Abstract
Activation of the RET (rearranged during transfection) receptor by glial cell-line-derived neurotrophic factor (GDNF) has been identified as an important differentiation and survival factor for dopaminergic neurons of the midbrain in preclinical experiments. These encouraging results have led to clinical trials of GDNF in patients with Parkinson's disease, which have resulted in conflicting findings. To investigate the potential benefit of Ret-dependent signaling on the challenged dopaminergic system, we tested the effect of tissue-selective ablation of the Ret gene on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) toxicity in mice, the most widely used animal model for Parkinson's disease. Ablation of Ret did not modify the MPTP-induced loss of dopaminergic neurons in the substantia nigra pars compacta and the dopaminergic innervation of the striatum at 14 days. However, Ret ablation abolished the regeneration of dopaminergic fibers and terminals, as well as the partial recovery of striatal dopamine concentrations, that was observed in control mice between days 14 and 90 after MPTP treatment. We therefore conclude that RET signaling has no influence on the survival of dopaminergic neurons in the MPTP model of Parkinson's disease but rather facilitates the regeneration of dopaminergic axon terminals.
Keywords: Parkinson's disease; 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; glial cell-line-derived neurotrophic factor; neuroprotection
Endogenous neurotrophic factors regulate physiological cell death during neuronal development, facilitate target innervation, and maintain the survival of neuronal networks during postnatal life. Glial cell-line-derived neurotrophic factor (GDNF) is one of four members of the GDNF family of neurotrophic factors that signal mainly through a two-component receptor complex consisting of the RET (rearranged during transfection) receptor tyrosine kinase and the GPI-linked GDNF family receptor (GFR)α. For the activation of the RET receptor tyrosine kinase, the ligand must first bind to a GFRα (1). Four different GFRα receptors have been identified (GFRα1–4), which determine ligand specificity. GDNF binds to GFRα1, which then forms a complex with RET (1, 2). GDNF was first characterized as a trophic factor that supports differentiation and survival of midbrain dopaminergic neurons (3). GDNF also supports motor neurons (4), noradrenergic neurons (5), and sensory and autonomic neurons (6). In culture, GDNF is a survival factor for primary mesencephalic dopaminergic neurons and protects them against a number of toxic insults [reviewed elsewhere (7)]. In vivo, some controversy has remained as to whether GDNF provides neuroprotection in addition to its well established neurorestorative effects. The protective effects of GDNF against 6-hydroxydopamine-induced and axotomy-induced loss of dopaminergic nigrostriatal neurons appear to be without controversy (8–10). However, several reports (11–13) but not all (14, 15) using either virus-mediated GDNF expression or direct intraparenchymal GDNF delivery into the striatum show protective effects against MPTP/1-methyl-4-phenylpyridine (MPP+) toxicity in mice and monkeys. In addition to the toxin used to challenge the dopaminergic system, the site of GDNF administration (midbrain vs. striatum) appears to be a main determinant. The benefit of GDNF delivery to the striatum is widely accepted [reviewed elsewhere (16)]. In contrast, functional restoration after midbrain administration has been described only by some groups in rodent (17, 18) and monkey (19) models, whereas other results obtained in rodents suggest that midbrain GDNF treatment does not protect against toxic insults (20–22).
Because of its potential to maintain survival and function of dopaminergic neurons, GDNF application has been evaluated in several clinical trials for its effects on Parkinson's disease, which is caused by selective degeneration of these neurons. In the first trial, intracerebroventricular injections of GDNF in humans did not result in symptomatic benefit or slowing of disease progression, likely because of limited intraparenchymal diffusion of GDNF (23). Unilateral or bilateral direct intraparenchymal, putaminal infusions led to substantial beneficial effects in two phase I open-label safety trials (24, 25). However, a similar paradigm was not successful in 34 Parkinson's disease patients in a double-blinded placebo controlled study (26). Not only do methodological questions remain to be resolved (27) but also in-depth studies must be performed on the molecular and signaling effects of GDNF to better characterize and dissect its protective and/or regenerative potential for dopaminergic neurons.
Until recently, neither GDNF nor its receptors have been used in gene ablation analyses to study the effects of GDNF on dopaminergic neurons in vivo, because all of the engineered mice die at birth [reviewed elsewhere (28)]. Two recent studies surprisingly have demonstrated that conditional ablation of RET by using Cre recombinase under control of the dopamine transporter (DAT) does not disturb the development of the nigrostriatal dopaminergic pathway (29, 30). Whereas one study reports no degeneration of the nigrostriatal pathway up to 12 months of age (29), the other study reports a loss of tyrosine hydroxylase (TH)-positive neurons in the substantia nigra pars compacta (SNpc) and TH-positive terminals in the striatum starting at 12 months and progressing thereafter (30). By using the latter mouse model, we addressed the question of whether selective ablation of the Ret receptor in dopaminergic neurons modulates MPTP-induced degeneration of these neurons and their terminals in the striatum and whether it affects the regenerative capacity of the nigrostriatal system.
