Abstract
Bacterial type IV secretion system (T4SS) belongs to a growing class of evolutionarily conserved transporters that translocate DNA and proteins into a wide variety of organisms including bacterial and eukaryotic cells. Archetypal is the Agrobacterium tumefaciens VirB/D4 T4SS that transfers oncogenic T-DNA to various eukaryotic cells, which is transferred as a nucleoprotein T-complex with VirD2 as the pilot protein. As a derivative of plasmid conjugation systems, the VirB/D4 T4SS can also transfer certain mobilizable plasmids and bacterial proteins like VirE2 and VirF, although it is unknown how the membrane-bound T4SS recruits different transfer substrates. Here, we show that a cytoplasmic VirD2-binding protein (VBP) is involved in the recruitment of the T-complex to the energizing components of the T4SS, including VirD4, VirB4, and VirB11. VBP is also important for the recruitment of a conjugative plasmid to a different transfer system independent of VirB/D4. These data indicate that VBP functions as a previously unrecognized recruiting protein that helps couple nucleoprotein substrates to the appropriate transport sites for conjugative DNA transfers. VBP has three functionally redundant homologs, and similar homologs can be found in different bacterial genomes, suggesting a previously uncharacterized class of proteins involved in conjugative DNA transfers.
Keywords: bacterial type IV secretion system, T-DNA, virulence
Bacteria are equipped with different secretion systems that export a wide spectrum of substrates into the surroundings or interacting organisms. One secretion system, named type IV secretion system (T4SS), is widely used by bacteria to translocate DNA and protein macromolecules to a diverse range of bacterial and eukaryotic cells (1, 2). The importance of T4SS is highlighted by the expanding list of bacterial pathogens (such as Helicobacter pylori and Legionella) that use T4SS to inject proteins and nucleoprotein complexes into eukaryotic host cells (1–3). An archetypal T4SS is the Agrobacterium tumefaciens VirB/D4 apparatus, which is responsible for the transfer of T-DNA from A. tumefaciens to the natural host plant cells, as well as laboratory hosts like bacterial, yeast, and fungal cells (4, 5).
T4SS shares a common ancestry with bacterial conjugation systems (1, 2). Proteins involved in conjugative DNA transfers are designated as Tra proteins and can be divided into three functionally distinct subsets (1, 2, 6). The first subset is involved in the processing and packing of transferred DNA intermediate. For A. tumefaciens T-DNA transfer system, this subset comprises the VirD2 relaxase, VirD1 and VirC proteins, and may also include VirE2, VirE1, and VirF (4, 5, 7). VirD2 cleaves the bottom strand of the T-DNA at the two border sequences to generate single-stranded (ss) T-strand (8, 9) and remains covalently associated with the 5′ end of the T-strand (10). VirE2 is a nonspecific ssDNA-binding protein that can coat the length of the T-strand in vitro (11).
The second subset of Tra proteins for T-DNA transfer comprises 11 VirB proteins (VirB1 to VirB11), which form the transport apparatus (1, 2, 4). This apparatus is known to translocate diverse macromolecule substrates, including VirD2-T-strand complex and virulence proteins like VirE2 and VirF, into plant cells (4, 5, 7, 12, 13).
The third subset of Tra proteins are the “coupling proteins (CPs),” which mediate specific interaction between a substrate and the transport apparatus (1, 2). As a member of this large protein family, A. tumefaciens VirD4 CP is an inner-membrane protein required for the transfer of both T-strand and VirE2 to host cells (14–16). The VirD4 CP can recruit protein substrate VirE2 to the bacterial cell poles (6).
However, there is no experimental evidence showing that the same VirD4 CP can recruit the bulky nucleoprotein T-complex. It remains elusive how a nucleoprotein substrate like T-complex is recruited to the transport site. Here, we report a subset of proteins defined as “recruiting proteins” that is involved in the recruitment of nucleoprotein substrate complex to the energizing components of the transport apparatus. We also show that a recruiting protein is important for the transfer of different nucleoprotein substrates.
Results
VBP-VirD2 Interaction Is Important for T-DNA Transfer.
