative trans effect has been reported for the Δ32–71 mutant growth hormone and several rhodopsin mutants (22, 23). However, expression of the C18R PPTH failed to demonstrate such a dominant effect on cotransfecting wild-type PPTH (8). Although one cannot rule out the possibility that this mutant hormone might exert some interfering effect under in vivo conditions, the existing data from cell culture models provided no support for this hypothesis. Instead, our finding that the C18R PPTH can inflict cellular injury and cause cell death by the apoptotic pathway provides a more plausible mechanistic explanation for the dominant phenotype of the disease. In the dying cells, the ER stress markers, BiP and PERK, and the proapoptotic transcription factor, CHOP, were clearly up-regulated, suggesting continuous overload of the ER with the unprocessed mutant hormone.
The ER is the site of synthesis, folding, posttranslational processing, and transport of secretory and membrane-bound proteins in eukaryotic cells (24). A compromised protein-folding environment can result in ER stress, to which the cells respond by triggering UPR signaling pathways (17). However, if the ER stress is excessive or prolonged, pathways leading to cell death are activated (25). These pathways have been suggested to be involved in the pathogenesis of many protein-folding diseases. In most of these cases, a conformation-destabilizing mutation in the primary sequence of the mature protein appears to be the culprit (26). Instances of cytotoxic insults caused by a mutation in the signal sequence of the protein have been reported for two dominantly inherited human diseases, neurohypophyseal diabetes insipidus and one form of retinitis pigmentosa (RP17). These diseases are caused by a mutation in the signal sequences of the preprovasopressin and carbonic anhydrase IV genes, respectively. As we found here for C18R PPTH, both mutant proteins exhibited impaired processing and induced ER stress. Patients carrying these mutations suffer from progressive degeneration of the associated tissues (neurohypophyseal neurons and retina, respectively) (15, 16). We envisage a similar mechanism for the pathogenesis of AD-FIH and propose that ER stress leading to apoptosis in parathyroid tissue is the primary reason for the dominant manifestation of the disease.
In recent years, chemical chaperones have emerged as attractive candidates for rescuing trafficking-defective proteins perhaps by stabilizing improperly folded intermediates (27). We hypothesized that the general chemical chaperone, PBA, would correct the apoptosis-inducing effects of the C18R mutation in PPTH. The selection of PBA was based on its Food and Drug Administration approval for use in certain unrelated metabolic diseases and relatively low toxicity and successful application in other disease models (20, 28). In the presence of PBA, C18R PPTH appears to be efficiently processed, as evidenced from its improved secretion from barely detectable to almost normal levels in the media. Clearance of the intracellularly trapped hormone, alleviation of ER stress, and reduction in cell death were all seen in the PBA-treated cells. These results suggest that treatment with a chemical chaperone might provide an effective strategy for clinical management of this disease.
PBA also increased secretion in cells expressing the wild-type hormone. The quantitative RT-PCR measurements showing increased transcript levels suggest that this enhanced secretion results from a transcriptional enhancement, rather than from improvements in posttranslational processing of the wild-type hormone.
Although our data support the therapeutic potential of PBA or a similar chemical chaperone in AD-FIH, the precise mechanism by which it acts remains to be clarified. Earlier studies showed that the C18R mutation interfered with the targeting and processing of the signal sequence by ER membranes (8). Thus, the ER is a likely target for the action of PBA. Because PBA has been reported to prevent aggregation of at least one protein under in vitro conditions (29), it is plausible that PBA might directly interact with the mutant hormone and induce a conformational change, which favors its efficient processing in the ER by membrane-bound signal peptidases. An alternate hypothesis is that PBA, in its capacity as a transcriptional regulator (21, 30), alters the expression of chaperones or cochaperones that enhance the processing of the mutant hormone. In fact, up-regulation of chaperone proteins, including heat shock proteins 90 and 70, was observed in microarray analyses of the transcript levels in PBA-treated bronchial epithelial cells (IB3–1) (21). In a less likely scenario, PBA or the up-regulated chaperones could accompany PPTH to the Golgi, where the prepro sequences could be cleared off by the usual convertases. The cell culture model described here should be useful in distinguishing between these hypotheses and clarifying the mechanism of corrective action of PBA in this disorder and other protein-folding diseases.
