Abstract
Amputees cannot feel what they touch with their artificial hands, which severely limits usefulness of those hands. We have developed a technique that transfers remaining arm nerves to residual chest muscles after an amputation. This technique allows some sensory nerves from the amputated limb to reinnervate overlying chest skin. When this reinnervated skin is touched, the amputees perceive that they are being touched on their missing limb. We found that touch thresholds of the reinnervated chest skin fall within near-normal ranges, indicating the regeneration of large-fiber afferents. The perceptual identity of the limb and chest was maintained separately even though they shared a common skin surface. A cutaneous expression of proprioception also occurred in one reinnervated individual. Experiments with peltier temperature probes and surface electrical stimulation of the reinnervated skin indicate the regeneration of small diameter temperature and pain afferents. The perception of an amputated limb arising from stimulation of reinnervated chest skin may allow useful sensory feedback from prosthetic devices and provides insight into the mechanisms of neural plasticity and peripheral regeneration in humans.
Keywords: regeneration, neural machine interface, touch, artificial limbs
The loss of an arm is a singularly debilitating injury. Improving the function of artificial arms remains a considerable challenge, especially for high-level amputations where the disability is greatest. A primary impediment to better function is that current prostheses provide very little sensory feedback. The amputees must rely primarily on vision to manipulate objects, and they cannot feel what they touch with motorized prosthetic hands. This limitation greatly increases the cognitive burden on the amputee and impedes the use of the artificial limb.
We have developed a neural–machine interface called targeted reinnervation (TR) that provides enhanced motor control and the potential for meaningful sensation feedback for artificial arms (1–3). The amputated brachial plexus nerves that once provided motor control and sensory feedback in the missing limb are transferred to arm and chest muscles that remain after the amputation. Once reinnervated, these muscles produce electromyogram (EMG) signals that correspond to the original arm control signals sent from the brain down the brachial plexus nerves. This process provides improved and more intuitive control of a motorized artificial arm (4–6). The contractions of the reinnervated muscles function as biological amplifiers for the motor commands transmitted by the amputated arm nerves. Concurrently, the sensory nerve fibers in these amputated nerves appear to reinnervate the skin overlying the target muscles. When this reinnervated skin is touched, the amputee feels as if the missing hand is being touched. This skin reinnervation may provide a direct portal to the sensory pathways of the amputated arm and hand. It could potentially provide an amputee with the ability to feel what he touches with a prosthetic hand as although it were his own hand. In addition, TR is a unique somatosensory model that will help us to understand better the mechanisms of peripheral nerve regeneration and neural plasticity. Here, we provide evidence that amputated-hand nerves can establish a cutaneous expression of sensation, referred to the missing limb, in foreign skin. These regenerating afferents appear to traverse through muscle and breast tissue to the skin and occupy skin territory that is also innervated by native-chest afferents. This work examines the intriguing sense of touch, temperature, and pain from the hand that developed in the chest skin of two patients who had undergone TR surgery after amputations at the shoulder.
Results
Touch Sensation.
Patient BSD was a 54-year-old man who lost his arms at the shoulder because of electrical burns. At 9 months after injury, his remaining median, ulnar, radial, and musculocutaneous nerves were transferred to different segments of his left pectoral chest muscles (1, 2). All of the s.c. fat on his chest was removed to bring the skin in contact to the muscle to maximize the strength of the EMG signals. His chest skin was not purposely denervated, and no insensate areas developed (Fig. 1A). Within 5 months he developed sensations, referred to his missing limb, when he was touched on this chest. These evoked sensations were perceived as occurring on his phantom limb. He was able to feel these sensations over an area 15 cm across × 17 cm high. This new limb sensation area was mapped by using a cotton-tipped probe that indented the skin with 300 grams applied force (gAF) (Fig. 2). When touched at a single point, he generally felt pressure in large areas of his hand, localized to either the palmar or dorsal sides of his missing limb.
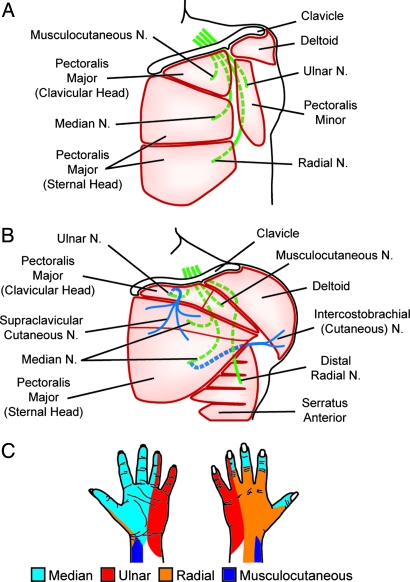
Fig. 1.