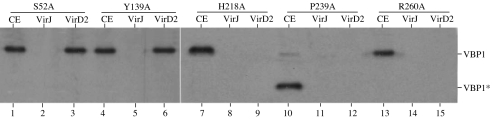
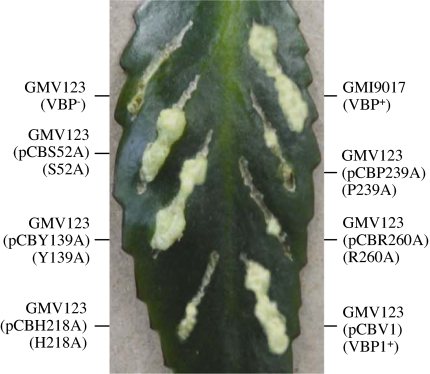
Previously, we identified a VirD2-binding protein VBP1, which can bind VirD2 directly and is involved in Agrobacterium tumorigenesis (17). A. tumefaciens contains two additional genes encoding proteins highly homologous to VBP1, which are designated VBP2 and VBP3 [supporting information (SI) Table 1]. All of the three VBP proteins can bind to VirD2 (17). To determine whether the VBP-VirD2 interaction is important for T-DNA transfer, we created vbp1 point-mutations by site-directed mutagenesis using PCR. Five highly conserved amino acid residues (S52, Y139, H218, P239, and R260) of VBP1 were changed to alanine (A) (Fig. 1). Y139, H218, P239, and R260 are conserved among all of the VBP homologs. S25 is conserved in 13 of the 14 VBP homologs. We then tested the functionality of these VBP mutant proteins, after introducing the corresponding genes into the vbp triple mutant GMV123 (lacking any functional vbp). The bacterial cells were used to test the function to transfer T-DNA, as detected by the tumorigenesis assay; the crude cell extracts were used in a pull-down assay to assess the function for VBP1–VirD2 interaction, because previous experiments have shown that purified VBP1 can bind to purified VirD2 (17). Mutations at S52 and Y139 did not affect the VBP1–VirD2 interaction (Fig. 2, lanes 1–6) or the T-DNA transfer (Fig. 3). However, mutations at H218 and R260 abolished the VBP1–VirD2 interaction (Fig. 2, lanes 7–9 and 13–15) and reduced the bacterial ability to transfer the T-DNA (Fig. 3). Among the three function-deficient mutants, mutations at H218 and R260 did not affect the stability of VBP1 accumulated inside A. tumefaciens cells (Fig. 2, lanes 7 and 13), although the P239A mutant appeared to be unstable because a smaller version of VBP1 was detected inside the bacterial cells (Fig. 2, lane 10). These indicate that the amino acid residues H218 and R260 affected both the VBP1–VirD2 interaction and the T-DNA transfer, whereas S52 and Y139 did not. The data strongly suggest that the VBP1–VirD2 interaction is important for T-DNA transfer, and VBP plays a role in the transfer process.
Fig. 1.
Sequence alignment of VBP homologs. The VBP homologs and the bacterial species encoding them are described in SI Table 1. The identical and conserved residues are boxed in black and colors, respectively. The arrowheads indicate the VBP1 point mutations at amino acid positions 52 (S52A), 139 (Y139A), 218 (H218A), 239 (P239A), and 260 (R260A). Atu, A. tumefaciens.
Fig. 2.
The effect of VBP1 mutations on the VBP1–VirD2 interaction. Plasmids carrying vbp1 were introduced into the vbp triple-mutant GMV123 to complement the vbp function deficiency. The crude extracts (CE) of A. tumefaciens GMV123(pCBS52A) (S52A), GMV123(pCBY139A) (Y139A), GMV123(pCBH218A) (H218A), GMV123(pCBP239A) (P239A), or GMV123(pCBR260A) (R260A) were used for the pull-down assays using MBP-VirJ (VirJ) and MBP-VirD2 (VirD2). The pulled-down proteins were analyzed by Western blot using VBP1 antibody. VBP1* represents a VBP1 degradation product.
Fig. 3.
The effect of VBP1 mutations on tumorigenesis. A. tumefaciens strains were grown in MG/L medium at 28°C overnight. The cell density was adjusted to 108 cells per milliliter. Then 2 μl of cell suspension was inoculated onto each wound site on the leaves of Kalanchoe plants. The tumors were photographed 35 days after inoculation.
VBP1 Interacts with both T-Complex and Some T4SS Components.
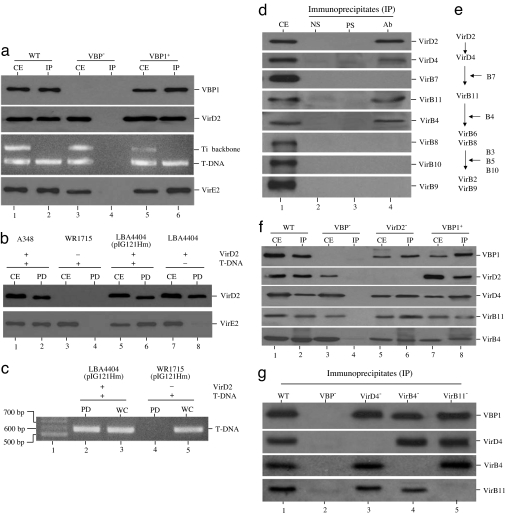
To determine whether VBP1 could interact with VirD2-bound T-complex, we used VBP1 antibody to immunoprecipitate proteins from crude extracts of A. tumefaciens cells, in which the vir genes had been induced by acetosyringone (AS) (4, 5). As shown in Fig. 4a, VBP1 antibody could immunoprecipitate both VirD2 and T-strand from the wild-type strain C58 (Fig. 4a, lane 2), but not from the vbp triple mutant (VBP−) (Fig. 4a, lane 4). When the vbp triple mutant was complemented with vbp1, the VBP1 antibody could then immunoprecipitate both VirD2 and T-strand (Fig. 4a, lane 6). The VBP1 antibody did not immunoprecipitate the Ti plasmid backbone. The results indicated that VBP1 can interact with the T-complex.
Fig. 4.