Materials and Methods
Plasmids, Antibodies, and Other Reagents.
The plasmids (pCDM-PTH-1 and pCDM-PTH-190) containing the wild-type and C18R mutant human PPTH cDNA, respectively (3), were kindly provided by Thomas Gardella and Henry M. Kronenberg (Massachusetts General Hospital, Boston, MA). Rabbit anti-human PTH antibody and PTH immunoassay kit were purchased from Bachem Peninsula Laboratories. Goat anti-BiP, goat anti-PERK, mouse anti-CHOP, chicken anti-rabbit IgG-Alexa Fluor 488, donkey anti-goat IgG-Alexa Fluor 546, and goat anti-mouse IgG-Alexa Fluor 546 were from Santa Cruz Biotechnology. Goat anti-rabbit IgG-peroxidase, goat anti-rabbit IgG-tetramethylrhodamine isothiocyantate (TRITC), and 4-PBA were from Sigma–Aldrich.
HEK293 Cell Culture and Transient Transfection.
HEK293 cells (American Type Culture Collection) were grown in DMEM (Cell Culture Systems) supplemented with l-glutamine and 10% heat-inactivated FBS. Cells grown in P-60 plates or chamber slides were transfected with 5 or 1 μg of cDNA, respectively, by using Transfast transfection reagent (Promega) according to the manufacturer's suggested protocol. At 72 h after transfection, the cells were either harvested or processed for immunohistochemistry. Media were collected for further analysis wherever necessary. RNA also was isolated at this time point. Where indicated, cells were incubated with 2 mM PBA during the entire posttransfection period.
SDS/PAGE and Western Blot Analysis.
The cell lysates, produced by sonication of HEK293 cells in ice-cold PBS (pH 7.5) containing protease inhibitors (1 mM phenylmethanesulfonyl fluoride, 1 mM ortho-phenanthroline, 1 mM EDTA, and 1 mM benzamidine chloride), were analyzed by SDS/PAGE under nonreducing conditions according to Laemmli's procedure (31). The protein concentration of each cell lysate was determined by micro Lowry assay using BSA as a standard (32).
Measurement of Secreted PTH.
The secreted PTH in the culture media was quantified by using a competitive immunoassay kit according to the supplier's recommendation with appropriate controls and standards. The total amount of cell protein for each sample was measured to allow normalization. Each assay was repeated at least three times, and standard deviations were calculated.
RNA Extraction and Quantitative RT-PCR.
Total RNA was extracted from the cultured cells by using RNA-Bee RNA isolation reagent (Tel-Test) following the manufacturer's protocol. Then ≈1 μg of the total RNA was exposed to RNase-free DNase I (Invitrogen) for 20 min at room temperature to remove DNA contamination. The DNase-treated RNA was then reverse-transcribed by using oligo d(T)16 and MuLV reverse transcriptase (RT) (Applied Biosystems) in a final volume of 20 μl. To estimate the relative expression of the PPTH mRNAs, quantitative RT-PCR analysis was carried out by using the DNA Engine Opticon 2 continuous fluorescence detection system (Bio-Rad). The human β-actin gene was used as an endogenous control to normalize the results obtained with the PPTH gene expression. The oligonucleotide primers PTH forward (5′-GATGATACCTGCAAAAGACATGGC-3′) and PTH reverse (5′-CACTGGGATTTAGCTTTAGTTAATAC-3′) were used to amplify a 348-bp fragment of the PPTH gene. The primers β-actin forward (5′-AGCCATGTACGTAGCCAT CCAGGCTGTG-3′) and β-actin reverse (5′-TGGGTACATGGTGGTACCACCAGACAGC-3′) amplified the 533-bp fragment of the human β-actin gene. Each quantitative RT-PCR mixture contained 0.12 μl of ×100 SYBR Green I (Molecular Probes), 0.2 μM forward and reverse primers, 1.5 mM MgCl2, 0.5 μl of Taq DNA polymerase, and 5 μl of the respective RT reaction mixtures in a final volume of 25 μl. Appropriate negative controls were performed with reaction mixtures that lacked the RT. The thermal cycling conditions were 95°C for 5 min and 30 cycles of 95°C for 45 s, 60°C for 45 s, and 68°C for 45 s. All reactions were carried out in triplicate. The cycle threshold values of the samples were determined by using MJ Opticon monitor 3 software (Bio-Rad), and the relative transcript level of the target gene (PPTH) was calculated according to Pfaffl's method (33).