Results
Ret Deficiency Does Not Increase MPTP Vulnerability.
There was no difference in the number of TH-positive and Nissl-positive SNpc cells between the three genotypes investigated at 14 days and 90 days after saline treatment (Fig. 1 and Table 1). At these time points mice were 12–16 weeks and 25–29 weeks old, respectively. MPTP treatment led to a robust decrease in the number of TH-positive neurons at 14 days after the final MPTP injection. As shown by retrograde labeling of the nigrostriatal pathway, the decrease of TH-positive cells after MPTP treatment reflects the loss of dopaminergic neurons in the SNpc at this time point (14). There was no further change in the number of TH-positive cells between days 14 and 90 after MPTP treatment (Table 1). Similar effects were observed for Nissl-positive neurons in the SNpc, ruling out differences in TH expression (Table 1). These results demonstrate that, at this age, mice with a deficiency of RET do not show a higher cellular vulnerability against MPTP and that MPTP does not trigger an ongoing neurodegeneration in these mice. In addition, these data confirm that there is no spontaneous degeneration of dopaminergic neurons in RET-deficient (DAT-Retlx/lx) mice at the age of 25–29 weeks.
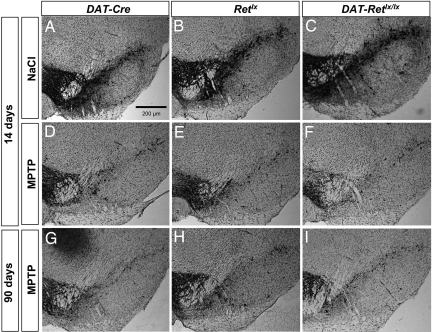
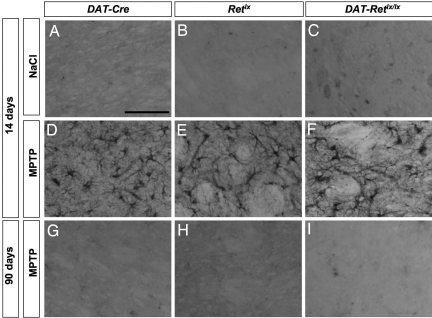
Fig. 1.
MPTP-induced loss of TH-positive SNpc neurons. (A–C) In saline-treated control mice, there was no difference in the number of TH-positive SNpc neurons among the genotypes DAT-Cre (A), Retlx (B), and DAT-Retlx/lx (C). (D–I) In addition, MPTP-induced depletion of TH-positive SNpc neurons did not differ among the genotypes DAT-Cre (D and G), Retlx (E and H), and DAT-Retlx/lx (F and I) at 14 days (D–F) and 90 days (G–I). Furthermore, there was no difference between 14 and 90 days for each of the three genotypes.
Table 1.
Stereological counts for TH-positive and Nissl-positive cells in the SNpc
| Genotype and treatment | TH+ neurons |
Nissl+ cells |
||
|---|---|---|---|---|
| 14 days | 90 days | 14 days | 90 days | |
| DAT-Retlx/lx | ||||
| NaCl | 8,580 ± 460 | 9,390 ± 448 | 11,940 ± 1,029 | 13,300 ± 1,157 |
| MPTP | 4,350 ± 266 | 4,394 ± 1466 | 6,700 ± 359 | 6,366 ± 1,241 |
| Retlx | ||||
| NaCl | 9,650 ± 556 | 8,940 ± 794 | 13,640 ± 1,021 | 13,010 ± 1,374 |
| MPTP | 4,390 ± 938 | 5,634 ± 686 | 7,570 ± 562 | 8,173 ± 1,208 |
| DAT-Cre | ||||
| NaCl | 9,130 ± 942 | 9,220 ± 344 | 12,720 ± 1,780 | 12,870 ± 453 |
| MPTP | 4,256 ± 370 | 4,334 ± 242 | 6,563 ± 732 | 6,813 ± 580 |
Values represent means ± SD. The decrease of TH-positive and Nissl-positive cells after MPTP treatment is significant (P < 0.001; ANOVA followed by Tukey's post hoc test) for each genotype. However, there are no differences for the factors genotype and time.
Requirement of Ret for Regeneration of the Dopaminergic System in the Striatum.
As a neurotrophic factor that is expressed in the striatum, GDNF is considered important for maintaining the integrity of dopaminergic synapses and for the sprouting of nigrostriatal fibers after partial denervation by MPTP. As a measure for the number and function of dopaminergic fibers in the striatum, we quantified the density of TH-stained fibers in the striatum (Fig. 2), quantified the density of DAT positive fibers (Fig. 3), and measured the concentration of dopamine and its metabolites, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), in the striatum (Fig. 4).