VBP1 could bind to both T-complex and some components of the VirB/D4 T4SS. Crude extracts of AS-induced A. tumefaciens cells were immunoprecipitated or pulled down and then analyzed by Western blot using the antibodies against the proteins indicated on the right. The T-DNA was detected by PCR amplification using the primers that anneal to the T-DNA, and Ti plasmid backbone was amplified as the control by using the primers that anneal to the backbone. CE, crude extracts; IP, immunoprecipitates; PD, pull-down samples; WC, whole cells; WT, wild-type A348 or C58; VBP−, GMV123 strain; VBP1+, GMV123(pCBV1) strain; VirD2−, WR1715 strain; VirD4−, At12506 strain; VirB4−, At12044 strain; VirB11−, At10011 strain. (a) Coimmunoprecipitation of T-complex by VBP1 antibody. (b and c) Pulling down of T-complex by His-VBP1. (d) Coimmunoprecipitation of T4SS components by VBP1 antibody; precleared crude extract (CE) of AS-induced A348 cells was immunoprecipitated with no serum (NS), preimmune serum (PS), or VBP1 antibody (Ab). (e) A postulated contact pathway for the T-strand translocation through the VirB/D4 T4SS (18); the proteins listed on the right indicate the requirement of the corresponding proteins for the passage of T-strand at the steps indicated. (f and g) The coimmunoprecipitation of T4SS components by VBP1 antibody depends on VBP1 and independent of the interactions among VirD2, VirD4, VirB4, and VirB11.
In the coimmunoprecipitation, the VBP1 antibody also immunoprecipitated VirE2 (Fig. 4a). To confirm the results, we used His-tagged VBP1 (His-VBP1) as the bait to identify VBP1-interacting molecules. As shown in Fig. 4b, His-VBP1 pulled down both VirD2 and VirE2. Pulling-down of VirE2 by VBP1 required the presence of both VirD2 and T-strand, because no VirE2 was pulled down from either the virD2− strain WR1715 (Fig. 4b, lane 4) or the T-DNA− strain LBA4404 (Fig. 4b, lane 8). When plasmid pIG121Hm carrying a recombinant T-DNA was introduced into LBA4404, VirE2 was pulled down by VBP1 (Fig. 4b, lane 6). Consistent with this, T-strand was detected by PCR in the pull-down complex from LBA4404(pIG121Hm), but not from WR1715(pIG121Hm) (Fig. 4c). These experiments demonstrated that VBP1 could pull down the VirD2-bound T-complex. VBP1 pulled down VirE2 through the T-strand. VBP1 did not bind to VirE2 directly, indicating that VBP1 was not directly involved in the recruitment for VirE2.
Subsequently, we determined whether VBP1 could bind to any component(s) of the T4SS transport apparatus. We used 0.5% Triton X-100 to solubilize the T4SS components. As shown in Fig. 4d, the VBP1 antibody coimmunoprecipitated VirD2, VirD4, VirB4, and VirB11 but not other T4SS components like VirB7, VirB8, VirB9, and VirB10 (Fig. 4d, lane 4). This is consistent with the T-strand translocation pathway (Fig. 4e) postulated previously based on transfer DNA immunoprecipitation (18). The pull-down experiments showed that VBP1 pulled down only the components of early translocation stage (Fig. 4 d and e), which are the inner-membrane components with cytoplasmic domains and not the channel components without cytoplasmic domains (1, 4, 18). It is still unknown whether VBP might be translocated into the T4SS channel, because it is possible that the detergent may have disrupted the interaction of VBP1 with the T4SS core components (like VirB7, VirB8, VirB9, and VirB10) during the assay. Nevertheless, the data suggest that VBP1 functions inside the cytoplasm, consistent with the fact that it is a cytoplasmic protein, as shown later.
We further wanted to know whether the coimmunoprecipitation of T4SS components by VBP1 antibody is VBP1-specific and whether the coimmunoprecipitation depends on VirD2. As shown in Fig. 4f, the VBP1 antibody did not coimmunoprecipitate VirD2, VirD4, VirB4, and VirB11 from the vbp triple mutant (Fig. 4f, lane 4). When the mutant was complemented with vbp1, all four proteins (VirD2, VirD4, VirB4, and VirB11) were coimmunoprecipitated (Fig. 4f, lane 8), indicating that their coimmunoprecipitation by the VBP1 antibody is VBP1-specific. VirD4, VirB4, and VirB11 were also coimmunoprecipitated from the virD2− mutant (Fig. 4f, lane 6), indicating that VirD2 was not required for the coimmunoprecipitation of the T4SS components. These suggest that VBP1 interacts with VirD2-bound T-complex and T4SS components independently, presumably through two binding domains, one for VirD2 and the other for T4SS.
The Role of VBP1 in Recruitment of T-Complex to T4SS Transport Site.
The data prompted us to hypothesize that VBP1 helps to recruit T-complex to the T4SS transport site. Because VirD4 was previously shown to interact with VirB4 and VirB11 (19), we wanted to know whether these interactions affect the VBP1 access to the T4SS. As shown in Fig. 4g, VBP1 bound to VirB4 and VirB11 in the VirD4− background (Fig. 4g, lane 3); VBP1 also bound to VirD4 and VirB11 in the VirB4− background (Fig. 4g, lane 4); and it bound to VirD4 and VirB4 in the VirB11− background (Fig. 4g, lane 5). This suggests that the binding of VBP1 to VirD4, VirB4, or VirB11 still occurred even when one of the three interacting proteins (VirD4, VirB4, and VirB11) was missing. This is consistent with the previous observation that VirD4, VirB4, and VirB11 interact independently with each other (19).