Schematics of the TR surgeries. Green, arm nerves; blue, skin sensory nerves; dotted lines, nerves routed beneath muscles. (A) Patient BSD. The pectoralis muscles were denervated, s.c. fat over his chest muscle was removed, and the four major arm nerves were sewn to the remnant nerves of each muscle segment. (B) The nerve transfers were performed differently in patient STH. To increase the likelihood of sensory reinnervation, skin nerves were cut, and the distal ends were sewn to the hand nerves to provide neural conduits for the regenerating nerve fibers. (C) Diagrams of skin sensation provided by each nerve in the normal hand (53).
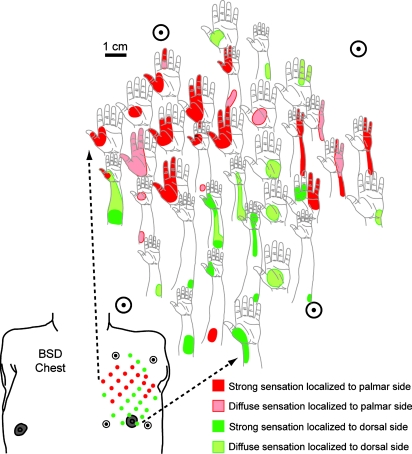
Fig. 2.
The reinnervated chest skin of patient BSD showing sensations referred to the missing limb elicited by indentation of the skin by a cotton-tipped probe (300 gAF). Referred sensations localized to either the palm side (red) or the back side (green) of the missing limb. Circled points at the corners serve as registers to orient the diagram.
Touching points on the medial aspect of this reinnervated region produced evoked sensations that were projected to the lateral palmer hand, and digits 1–4 while points touched on the lateral aspect evoked sensations referred to the medial palmer hand, the fourth and fifth digits. Touching points on the inferior aspect of this area elicited sensations referred to the back of the hand and posterior aspect of the forearm. Sensation referred to the lateral forearm was evident at a single point at the superior aspect of the reinnervation area. The expression of referred-limb sensation in patient BSD appeared to project to skin surfaces normally innervated by the four large transferred nerves (for comparison with the sensory innervation of a normal hand, see Fig. 1C). The regions of referred sensation also corresponded well with the positions of these underlying transferred nerves. However, the referred sensations evident within each region did not appear to display any somatotopic organization.
Patient STH was a 24-year-old woman with a left upper arm amputation resulting from a motor vehicle collision. At 15 months after injury, her median, ulnar, musculocutaneous, and distal radial nerves were redirected to segments of her pectoralis and serratus anterior muscles (3) (Fig. 1B). Her supraclavicular cutaneous nerve was cut and sewn to the side of her ulnar nerve to provide a neural conduit to the skin. Cutting this nerve created a numb area 11 cm across × 9 cm high on the front of her chest. The intercostal brachial cutaneous nerve was cut and sewn to the side of the median nerve in an effort to encourage skin reinnervation; however, this nerve transfer appeared to be mostly unsuccessful (3). Within 4 months, the numb area on front of her chest developed touch sensation referred to regions of the hand and wrist. As with BSD, these sensations were perceived by STH as occurring on her phantom limb. When touched, STH perceived a tingling sensation, as opposed to a feeling of pressure. The tingling sensation increased in intensity with increasing pressure.
Patient STH generally felt small, discreet, and detailed areas of referred-hand sensation when touched at single points on her chest (Fig. 3). However, she did have specific points where sensation was referred to larger areas of her hand and even noncontiguous areas. Pushing on most points caused STH to feel a distinct sensation referred to the palm side of her hand; however, at some points the evoked percepts were less discreet and difficult to localize specifically to one side or the other (see red and blue areas of Fig. 3). She did not report evoked sensations referred to the back surface of her hand. Touching points within the medial and superior regions of the reinnervated chest skin evoked clear sensations of the first and second digits. A smaller group of points within the inferior and lateral region of chest skin elicited sensations of the fourth and fifth digits. Pressing a single point at the lateral border of the skin overlying the nerve transfer sites evoked sensation that was referred to the distal forearm. The sensations evoked by stimulating her chest were referred to regions of her missing hand that were normally served by the median, ulnar, and musculocutaneous nerves (for comparison with the sensory innervation of a normal hand, see Fig. 1C). The points on the chest that evoked sensations referred to median-nerve territories were in good register with the underlying median-nerve transfer site. However, the points where sensations were referred to areas of the hand, normally innervated by the ulnar nerve, were displaced to a position far from the ulnar-nerve transfer site near her clavicle. As with BSD, there did not appear to be any specific somatotopic order to the referred-hand sensations.