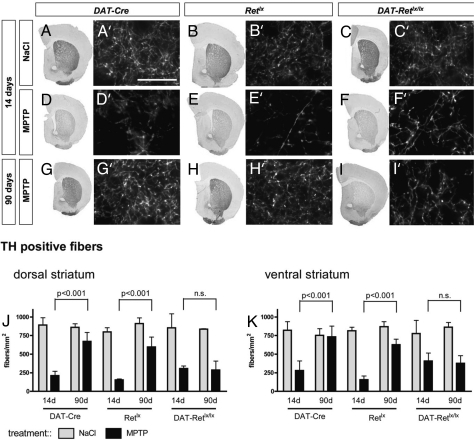
Fig. 2.
MPTP-induced loss of TH-positive striatal fibers. In saline-treated control mice, there was no difference in the number of striatal TH-positive fibers among the genotypes DAT-Cre (A and A′), Retlx (B and B′), and DAT-Retlx/lx (C and C′). In addition, MPTP-induced depletion of TH-positive striatal fibers did not differ among the genotypes DAT-Cre (D and D′), Retlx (E and E′), and DAT-Retlx/lx (F and F′) at 14 days. Striatal fibers showed regeneration between 14 and 90 days in DAT-Cre (G and G′) and Retlx (H and H′) mice but not in DAT-Retlx/lx mice (I and I′). (A–I) The entire frontal sections through the striatum stained for TH by using DAB. (A′–I′) Representative higher-resolution images used for the quantification of striatal fibers. (J and K) TH was stained by immunofluorescence. (Scale bar, 25 μm.) The bar graphs summarize the data for the dorsal (J) and ventral (K) striatum from three to four mice per group. Numbers are means ± SD; ANOVA followed by Tukey's post hoc test. n.s., nonsignificant.
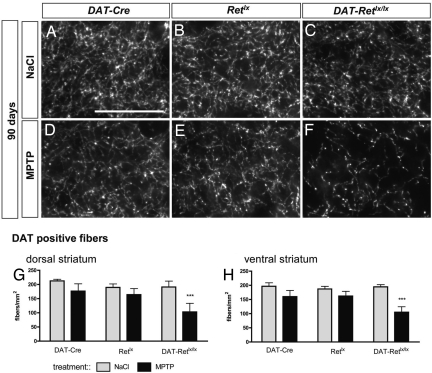
Fig. 3.
DAT fiber density in the striatum. (A–C) At 90 days, there was no difference in the amount of striatal DAT-positive fibers for saline-treated DAT-Cre (A), Retlx (B), and DAT-Retlx/lx (C) mice. (D–F) At 90 days after MPTP treatment, DAT staining was reduced in DAT-Retlx/lx mice (F) but partially recovered in DAT-Cre (D) and Retlx mice (E). (Scale bar, 25 μm.) (G and H) The bar graphs summarize the data for the dorsal (G) and ventral (H) striatum from three to four mice per group at 90 days after vehicle or MPTP treatment. Numbers are means ± SD. ***, P < 0.0001 compared with MPTP-treated DAT-Cre or Retlx mice; ANOVA followed by Tukey's post hoc test.
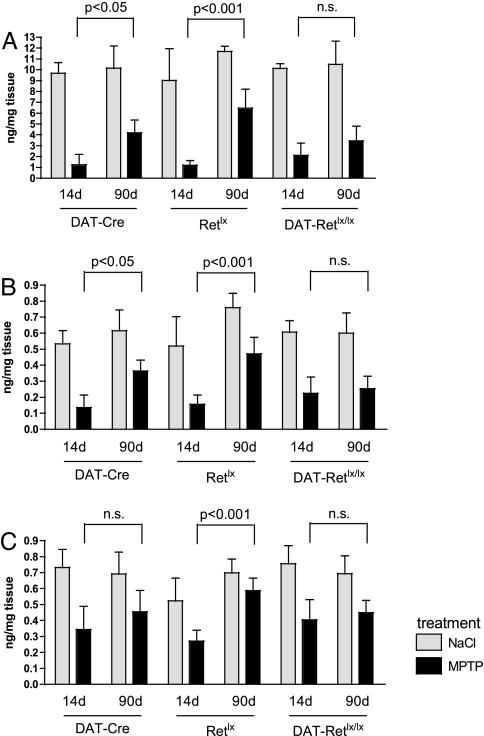
Fig. 4.