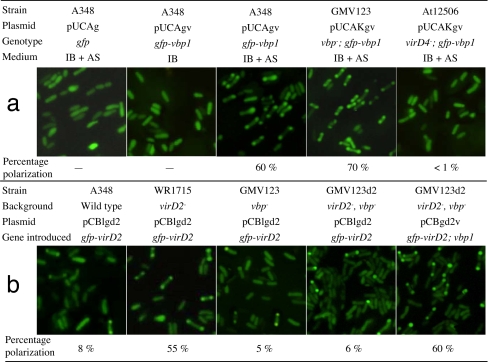
To provide in vivo evidence for the role of VBP in the recruitment of T-complex, we studied the subcellular localization of VBP and the effect of VBP on the VirD2 access to the T4SS transporter site. VBP1 was fused onto the C terminus of GFP, and GFP-VBP1 was expressed in A. tumefaciens cells. As shown in Fig. 5a, the subcellular localization of GFP-VBP1 depended on the presence of T4SS. In the absence of T4SS (without the vir gene induction by AS), GFP-VBP1 was evenly distributed in the cells, indicating that VBP1 is a cytoplasmic protein. When T4SS was induced by AS, GFP-VBP1 localized to the cell poles, whereas the control GFP did not. The polar localization of GFP-VBP1 appeared similar to that of GFP-VirE2 (6). When GFP-VBP1 was expressed in a virD4− strain, GFP-VBP1 did not localize to the cell poles. These experiments demonstrated that VirD4 was required for the VBP1 access to the cell poles, where VirD4 and T4SS localize (16, 20).
Fig. 5.
Localization of GFP-VBP1 and GFP-VirD2 at the cell poles. A. tumefaciens cells harboring the indicated plasmids were grown overnight in IB in the presence or absence of 200 μM AS. The cells were photographed under fluorescence microscopy. The percentage of polarization represents the percent of the cells with the polar localization of GFP-VBP1 or GFP-VirD2. (a) The effect of VirD4 on polar localization of GFP-VBP1. (b) The effect of VBP1 on polar localization of GFP-VirD2.
Then we determined the effect of VBP on VirD2 access to the T4SS transporter site. When GFP-VirD2 was expressed in the wild-type strain A348 and the cells were induced by AS, only 8% of the cells showed polar fluorescence (Fig. 5b). However, when expressed in a virD2− strain, 55% of the cells showed polar fluorescence, suggesting that native VirD2 could competitively inhibit the access of GFP-VirD2 to the T4SS. The high level of dominance by native VirD2 over GFP-VirD2 indicates that the native form of VirD2 functions better than the fusion protein. This is consistent with our observation that the GFP-VirD2 fusion could not complement a virD2− mutation in the tumorigenesis assay (data not shown). Nevertheless, the GFP-VirD2 fusion was useful to study VirD2 localization, because its localization was similar to the FLAG-tagged VirD2 that is fully functional for T-DNA transfer (21).
To determine whether the polar localization of GFP-VirD2 is VBP-dependent, GFP-VirD2 was expressed in the mutant lacking any vbp and virD2. As shown in Fig. 5b, only 6% of the cells showed fluorescence at the cell poles. When the vbp1 gene was introduced to the cells, ≈60% of them showed polar fluorescence. These results suggest that VBP1 is important for the VirD2 access to the cell poles, where the T4SS localizes. All of the data consistently suggest that VBP is involved in the recruitment of VirD2-bound T-complex to the T4SS apparatus.
VBP1 Is Involved in Plasmid Conjugation.
VBP has three functionally redundant homologs; one of them is encoded on the cryptic plasmid (17). We further determined whether VBP is important for plasmid conjugation, because T-DNA transfer is mechanistically related to plasmid conjugation (1, 4, 22 23).
It has been shown that A. tumefaciens is capable of mobilizing the IncQ plasmid RSF1010 under noninducing conditions (24, 25), indicating that RSF1010 can be mobilized by an A. tumefaciens transfer system independent of VirB/D4. We examined the effects of VBP proteins on the mobilization of a RSF1010 derivative plasmid pML122 (26) from A. tumefaciens to Escherichia coli under noninducing conditions. As shown in SI Table 2, the efficiency of the vbp triple mutant to transfer pML122 from A. tumefaciens to E. coli in the absence of any helper strain was reduced at least 50-fold as compared with the strains harboring any of the three vbp genes. We assume that the conjugative transfer of pML122 is independent of the VirB/D4 T4SS, because the conjugation conditions did not allow the expression of T4SS genes including the 11 VirB proteins and VirD4. In addition, mutations at VirB4 and VirB11 did not affect the efficiency of pML122 conjugation from A. tumefaciens to E. coli (SI Table 2). The data indicate that VBP plays a role in RSF1010 conjugation independent of the VirB/D4 T4SS in addition to its role in T-DNA transfer mediated by the VirB/D4 T4SS.