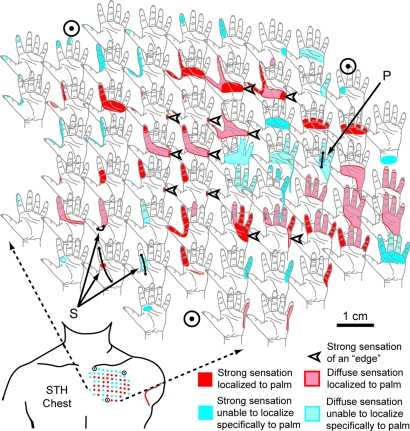
Fig. 3.
The reinnervated chest skin of patient STH showing sensations referred to the missing limb elicited by indentation of the skin by a cotton-tipped probe (300 gAF). Red, referred sensation points localized to the palm side of the hand. Blue, points where a general diffuse feeling of pressure was felt within the hand. Circled points orient the diagram. P, proprioceptive sensation of fourth finger joint position. S, sensation of skin stretch. Double-headed arrows, direction of stretch. Arrowheads, edge sensation.
STH also had different types of evoked sensations when her chest was touched. When a particular point on her chest was touched, STH reported the distinct sensation that her fourth finger was bent back (see Fig. 3, P). Touching a number of different points evoked the sensation that the ulnar border of her hand was being pressed onto an edge (see Fig. 3, arrowheads). Finally, she had evoked sensations of skin stretching (see Fig. 3, S), where she reported that the skin of the second digit was being tugged in line with the digit and that the webbing between the first and second digits was being stretched.
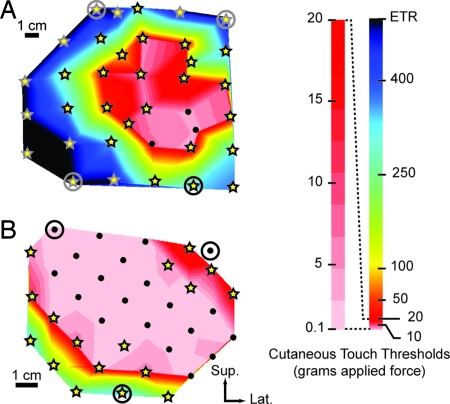
The reinnervated chest skin was tested with Semmes–Weinstein monofilaments to determine how light of a touch the patients could perceive. In patient BSD, touch thresholds of sensation referred to the missing hand ranged from ≈0.6 to 446.7 gAF (Fig. 4A). Fifty percent of all measured thresholds were at levels ≤8.5 gAF. At 92% of the examined points on the chest of BSD, there was an overlap between the native-chest sensation and the new referred-hand sensation (see Fig. 4A, stars). At three points within the region of lowest thresholds the sensation of only the missing limb was present with no evidence of native-chest sensation (see Fig. 4A, black dots). BSD reported that he was able to distinguish clearly between chest and referred-hand sensations, and at most of the points where overlap occurred the patient felt sensation in his chest first at a lower threshold and then referred-hand sensations at a higher threshold (see Fig. 4A, stars). In the areas of lower thresholds, the difference between referred-hand and chest thresholds was small (0–4 gAF). However, the difference in thresholds between referred hand and chest increased dramatically (up to 338 gAF) at points near the outer margins. At one point, within the lowest threshold region, the referred-hand sensation was felt at a lower average threshold than the chest (see Fig. 4A, light pink region). The average touch threshold at a point on the contralateral side of his chest was 1.1 gAF.
Fig. 4.
Contour plots of the average amount of force required for patients to feel touch projected to the missing limb. (A) BSD. (B) STH. Sup., superior; Lat., lateral. Black dots, points where only the missing limb was felt. Stars, points where chest was felt first at lower thresholds, and then the missing limb was felt at higher thresholds. Color bar is expanded from 0.1 to 20 gAF to provide detail. Circled points orient the plot (see Figs. 2 and 3). ETR, exceeded testing range.
Referred-touch sensation thresholds in patient STH ranged from ≈0.4 to 300 gAF. Fifty percent of all thresholds were at levels ≤2 gAF. These points were mostly referred only to the missing hand, with no evidence of chest sensation (see Fig. 4B, black dots). At 53% of the measured points on the chest of STH, there was an overlap between the native-chest sensation and referred-hand sensation (see Fig. 4B, stars). STH was also able to distinguish clearly between the two types of simultaneously occurring sensation. Generally, she felt her chest first at a lower threshold and then referred-hand sensations at a higher threshold; occasionally, both sensations were felt at the same threshold. Where referred-hand and native-chest sensations overlapped, in the lowest threshold region, the differences in force were quite small (0.2–0.4 gAF). At points closer to the margins of the regenerated sensation, the difference between hand and chest thresholds increased considerably, up to 299 gAF. Average touch thresholds on her contralateral chest and index finger were 0.87 gAF and 0.16 gAF, respectively.