Striatal catecholamine concentrations. MPTP reduces striatal dopamine (A), DOPAC (B), and HVA (C) concentrations, which partially recover between 14 and 90 days after MPTP treatment in DAT-Cre and Retlx but not in DAT-Retlx/lx mice. Numbers are means ± SD. For dopamine, DOPAC, and HVA, there were no significant differences among genotypes at 14 days after MPTP treatment. At 90 days, dopamine, DOPAC, and HVA from DAT-Retlx/lx mice were different from Retlx mice (P < 0.01) but not from DAT-Cre mice (two-way ANOVA followed by Tukey's post hoc test).
Treatment with MPTP led to a similar decrease of TH-positive fiber density in all three genotypes at 14 days (Fig. 2), consistent with the reduction in the numbers of SNpc neurons. At 90 days after MPTP treatment, the number of TH-positive fibers (Fig. 2) and DAT-positive fibers (Fig. 3) significantly recovered in the WT DAT-Cre and Retlx mice but not in the Ret-deficient DAT-Retlx/lx mice. Similarly, the striatal concentrations of dopamine, DOPAC, and HVA were substantially decreased at 14 days after MPTP treatment in all three genotypes. Striatal catecholamines substantially recovered in DAT-Cre and Retlx but not in DAT-Retlx/lx mice (Fig. 4). At 90 days, concentrations of dopamine, DOPAC, and HVA were significantly increased as compared with 14 days in DAT-Retlx/lx mice and Retlx mice but not DAT-Cre mice. Because our behavioral analysis did not reveal significant alterations in MPTP vs. saline-treated animals even at 14 days, we were not able to use behavior to demonstrate the functional relevance of this recovery at 90 days [see supporting information (SI) Text]. In summary, the regeneration of striatal dopaminergic axon terminals and the recovery of the dopamine content appears to depend on physiological RET expression in dopaminergic neurons.
In addition to the fiber density in the striatum, which is the terminal field of nigrostriatal fibers, we also measured their density close to their origin, ≈200 μm rostral to the SNpc. The number of TH-positive fibers, as well as the area covered by the nigrostriatal fiber tract, was substantially reduced at 14 days after MPTP treatment and did not recover after 90 days. For both parameters and at both time points, there was no difference among genotypes. These data suggest that recovery does not occur by outgrowth of new axons from the SNpc but likely by sprouting of new axon terminals in the striatum (SI Fig. 6).
Ret-Deficient Mice Show a Normal MPTP Metabolism and DAT and TH Expression.
The toxicity of MPTP depends on its metabolism to MPP+, mediated by the activity of monamine oxidase-B. To rule out possible differences in MPTP metabolism among the genotypes used, we measured striatal MPP+ concentrations at 90 min after an i.p. injection of 30 mg/kg MPTP. The concentrations observed for Retlx (n = 3; 544.6 ± 53.8), DAT-Cre (n = 4; 504 ± 50.5), and DAT-Retlx/lx (n = 3; 501 ± 14.2) mice were not different among groups and did not explain the group differences that we observed in the recovery of dopaminergic markers. Because DAT protein levels might limit the uptake of MPP+ into dopaminergic neurons, we also analyzed these in our mice. In agreement with our previous report (30), the expression of DAT was unaffected at 3 months in mice carrying the DAT–Cre knockin construct (DAT-Cre controls and DAT-Retlx/lx mutants) (SI Fig. 7). Therefore, the observed regeneration defect in the Ret-deficient mice should not be attributable to an altered MPP+ generation or uptake but should mainly be caused by the loss of Ret protein expression.
GDNF-mediated Ret signaling has been implicated in the regulation of TH expression (31). We therefore investigated whether deficiency of Ret had an influence on the mRNA and protein expression of TH. In DAT-Retlx/lx mice, there was no alteration in the expression of TH mRNA and protein (SI Fig. 8).
MPTP-Treated Ret-Deficient Mice Do Not Show a Persistent Gliosis.
In agreement with earlier results, treatment with MPTP led to an activation of GFAP expression in astrocytes (Fig. 5) at 14 days after MPTP treatment in the striatum. At 90 days after MPTP, this induction was no longer detectable. Neither at 14 days nor at 90 days after MPTP treatment was there a difference among the genotypes. Despite the pronounced defect in regeneration in Ret-deficient mice, they did not show a persistent gliosis in the striatum.
Fig. 5.
MPTP-induced gliosis. (A–C) Immunohistochemical staining for GFAP in the striatum in DAT-Cre (A), Retlx (B), and DAT-Retlx/lx (C) mice. (D–F) There is a robust increase in GFAP-positive reactive astrocytes at 14 days after MPTP treatment, with no difference among genotypes. (G–I) At 90 days, the number of GFAP-positive astrocytes is back to baseline levels. (Scale bar, 100 μm.)