We also tested the conjugation of a broad-host-range IncP plasmid pSW172 (27) from A. tumefaciens to E. coli. As shown in SI Table 3, the conjugation of pSW172 was not detectable in the absence of any helper strain. To confirm that the conditions were conducive for conjugation, we introduced a helper strain MT616 harboring the plasmid pRK600, which contains the RK2 (also an IncP plasmid) (28) transfer genes that can mobilize OriT-containing plasmids (29). As shown in SI Tables 2 and 3, the conjugation efficiency was dramatically increased when the conjugation was conducted in the presence of the helper strain MT616. But the vbp mutation did not affect the conjugation mediated by the RK2 transfer genes. These data suggest that VBP plays a role in conjugation of the IncQ plasmid RSF1010 mediated by a VirB/D4-independent transfer system but not in conjugation of the IncP plasmid pSW172 mediated by the RK2 system. The RSF1010 plasmid can be transferred by both the VirB/D4-dependent (30, 31) and -independent (24, 25) gene transfer systems, both of which are targeted by VBP (SI Table 2). The data suggest that VBP plays a role in different gene transfer systems that can transfer a common substrate(s).
VBP1 Interacts with a Plasmid Conjugative Intermediate.
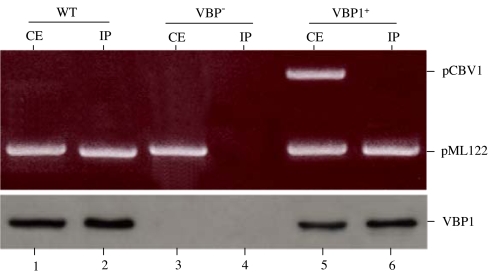
Subsequently, we wanted to know whether VBP functions as a recruiting protein in the plasmid conjugation. We used VBP1 antibody to incubate with the cell extracts of A. tumefaciens strains that harbor pML122 and then determined whether VBP1 antibody could immunoprecipitate nucleoprotein complex derived from a plasmid. As shown in Fig. 6, pML122 plasmid DNA was coimmunoprecipitated by the VBP1 antibody from the wild-type A348 (Fig. 6, lane 2) but not from the vbp triple mutant (Fig. 6, lane 4), whereas introduction of vbp1 restored the coimmunoprecipitation of pML122 (Fig. 6, lane 6). In addition, VBP1 antibody did not coimmunoprecipitate pCBV1, which is incapable of conjugative transfer because it lacks OriT (32). These results indicated that VBP antibody can immunoprecipitate pML122 plasmid DNA that the VBP proteins help to transfer. This further suggests that VBP proteins could bind to the nickase of the pML122 plasmid DNA so that pML122 DNA was coimmunoprecipitated by the VBP1 antibody (Fig. 6), because the VBP1 did not bind to DNA nonspecifically (Figs. 4 and 6). This is consistent with the observations that RSF1010 can be transferred by the VirB/D4 T4SS (30, 31), and its transfer intermediate inhibits the binding of T-DNA to the VirD4 receptor (33). It appeared that T-DNA and RSF1010 transfer systems are related. Presumably, pML122 was processed by its corresponding nickase, which provided the specificity for the VBP1–pML122 binding; thus, pML122 was transferred by a VirB/D4-independent transfer system in a way that involves VBP and is similar to the T-DNA transfer. It should be of interest to study how the RSF1010 transfer is facilitated in other bacterial species that may not possess a VBP homolog.
Fig. 6.
Plasmid pML122 DNA was coimmunoprecipitated by VBP1 antibody. Plasmid pML122 was introduced into A. tumefaciens A348 (WT), GMV123 (VBP−), and GMV123(pCBV1) (VBP1+); the bacteria were grown in the noninducing conditions (no vir gene expression). The crude extracts from the cells were coimmunoprecipitated by VBP1 antibody. VBP1 in the crude extracts (CE) and immunoprecipitates (IP) was detected by Western blot. The plasmid DNAs were amplified by PCR using the primers annealed to pML122 or pCBV1.
Discussion
Acquiring new genetic information is a critical way for a cell to adapt to the changing environment. This is particularly prevalent in bacteria because they exchange DNA molecules like plasmids at high frequencies. The machinery for plasmid conjugation is highly conserved among bacteria and is known as T4SS. During evolution, T4SS has been modified to export other substrates, like DNA and proteins, into a wide variety of organisms, including bacterial and eukaryotic cells.
A. tumefaciens VirB/D4 T4SS is archetypal; it can transfer both DNA and protein substrates to various eukaryotic cells (1, 2, 4, 5). Recently, it was reported that VirC1 can spatially coordinate the early conjugative DNA transfer reactions, including the recruitment of T-complex to cell poles (21). However, VirC1 recruits T-complex to the cell poles independently of the T4SS components (VirD4 substrate receptor and VirB channel subunits) (21); it is not clear how the VirD2–VirD4 interaction is mediated. Therefore, it is still unknown how the membrane-bound T4SS apparatus recruits the VirD2-guided T-complex. Here, we show that a cytoplasmic protein VBP can facilitate the VirD2–VirD4 interaction and that VBP is involved in the recruitment of the nucleoprotein substrate (VirD2–T-DNA complex) to the T4SS apparatus. The role of VBP in the recruitment of T-complex is supported by several lines of evidence. (i) The mutation and tumorigenesis experiments substantiated the importance of the physical interaction between VBP and VirD2 in T-DNA transfer; (ii) The coimmunoprecipitation and pull-down assays demonstrated that VBP could bind to both the T-complex and the energizing components of the VirB/D4 T4SS; (iii) the in vivo microscopy experiments showed that VBP played a role in the recruitment of VirD2 to the bacterial cell poles (where the VirB/D4 T4SS is located) in a VirB/D4-dependent manner.