Immunohistochemical Staining and Detection of Apoptotic Cells.
HEK293 cells were transfected with wild-type or C18R PPTH cDNA. At 72 h after transfection, the cells were fixed with 2% paraformaldehyde for 40 min and permeabilized with 0.1% Triton X-100 for 2 min on ice. The cells were rinsed in PBS and incubated with 0.2% gelatin for 15 min to block nonspecific binding sites. Primary antibodies were used at 1:100 dilutions for BiP, PERK, and CHOP and at 1:200 for PPTH for 1.5 h at room temperature. All secondary antibodies were used at 1:200 dilutions for 1 h at room temperature. The slides were washed with 0.2% gelatin, followed by PBS and water. The slides were embedded in antiquencher solution (1,4-diazabicyclo[2.2.2]octane; Fluka/Sigma–Aldrich) containing DAPI, and coverslip edges were sealed with nail polish. The slides were examined, and images were captured on an Olympus Vanox AHBT3 fluorescence microscope integrated with an Olympus C-35AD-4 digital camera.
The apoptotic cells were detected by analyzing the extent of nuclear fragmentation by using a TUNEL assay kit (Roche Applied Science) and by the externalization of phosphatidylserine on the outer membrane of the cells monitored by the binding of Annexin V–FITC by using an Annexin-V–FLUOS staining kit (Roche Applied Science) according to the manufacturer's protocol.
Images were quantified by counting the cells positive for PPTH, ER stress-responsive proteins, and TUNEL/Annexin V-positive cells from three to five fields from at least three different transfections. The number of DAPI-stained cells accounted for the total cells in the field. For quantification of cells expressing the ER stress markers, the values were expressed as the percentage of cells positive for the presence of the hormone (to ensure that only the transfected cells are taken into account).
Statistical significance of the data were analyzed by Student's t test or one-way ANOVA by using the Epistat package, where P < 0.05 represents a statistically significant difference. The data are expressed as mean ± SD.
Acknowledgments
We thank Dr. Eduardo Slatopolsky (Washington University School of Medicine, St. Louis, MO) for the chicken anti-human PTH antibody, Drs. Thomas Gardella and Henry M. Kronenberg for wild-type and C18R PPTH plasmids, and Tracey Baird for editorial assistance. This work was supported by National Institutes of Health Grants DK40163 and GM34182 (to W.S.S.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Marx SJ. N Engl J Med. 2000;343:1863–1875. doi: 10.1056/NEJM200012213432508. [DOI] [PubMed] [Google Scholar]
- 2.Garfield N, Karaplis AC. Trends Endocrinol Metab. 2001;12:288–294. doi: 10.1016/s1043-2760(01)00435-0. [DOI] [PubMed] [Google Scholar]
- 3.Arnold A, Horst SA, Gardella TJ, Baba H, Levine MA, Kronenberg HM. J Clin Invest. 1990;86:1084–1087. doi: 10.1172/JCI114811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parkinson DB, Thakker RV. Nat Genet. 1992;1:149–152. doi: 10.1038/ng0592-149. [DOI] [PubMed] [Google Scholar]
- 5.Sunthornthepvarakul T, Churesigaew S, Ngowngarmratana S. J Clin Endocrinol Metab. 1999;84:3792–3796. doi: 10.1210/jcem.84.10.6070. [DOI] [PubMed] [Google Scholar]
- 6.MacGregor RR, Cohn DV. Clin Orthop Relat Res. 1978:244–258. [PubMed] [Google Scholar]
- 7.Ahn TG, Antonarakis SE, Kronenberg HM, Igarashi T, Levine MA. Medicine (Baltimore) 1986;65:73–81. [PubMed] [Google Scholar]