Discussion
By using a conditional Ret-deficient mouse model, we show here that Ret-dependent signaling does not modulate the MPTP-induced degeneration of dopaminergic neurons in the SNpc and dopaminergic innervation of the striatum. In other words, signaling through RET mediated by physiological concentrations of neurotrophic factors does not provide protection against MPTP-induced loss of dopaminergic neurons and its terminals. However, synaptic markers, consisting of catecholamines and number of TH-positive fibers, increased between the early (14 days) effects of MPTP toxicity and the later time point (90 days) in mice expressing RET (DAT-Cre, Retlx) but not in mice with a deficiency of RET (DAT-Retlx/lx). RET signaling induced by endogenous concentrations of neurotrophic factors therefore does not provide protection against MPTP toxicity but facilitates regrowth of dopaminergic axon terminals. The mechanism likely involves a sprouting response in the terminal field of the nigrostriatal pathway but not the outgrowth or recovery of axons originating from the somata of injured cells or from newborn cells in the SNpc. Although initially controversial, there is to date no evidence for neurogenesis of dopaminergic neurons after lesioning of the nigrostriatal pathway (32). In addition, an increase of dopaminergic interneurons in the striatum after MPTP intoxication, resulting from a phenotypic shift and not from neurogenesis, is only seen in primates but not in rodents (33).
Besides activation of RET signaling by GDNF and other members of the GDNF family of ligands, other neurotrophic signaling pathways might also be involved in the recovery of the dopaminergic system after MPTP treatment. Experiments addressing, for example, the function of BDNF-dependent signaling may provide insights into how different trophic systems cooperate in regenerating the dopaminergic system after toxic insults.
Our results are unlikely to be influenced by factors that may alter MPTP toxicity because (i) the number of dopaminergic neurons, the density of TH-positive and DAT-positive fibers, and the expression of the DAT, as measured by Western blot, were not different among genotypes; (ii) the conversion of MPTP to MPP+, the active metabolite, was not different among the genotypes; and (iii) the glial cell response was not different among genotypes. Interestingly in all MPTP-treated mice (including the DAT-Retlx/lx mice, which do not show efficient recovery of the dopaminergic system) the gliosis disappears again at the late time point. Astrocytes thus are activated only during active degeneration in this mouse model.
The results presented here are valid for a specific mouse model of Parkinson's disease, the MPTP model, but may differ in other models. In the absence of a perfect transgenic mouse model for Parkinson's disease that would combine the cytoplasmic aggregation of insoluble α-synuclein with degeneration of dopaminergic synapses and neurons, the MPTP model still appears to be the superior model of Parkinson's disease to study mechanisms of cell death, as well as strategies for degeneration and regeneration in vivo (34, 35). It replicates many of the biochemical and neuropathological features of Parkinson's disease, including the inhibition of complex I of the mitochondrial electron transport chain, generation of reactive oxygen species, induction of inflammation, and degeneration of dopaminergic neurons and neurites. In several patients, MPTP i.v. injections in humans has been shown also to cause a Parkinsonian syndrome that is almost indistinguishable from Parkinson's disease. In contrast, the toxicity of 6-hydroxydopmine is mediated primarily by the generation of reactive oxygen species. Both toxins rely on being taken up through the DAT to selectively kill dopaminergic neurons at low concentrations. Whereas the protective and regenerative effects of GDNF against 6-hydroxydopamine toxicity are not disputed, only few reports clearly describe protective effects of GDNF against MPTP/MPP+-induced death of dopaminergic neurons in addition to regenerative effects (11–13). Moreover, the protective effects of GDNF against MPTP toxicity may require TGF-β (13), likely to recruit GFRα1 to the plasma membrane (36). We have reported recently that we did not observe any effect on cell survival of dopaminergic neurons in mice after a subchronic MPTP intoxication paradigm of 5 × 30 mg/kg MPTP spaced by 24 h when using adenovirus-mediated expression of bioactive GDNF in the striatum (14). Nevertheless, we observed in the same mice a robust protection against the MPTP-induced decrease in striatal catecholamine concentrations. These data are congruent with the results presented here showing that GDNF-induced RET signaling does not provide protection against MPTP toxicity. In our earlier study, the additional therapy with an adenovirus-mediated expression of the anti-apoptotic X-chromosome-linked inhibitor of apoptosis (XIAP) was necessary to also block MPTP-induced death of dopaminergic SNpc neurons. A similar synergistic interplay of GDNF and XIAP has been observed in motor neurons (37).
In contrast to the lack of RET-signaling effects in MPTP toxicity among the ages of 3 and 7 months, RET signaling is required for long-term maintenance of the nigrostriatal system during aging (30). In this study, loss of dopaminergic neurons in the SNpc and dopaminergic fibers in the striatum was first observed at 12 months of age and progressed thereafter, whereas in another study, no differences compared with controls are observed at 12 months of age (29). We intentionally did not use older mice for our study of MPTP toxicity to avoid time points at which degenerative processes in Ret-deficient mice were already occurring.