GFP-VirD2 localized to both poles in most of the bacterial cells in our localization experiments, in which the GFP-VirD2 expression was under the lac promoter control. In contrast, it is reported that VirD2 localizes at one pole in most of the cells in previous studies, in which the expression of FLAG-tagged VirD2 was under the virB promoter control (21). Our time-course study on GFP-VirD2 localization indicated that the discrepancy is likely due to the difference in gene expression levels. As shown in SI Fig. 7, more cells exhibited unipolar localization of GFP-VirD2 at the early stage of vir induction (3 h); more cells exhibited bipolar localization at the later stage of vir induction (16 h). This further confirmed that GFP-VirD2 localization depends on vir induction.
Because VirE2 is also a substrate for the VirB/D4 transfer (6), the VBP1–VirE2 interaction was studied. Our experiments suggest that VirE2 can be bound to the VirD2-T-strand complex in the bacterial cell extracts (Fig. 4 A and B). However, the experiments could not conclusively determine whether VirE2 is part of T-complex inside bacterial cells or becomes associated with the T-complex upon lysis of the bacteria, although there is evidence that VirE2 is not part of the T-complex inside bacterial cells (4, 18). Nevertheless, our experiments demonstrated that VBP1 is not directly involved in the VirE2 recruitment.
A recruiting protein like VBP that can strongly interact with both VirD2 and VirD4 can effectively enhance the recruitment of T-complex to the T4SS apparatus. In addition, the polar positioning of T-complex facilitated by VirC1 can be also helpful for the recruitment of T-complex to the T4SS apparatus (21). VirC1 antibody immunoprecipitated both T-strand and Ti-plasmid (21), suggesting that VirC1 binds to VirD2 during relaxosome assembly and remains bound during transit to the T4SS apparatus. On the other hand, VBP1 antibody immunoprecipitated only T-strand (Fig. 4), suggesting that VBP1 recruits the VirD2-T-strand complex after the complex is released from Ti-plasmid. Presumably, VirC1-facilitated positioning of T-strand-relaxosome complex is followed by VBP-mediated recruitment of free T-complex to the T4SS apparatus.
VirD4 plays an important role in recognizing T4SS-translocating substrate (1, 6, 16). VirB11 is the first VirB protein that contacts with the VirD2-T-strand complex after VirD4 CP (18). VirB11 is proposed to function as a gating molecule of T4SS at the inner membrane and to drive substrate export (34). VirD4, VirB11, and VirB4 are the energizing molecules for substrate export (1, 4, 19). The interaction of VBP with each of these energizing proteins further supports our hypothesis that VBP recruits the T-complex to the T4SS gate, because early translocation certainly requires energy input. The independent interactions of VBP1 with three energizing proteins indicate that the recruiting protein plays a significant role in the early-stage translocation and in a sequential manner, presumably starting from VirD4 to VirB11 and then VirB4. Therefore, it is important to further investigate whether the interaction of VBP with each of these energizing proteins is indeed involved in the T-DNA transfer process.
It is of considerable interest that the VBP proteins also affect the plasmid conjugation in A. tumefaciens, although it remains unknown how RSF1010 is transferred in the bacterial species that may not possess a VBP homolog. In addition, VBP1 antibody could specifically immunoprecipitate the plasmid that VBP1 helps to transfer. The data indicate that VBP1 can recruit different transfer substrates: T-complex and RSF1010. This is consistent with the fact that these two substrates can be transferred by the VirB/D4 T4SS (1, 4, 30, 31). We propose to name VBP as a “recruiting protein” to reflect its recruitment function.
It is not clear how VBP carries out the recruitment function. Computer analysis revealed that VBP proteins contain a putative nucleotidyltransferase (NT) motif in their N-terminal region and a putative higher eukaryotes and prokaryotes nucleotide-binding (HEPN) domain in their C-terminal region (17). The NT motif possesses two principal elements: the conserved glycine–serine (GS) doublet and the two conserved aspartates (DXD) (35). We noticed that one of the point mutations occurred at the conserved serine position (S52A) (Fig. 1) of the GS doublet (35). The S52A mutation did not affect the VBP1–VirD2 interaction (Fig. 2) or T-DNA transfer (Fig. 3), suggesting that the NT motif is not important for the VBP recruitment function. Interestingly, another point mutation occurred at one of the highly conserved amino acids (R260A) (Fig. 1) of the HEPN domain (36); the R260A mutation abolished the VBP1–VirD2 interaction (Fig. 2) and reduced the bacterial ability to transfer T-DNA (Fig. 3). This suggests that the HEPN domain is required for the VBP recruitment function. Because the HEPN domain is implicated in nucleotide binding (36), we speculate that the ATP binding might be involved in the VBP recruitment function. Interestingly, VBP1 interacts with each of the three energizing proteins VirD4, VirB4, and VirB11, which belong to the ATPase protein family.