- 8.Karaplis AC, Lim SK, Baba H, Arnold A, Kronenberg HM. J Biol Chem. 1995;270:1629–1635. doi: 10.1074/jbc.270.4.1629. [DOI] [PubMed] [Google Scholar]
- 9.Dobson CM. Trends Biochem Sci. 1999;24:329–332. doi: 10.1016/s0968-0004(99)01445-0. [DOI] [PubMed] [Google Scholar]
- 10.Horwich A. J Clin Invest. 2002;110:1221–1232. doi: 10.1172/JCI16781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattson MP, Gary DS, Chan SL, Duan W. Biochem Soc Symp. 2001:151–162. doi: 10.1042/bss0670151. [DOI] [PubMed] [Google Scholar]
- 12.Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA. J Neurosci. 2002;22:10690–10698. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oyadomari S, Araki E, Mori M. Apoptosis. 2002;7:335–345. doi: 10.1023/a:1016175429877. [DOI] [PubMed] [Google Scholar]
- 15.Russell TA, Ito M, Ito M, Yu RN, Martinson FA, Weiss J, Jameson JL. J Clin Invest. 2003;112:1697–1706. doi: 10.1172/JCI18616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rebello G, Ramesar R, Vorster A, Roberts L, Ehrenreich L, Oppon E, Gama D, Bardien S, Greenberg J, Bonapace G, et al. Proc Natl Acad Sci USA. 2004;101:6617–6622. doi: 10.1073/pnas.0401529101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. Nature. 1988;332:462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 18.Wu J, Kaufman RJ. Cell Death Differ. 2006;13:374–384. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- 19.Rubenstein RC, Zeitlin PL. Curr Opin Pediatr. 1998;10:250–255. doi: 10.1097/00008480-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Burrows JA, Willis LK, Perlmutter DH. Proc Natl Acad Sci USA. 2000;97:1796–1801. doi: 10.1073/pnas.97.4.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright JM, Zeitlin PL, Cebotaru L, Guggino SE, Guggino WB. Physiol Genomics. 2004;16:204–211. doi: 10.1152/physiolgenomics.00160.2003. [DOI] [PubMed] [Google Scholar]
- 22.Graves TK, Patel S, Dannies PS, Hinkle PM. J Cell Sci. 2001;114:3685–3694. doi: 10.1242/jcs.114.20.3685. [DOI] [PubMed] [Google Scholar]
- 23.Rajan RS, Kopito RR. J Biol Chem. 2005;280:1284–1291. doi: 10.1074/jbc.M406448200. [DOI] [PubMed] [Google Scholar]
- 24.Hegde RS, Lingappa VR. Trends Cell Biol. 1999;9:132–137. doi: 10.1016/s0962-8924(99)01504-4. [DOI] [PubMed] [Google Scholar]
- 25.Xu C, Bailly-Maitre B, Reed JC. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kopito RR, Ron D. Nat Cell Biol. 2000;2:E207–E209. doi: 10.1038/35041139. [DOI] [PubMed] [Google Scholar]
- 27.Ulloa-Aguirre A, Janovick JA, Brothers SP, Conn PM. Traffic. 2004;5:821–837. doi: 10.1111/j.1600-0854.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- 28.Bonapace G, Waheed A, Shah GN, Sly WS. Proc Natl Acad Sci USA. 2004;101:12300–12305. doi: 10.1073/pnas.0404764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubota K, Niinuma Y, Kaneko M, Okuma Y, Sugai M, Omura T, Uesugi M, Uehara T, Hosoi T, Nomura Y. J Neurochem. 2006;97:1259–1268. doi: 10.1111/j.1471-4159.2006.03782.x. [DOI] [PubMed] [Google Scholar]
- 30.Singh OV, Vij N, Mogayzel PJ, Jr, Jozwik C, Pollard HB, Zeitlin PL. J Proteome Res. 2006;5:562–571. doi: 10.1021/pr050319o. [DOI] [PubMed] [Google Scholar]
- 31.Laemmli UK. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 32.Peterson GL. Anal Biochem. 1979;100:201–220. doi: 10.1016/0003-2697(79)90222-7. [DOI] [PubMed] [Google Scholar]
- 33.Pfaffl MW. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]