Tyrosine kinases signal through the MAPK and/or the PI3-kinase pathways. It has been shown recently that when GDNF is injected into the striatum of rats, higher concentrations are necessary for the activation of PI3-kinase/AKT than for the activation of the MAPK ERK1/2, and only the higher concentrations provide protection against MPTP toxicity (38). GDNF may thus have qualitatively different effects on RET-induced signaling at different concentrations. Consistent with this finding, the use of different dosages is one of the potential explanations for the failure in a recent placebo-controlled trial of intraputaminal GDNF infusions to show efficacy in contrast to earlier open-label trials. Other possible explanations are underestimation of the placebo effect in earlier open-label studies, the use of different catheters influencing GDNF diffusion, and the development of neutralizing antibodies (27). Another explanation for the discrepant findings between GDNF administration and RET deficiency is that GDNF may also signal through RET-independent pathways. It has been shown in cell lines and primary neuronal cells that GDNF may signal independently of RET through GFRα-1 to activate Src family kinase (39, 40) or regulate neural cell adhesion molecule-mediated cell adhesion (41). Furthermore, GDNF-mediated presynaptic differentiation does not depend on RET but GFRα-1 (42). Therefore, it would be interesting to evaluate whether treatment with exogenous GDNF shows still effects in the dopaminergic system of RET-deficient mice.
Materials and Methods
Transgenic Animals.
To selectively disrupt the gene encoding RET in dopaminergic neurons, we used mice with a floxed allele of Ret (Retlx) (43) in combination with DAT-Cre mice (44), resulting in RET deficiency in dopaminergic neurons (DAT-Retlx/lx mice). A detailed characterization of these mice has been published recently (30). Mice carrying one copy of DAT-Cre and mice heterozygous or homozygous for the Ret floxed allele (Retlx) served as controls.
Experimental Animal Procedures.
Twelve- to 16-week-old male DAT-Retlx/lx mice, DAT-Cre mice, or Retlx mice were treated with either MPTP hydrochloride or saline. MPTP was administered in 0.1 ml of saline at a dose of 30 mg/kg i.p. (freebase) at 24-h intervals over 5 consecutive days. Half of the animals of each genotype were killed at 14 days after the last MPTP injection, and the other half were killed at 90 days. The left striata were rapidly dissected, frozen, and stored at −80°C until the catecholamine concentrations, dopamine, DOPAC, and HVA were measured by HPLC with electrochemical detection (14). The right striata and the posterior parts of the same brains, containing the substantia nigra, were fixed for 24 h in 4% PFA and cryoprotected in 30% sucrose for 2 days at 4°C. Brains were then frozen on dry ice and stored at −80°C. Four to seven mice were used for each genotype and each time point. MPTP handling and safety measures were in accordance with published guidelines (45). The animal care and use committees of the regional government (Braunschweig, Germany) approved all procedures in this study.
Histology, Immunohistochemistry, and Quantification.
For quantification of dopaminergic neurons, we obtained 30-μm serial cryosections of the entire substantia nigra. Every fourth section was stained for TH (Chemicon polyclonal 1:1,000) by using diaminobenzidine (DAB) (Vectastain ABC Kit Standard PK-4000, Vector Laboratories) and for Nissl. TH-positive and Nissl-positive neurons in the substantia nigra were counted by stereology by using the optical fractionator method (StereoInvestigator, MBF Bioscience). Counting was performed blinded for treatment, by using an oil immersion ×63 objective (Axioskop 2, Zeiss), a counting frame of 50 × 50 μm, and a grid size of 100 × 125 μm.
Dopaminergic fiber density in the striatum was assessed in on average six sections between bregma +1.10 and −0.10 mm by using staining for TH and the DAT as described previously (30), with minor modifications. All antibody incubation steps were performed overnight, and wash steps were always for 10 min. For every section, five pictures were acquired in the dorsal striatum and five pictures in the ventral striatum. To automatically delineate the fibers and to increase the signal-to-noise ratio, the images were first thresholded and subsequently quantified with an automatic counting-grid macro implemented in the Metamorph software (Molecular Devices). Assessment of GFAP-positive astrocytes was also carried out as described previously (30).
MPTP Metabolism.
MPP+ levels in the brain were determined by HPLC. Three to four mice of each genotype were injected with 30 mg/kg MPTP i.p. and killed 90 min later. Striata were dissected quickly on ice and homogenized in 20 μl of 0.1 M perchloric acid per milligram of tissue, and debris was removed by high-speed centrifugation. Twenty microliters of supernatant was injected onto a reverse-phase column (Nucleosil-100 C18, Knauer) and quantified by UV absorption at 300 nm (UVD340U, Dionex) by using Chromeleon 6.60 software. The mobile phase consisted of 697/1,000 ml acetonitrile in phosphate buffer (pH 2.5). The flow rate was 0.5 ml/min. Values represent picomoles of MPP+ per milligram of wet tissue.