VBP has three functionally redundant homologs in A. tumefaciens C58 (SI Table 1 and Fig. 1). Interestingly, the redundancy of VBP homologs appears to be common in some α-proteobacteria, because two VBP homologs are encoded in the genomes of Sinorhizobium meliloti strain 1021, Sphingopyxis alaskensis RB2256, and Rickettsia felis URRWXCal2; other taxonomically distant bacteria like Bacteroides thetaiotaomicron also appear to have VBP homologs (SI Table 1 and Fig. 1). Because a bacterial function is normally encoded by a single gene, and redundancy is rare in a bacterial genome, the redundancy of VBP homologs is particularly surprising and suggests the importance of this group of proteins.
VBP proteins and their homologs may be regarded as a newly recognized class of proteins that may be involved in the recruitment of conjugative DNA transfer intermediate to the membrane transport apparatus in diverse species. It is intriguing to speculate how conjugative plasmids are recruited in the bacteria that do not have a VBP homolog; it remains unknown whether other class(es) of proteins with no or low homology to VBP can also play a role in the recruitment in these bacteria. Because different plasmids from various sources may replicate in the same bacterial cells and they may be transferred into other cells, a recruiting protein may function as a “head hunter” to facilitate productive matching between the conjugative DNA available for transfer and the DNA translocation machinery.
Materials and Methods
Strains and Plasmids.
Strains and plasmids used in this study are listed in SI Table 4. Primers used in this study are described in SI Table 5. E. coli and A. tumefaciens strains were cultured as described (17).
Binding of VBP1 Mutant Proteins with VirD2.
Plasmids carrying the vbp1 mutant genes were introduced into the vbp triple-mutant GMV123 to complement the vbp function deficiency. The agrobacterial cells were then cultured and used for the pull-down assays as described (17). MBP-VirD2 fusion protein was used as the affinity ligand to pull down the VBP1 mutant proteins; MBP-VirJ fusion protein was used as the negative control.
Tumorigenesis Assay.
To test the effect of vbp mutations on T-DNA transfer, the GMV123 cells expressing the VBP1 mutant proteins were inoculated onto the leaves of Kalanchoe plants. The tumorigenesis assay was conducted as described (17) to detect the efficiency of T-DNA transfer into plant cells.
Binding of VBP1 to T-Complex.
To detect the binding between VBP1 and T-complex, VBP1 antibody or resin-bound His-VBP1 was used to immunoprecipitate or pull down the proteins from crude extracts of A. tumefaciens cells induced by AS (SI Text).
Binding of VBP to T4SS Components.
To detect the interaction between VBP1 and T4SS components, VBP1 antibody was used to immunoprecipitate the proteins from crude extracts of A. tumefaciens cells induced by AS; 0.5% Triton X-100 was used to solubilize the proteins (SI Text).
Microscopy for Agrobacterial Cells.
Plasmids encoding GFP, GFP-VBP1, and GFP-VirD2 were introduced into the A. tumefaciens strains. The cells were grown in MG/L to an OD600 ≈ 0.5 and then grown in IB with or without 200 μM AS overnight. The cells were observed under an Olympus fluorescence microscope.
Conjugation Assay.
The conjugation assay was conducted under noninducing conditions as described by Zyl et al. (37) with some modifications (SI Text).
Coimmunoprecipitation of Plasmid pML122 by VBP1 Antibody.
For coimmunoprecipitation of plasmid pML122, A. tumefaciens cells were grown in MG/L liquid and did not undergo AS-induction. The remaining procedure was performed as the coimmunoprecipitation of T-complex. Primers Prsf1 and Prsf2 were used to amplify the fragment of plasmid pML122. Primers PvbpCS1 and PvbpCS2 were used to detect the plasmid pCBV1.
Supplementary Material
Acknowledgments
We thank Joseph Heitman and Alexander Idnurm for reading the manuscript, Anath Das (University of Minnesota, Minneapolis) for providing At12506, and Charles Rosenberg (Centre National de la Recherche Scientifique–Institut National de la Recherche Agronomique, France) for providing GMI9017. This research was supported by National University of Singapore Academic Research Funds (R-154-000-142-112 and R-154-000-208-112) and Defense Science and Technology Agency of Singapore (R-154-000-178-422).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701738104/DC1.