Statistical Analysis.
Data are expressed as means ± SD. The statistical analysis was performed by ANOVA, followed by Tukey's post hoc test to compare group means with GraphPad Prism 4.0 (GraphPad Software). For the analysis of catecholamine concentrations in the striatum, we also used two-way ANOVA with factor 1 for treatment (MPTP or NaCl) and factor 2 for group defined by genotype and time after MPTP treatment. In all analyses, the null hypothesis was rejected at the 0.05 level.
Supplementary Material
Acknowledgments
We thank Roland Guckler (Visitron Systems GmbH, Puchheim, Germany) for providing the MetaMorph Macros that was used for quantifying the striatal innervation; P. Ghahraman, A. Dengler, and S. Englhart for technical assistance; and C. Ludwig for secretarial assistance. The study was supported by the Deutsche Forschungsgemeinschaft (research center, Molecular Physiology of the Brain) (J.B.S.), Deutsche Forschungsgemeinschaft Grant SFB596 (to R.K.), and the European Union through NeuroNE (R.K.) and a European Molecular Biology Organization Long-Term Fellowship (to E.R.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706177104/DC1
References
- 1.Airaksinen MS, Saarma M. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 2.Bespalov MM, Saarma M. Trends Pharmacol Sci. 2007;28:68–74. doi: 10.1016/j.tips.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 4.Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Simpson LC, et al. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- 5.Arenas E, Trupp M, Akerud P, Ibanez CF. Neuron. 1995;15:1465–1473. doi: 10.1016/0896-6273(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 6.Trupp M, Ryden M, Jornvall H, Funakoshi H, Timmusk T, Arenas E, Ibanez CF. J Cell Biol. 1995;130:137–148. doi: 10.1083/jcb.130.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krieglstein K. Cell Tissue Res. 2004;318:73–80. doi: 10.1007/s00441-004-0920-8. [DOI] [PubMed] [Google Scholar]
- 8.Beck KD, Valverde J, Alexi T, Poulsen K, Moffat B, Vandlen RA, Rosenthal A, Hefti F. Nature. 1995;373:339–341. doi: 10.1038/373339a0. [DOI] [PubMed] [Google Scholar]
- 9.Bilang-Bleuel A, Revah F, Colin P, Locquet I, Robert JJ, Mallet J, Horellou P. Proc Natl Acad Sci USA. 1997;94:8818–8823. doi: 10.1073/pnas.94.16.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi-Lundberg DL, Lin Q, Chang Y-N, Chiang YL, Hay CM, Mohajeri H, Davidson BL, Bohn MC. Science. 1997;275:838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- 11.Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, Olson L. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- 12.Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, et al. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- 13.Schober A, Peterziel H, von Bartheld CS, Simon H, Krieglstein K, Unsicker K. Neurobiol Dis. 2007;25:378–391. doi: 10.1016/j.nbd.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Eberhardt O, von Coelln R, Kügler S, Lindenau J, Rathke-Hartlieb S, Gerhardt E, Haid S, Isenmann S, Gravel C, Srinivasan A, et al. J Neurosci. 2000;20:9126–9134. doi: 10.1523/JNEUROSCI.20-24-09126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietz GP, Valbuena PC, Dietz B, Meuer K, Mueller P, Weishaupt JH, Bahr M. Brain Res. 2006;1082:61–66. doi: 10.1016/j.brainres.2006.01.083. [DOI] [PubMed] [Google Scholar]
- 16.Kirik D, Georgievska B, Bjorklund A. Nat Neurosci. 2004;7:105–110. doi: 10.1038/nn1175. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman AF, van Horne CG, Eken S, Hoffer BJ, Gerhardt GA. Exp Neurol. 1997;147:130–141. doi: 10.1006/exnr.1997.6571. [DOI] [PubMed] [Google Scholar]
- 18.Bowenkamp KE, Hoffman AF, Gerhardt GA, Henry MA, Biddle PT, Hoffer BJ, Granholm AC. J Comp Neurol. 1995;355:479–489. doi: 10.1002/cne.903550402. [DOI] [PubMed] [Google Scholar]
- 19.Gash DM, Zhang Z, Ai Y, Grondin R, Coffey R, Gerhardt GA. Ann Neurol. 2005;58:224–233. doi: 10.1002/ana.20549. [DOI] [PubMed] [Google Scholar]
- 20.Kirik D, Rosenblad C, Bjorklund A. Eur J Neurosci. 2000;12:3871–3882. doi: 10.1046/j.1460-9568.2000.00274.x. [DOI] [PubMed] [Google Scholar]
- 21.Kirik D, Rosenblad C, Bjorklund A, Mandel RJ. J Neurosci. 2000;20:4686–4700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connor B, Kozlowski DA, Schallert T, Tillerson JL, Davidson BL, Bohn MC. Gene Ther. 1999;6:1936–1951. doi: 10.1038/sj.gt.3301033. [DOI] [PubMed] [Google Scholar]
- 23.Kordower JH, Palfi S, Chen EY, Ma SY, Sendera T, Cochran EJ, Mufson EJ, Penn R, Goetz CG, Comella CD. Ann Neurol. 1999;46:419–424. doi: 10.1002/1531-8249(199909)46:3<419::aid-ana21>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 24.Gill SS, Patel NK, Hotton GR, O'Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P. Nat Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- 25.Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B. J Neurosurg. 2005;102:216–222. doi: 10.3171/jns.2005.102.2.0216. [DOI] [PubMed] [Google Scholar]
- 26.Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, et al. Ann Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- 27.Sherer TB, Fiske BK, Svendsen CN, Lang AE, Langston JW. Mov Disord. 2006;21:136–141. doi: 10.1002/mds.20861. [DOI] [PubMed] [Google Scholar]
- 28.Baloh RH, Enomoto H, Johnson EM, Jr, Milbrandt J. Curr Opin Neurobiol. 2000;10:103–110. doi: 10.1016/s0959-4388(99)00048-3. [DOI] [PubMed] [Google Scholar]
- 29.Jain S, Golden JP, Wozniak D, Pehek E, Johnson EM, Jr, Milbrandt J. J Neurosci. 2006;26:11230–11238. doi: 10.1523/JNEUROSCI.1876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kramer ER, Aron L, Ramakers GM, Seitz S, Zhuang X, Beyer K, Smidt MP, Klein R. PLoS Biol. 2007;5:e39. doi: 10.1371/journal.pbio.0050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenblad C, Georgievska B, Kirik D. Eur J Neurosci. 2003;17:260–270. doi: 10.1046/j.1460-9568.2003.02456.x. [DOI] [PubMed] [Google Scholar]
- 32.Frielingsdorf H, Schwarz K, Brundin P, Mohapel P. Proc Natl Acad Sci USA. 2004;101:10177–10182. doi: 10.1073/pnas.0401229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tande D, Hoglinger G, Debeir T, Freundlieb N, Hirsch EC, Francois C. Brain. 2006;129:1194–1200. doi: 10.1093/brain/awl041. [DOI] [PubMed] [Google Scholar]
- 34.Dawson T, Mandir A, Lee M. Neuron. 2002;35:219–222. doi: 10.1016/s0896-6273(02)00780-8. [DOI] [PubMed] [Google Scholar]
- 35.Beal MF. Nat Rev Neurosci. 2001;2:325–334. doi: 10.1038/35072550. [DOI] [PubMed] [Google Scholar]
- 36.Peterziel H, Unsicker K, Krieglstein K. J Cell Biol. 2002;159:157–167. doi: 10.1083/jcb.200203115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perrelet D, Ferri A, Liston P, Muzzin P, Korneluk RG, Kato AC. Nat Cell Biol. 2002;4:175–179. doi: 10.1038/ncb751. [DOI] [PubMed] [Google Scholar]
- 38.Lindgren N, Leak RH, Smith AD, Zigmond MJ. Soc Neurosci Abstr. 2006;756:7. [Google Scholar]
- 39.Poteryaev D, Titievsky A, Sun YF, Thomas-Crusells J, Lindahl M, Billaud M, Arumae U, Saarma M. FEBS Lett. 1999;463:63–66. doi: 10.1016/s0014-5793(99)01590-2. [DOI] [PubMed] [Google Scholar]
- 40.Trupp M, Scott R, Whittemore SR, Ibanez CF. J Biol Chem. 1999;274:20885–20894. doi: 10.1074/jbc.274.30.20885. [DOI] [PubMed] [Google Scholar]
- 41.Paratcha G, Ledda F, Ibanez CF. Cell. 2003;113:867–879. doi: 10.1016/s0092-8674(03)00435-5. [DOI] [PubMed] [Google Scholar]
- 42.Ledda F, Paratcha G, Sandoval-Guzman T, Ibanez CF. Nat Neurosci. 2007;10:293–300. doi: 10.1038/nn1855. [DOI] [PubMed] [Google Scholar]
- 43.Kramer ER, Knott L, Su F, Dessaud E, Krull CE, Helmbacher F, Klein R. Neuron. 2006;50:35–47. doi: 10.1016/j.neuron.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 44.Zhuang X, Masson J, Gingrich JA, Rayport S, Hen R. J Neurosci Methods. 2005;143:27–32. doi: 10.1016/j.jneumeth.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 45.Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akram M. J Neurochem. 2001;76:1265–1274. doi: 10.1046/j.1471-4159.2001.00183.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.