References
- 1.Christie PJ, Atmakuri K, Krishnamoorthy V, Jakubowski S, Cascales E. Annu Rev Microbiol. 2005;59:451–485. doi: 10.1146/annurev.micro.58.030603.123630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backert S, Meyer T. Curr Opin Microbiol. 2006;9:207–217. doi: 10.1016/j.mib.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, de Felipe KS, Clarke M, Lu H, Anderson OR, Segal G, Shuman HA. Science. 2004;303:1358–1361. doi: 10.1126/science.1094226. [DOI] [PubMed] [Google Scholar]
- 4.McCullen CA, Binns AN. Annu Rev Cell Dev Biol. 2006;22:101–127. doi: 10.1146/annurev.cellbio.22.011105.102022. [DOI] [PubMed] [Google Scholar]
- 5.Gelvin SB. Microbiol Mol Biol Rev. 2003;67:16–37. doi: 10.1128/MMBR.67.1.16-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atmakuri K, Ding Z, Christie PJ. Mol Microbiol. 2003;49:1699–1713. doi: 10.1046/j.1365-2958.2003.03669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzfira T, Vaidya M, Citovsky V. Nature. 2004;431:87–92. doi: 10.1038/nature02857. [DOI] [PubMed] [Google Scholar]
- 8.Yanofsky MF, Porter SG, Young C, Albright LM, Gordon MP, Nester EW. Cell. 1986;47:471–477. doi: 10.1016/0092-8674(86)90604-5. [DOI] [PubMed] [Google Scholar]
- 9.Stachel SE, Timmerman B, Zambryski P. Nature. 1986;322:706–712. [Google Scholar]
- 10.Ward E, Barnes W. Science. 1988;242:927–930. [Google Scholar]
- 11.Citovsky V, De Vos G, Zambryski P. Science. 1988;240:501–504. doi: 10.1126/science.240.4851.501. [DOI] [PubMed] [Google Scholar]
- 12.Regensburg-Tuink AJ, Hooykaas PJ. Nature. 1993;363:69–71. doi: 10.1038/363069a0. [DOI] [PubMed] [Google Scholar]
- 13.Vergunst AC, Schrammeijer B, Dulk-Ras AD, Vlaam CM, Regensburg-Tuink TJ, Hooykaas PJ. Science. 2000;290:979–982. doi: 10.1126/science.290.5493.979. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto S, Toyoda-Yamamoto A, Ito K, Takebe I, Machida Y. Mol Gen Genet. 1991;228:24–32. doi: 10.1007/BF00282443. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton CM, Lee H, Li PL, Cook DM, Piper KR, von Bodman SB, Lanka E, Ream W, Farrand SK. J Bacteriol. 2000;182:1541–1548. doi: 10.1128/jb.182.6.1541-1548.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar RB, Das A. Mol Microbiol. 2002;43:1523–1532. doi: 10.1046/j.1365-2958.2002.02829.x. [DOI] [PubMed] [Google Scholar]
- 17.Guo M, Hou Q, Hew CL, Pan SQ. Mol Plant-Microbe Interact. 2007;20:1201–1212. doi: 10.1094/MPMI-20-10-1201. [DOI] [PubMed] [Google Scholar]
- 18.Cascales E, Christie PJ. Science. 2004;304:1170–1173. doi: 10.1126/science.1095211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atmakuri K, Cascales E, Christie PJ. Mol Microbiol. 2004;54:1199–1211. doi: 10.1111/j.1365-2958.2004.04345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Judd PK, Kumar RB, Das A. Proc Natl Acad Sci USA. 2005;102:11498–11503. doi: 10.1073/pnas.0505290102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atmakuri K, Cascales E, Burton OT, Banta LM, Christie PJ. EMBO J. 2007;16:2540–2551. doi: 10.1038/sj.emboj.7601696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stachel SE, Zambryski P. Nature. 1989;340:190–191. doi: 10.1038/340190a0. [DOI] [PubMed] [Google Scholar]
- 23.Lessl M, Lanka E. Cell. 1994;77:321–324. doi: 10.1016/0092-8674(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 24.Cook DM, Farrand SK. J Bacteriol. 1992;174:6238–6246. doi: 10.1128/jb.174.19.6238-6246.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Chen Y, Wood DW, Nester EW. J Bacteriol. 2002;184:4838–4845. doi: 10.1128/JB.184.17.4838-4845.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labes M, Puhler A, Simon R. Gene. 1990;89:37–46. doi: 10.1016/0378-1119(90)90203-4. [DOI] [PubMed] [Google Scholar]
- 27.Chen C-Y, Winans SC. J Bacteriol. 1991;173:1139–1144. doi: 10.1128/jb.173.3.1139-1144.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas CM, Smith CA. Annu Rev Microbiol. 1987;41:77–101. doi: 10.1146/annurev.mi.41.100187.000453. [DOI] [PubMed] [Google Scholar]
- 29.Finan TM, Kunker B, De Vos GF, Signer ER. J Bacteriol. 1986;167:66–72. doi: 10.1128/jb.167.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buchanan-Wolloston V, Passiatore JE, Cannon F. Nature. 1987;328:172–175. [Google Scholar]
- 31.Beijersbergen A, Dulk-Ras AD, Schilperoort RA, Hooykaas PJ. Science. 1992;256:1324–1327. doi: 10.1126/science.256.5061.1324. [DOI] [PubMed] [Google Scholar]
- 32.Xiang C, Han P, Lutziger I, Wang K, Oliver DJ. Plant Mol Biol. 1999;40:711–717. doi: 10.1023/a:1006201910593. [DOI] [PubMed] [Google Scholar]
- 33.Cascales E, Atmakuri K, Liu Z, Binns AN, Christie PJ. Mol Microbiol. 2005;58:565–579. doi: 10.1111/j.1365-2958.2005.04852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savvides SN, Yeo H, Beck MR, Blaesing F, Lurz R, Lanka E, Buhrdorf R, Fischer W, Haas R, Waksman G. EMBO J. 2003;22:1969–1980. doi: 10.1093/emboj/cdg223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aravind L, Koonin EV. Nucleic Acids Res. 1999;27:1609–1618. doi: 10.1093/nar/27.7.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grynberg M, Erlandsen H, Godzik A. Trends Bio Sci. 2003;28:224–226. doi: 10.1016/S0968-0004(03)00060-4. [DOI] [PubMed] [Google Scholar]
- 37.Zyl LJ, Deane SM, Rawling DE. J Bacteriol. 2003;185:6104–6111. doi: 10.1128/JB.185.20.6104-6111.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.