Temperature Thresholds.
Select areas of the reinnervated chest skin of both patients were tested for thermal sensation thresholds. In BSD, in an area where monofilaments elicited only sensation referred to the missing limb, average thresholds for cold sensation, warm sensation, and heat pain were 28.8 ± 0.1°C (±standard error), 36.3 ± 0.2°C, and 45.4 ± 0.9°C, respectively. Average thresholds for cold sensation, warm sensation, and heat pain on his normal side were 24.3 ± 2.2, 38.4 ± 0.6, and 49.9 ± 0.1°C, respectively. The cold pain thresholds on both sides were <0.0°C (cutoff limit for the TSA II neurosensory analyzer). BSD's back was also tested to account for scarring of the normal chest that might affect thermal thresholds. Average thresholds for cold sensation, warm sensation, cold pain, and heat pain were 30.8 ± 0.1, 34.4 ± 0.3, <0.0, and 48.2 ± 1.2°C, respectively. Sensations associated with the thermal thresholds were referred to the back of his missing hand.
In STH, in an area where monofilaments elicited only sensation referred to the missing limb, average thresholds for cold sensation, warm sensation, cold pain, and heat pain were 31.0 ± 0.2, 32.9 ± 0.0, 11.1 ± 2.3, and 40.1 ± 0.7°C, respectively. On STH's contralateral normal chest, average thresholds for cold sensation, warm sensation, cold pain, and heat pain were 30.3 ± 0.1, 35.2 ± 0.1, 5.8 ± 2.7, and 43.7 ± 1.2°C, respectively. The average thresholds for cold sensation, warm sensation, cold pain, and heat pain on her intact thenar eminence were 30.0 ± 0.3, 34.2 ± 0.1, 1.0 ± 0.5, and 47.6 ± 0.6°C, respectively. Sensations associated with the thermal thresholds were referred to the base of the second digit of her missing hand.
Electrical Stimulation Induced Sensory and Pain Thresholds.
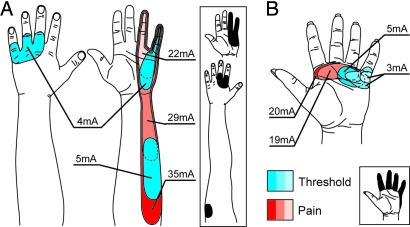
BSD and STH were tested for sensory and pain thresholds by using surface electrical stimulation. BSD was tested at five reinnervated chest locations and at one site on his contralateral chest. One reinnervated skin testing site was centered over points that elicited only referred sensation when pressed (Fig. 5A). At all measured sites, nonpainful sensations (sensory threshold) were elicited at currents ranging from 2.0 to 5.0 mA (reinnervated average, 3.7 ± 0.4 mA; contralateral normal average, 2.7 ± 2.7 mA). At the site where pressing the skin elicited only referred-limb sensations, the average sensory threshold was 4.3 ± 0.3 mA. At threshold levels, sensations were most often referred only to his missing hand and not his chest. At all measured sites, painful sensations were elicited at currents ranging from 11.0 to 36.0 mA (reinnervated average, 25.6 ± 3.9 mA; contralateral normal average, 36.3 ± 2.2 mA). The painful sensations on the reinnervated side were most frequently felt both on his chest and referred to his missing hand. In addition, as current levels increased, the surface area of the sensation referred to his missing limb increased.
Fig. 5.
Projected sensations elicited by electrical stimulation. (A) (BSD). (B) STH. Projected fields in blue indicate sensation (threshold) levels, and those in red indicate painful stimulation levels. Diagrams are of electrode positions within areas where sensation is projected to the missing limb only (determined by monofilaments). (Insets) Composites of mechanosensory projected fields (300 gAF) at points corresponding to the placement of the electrical stimulation electrodes.
STH was tested at four locations on her reinnervated chest and at single sites on her contralateral chest and index finger. Two of the four reinnervated skin testing sites were centered over points that elicited only referred sensation when pressed (Fig. 5B). At all measured reinnervated chest sites, sensory thresholds were elicited at currents ranging from 3.0 to 10.0 mA (average, 4.9 ± 1.3 mA; contralateral normal average, 2.0 ± 0.0 mA; index finger average, 3.0 ± 0.0 mA). At the two sites where monofilaments elicited sensations projected only to her missing limb, the average sensation thresholds were 4.0 ± 0.0 and 3.7 ± 0.7 mA. At sensory threshold levels, sensations were almost exclusively projected only to the missing hand and not the chest. At all measured reinnervated sites, painful sensations were elicited at currents ranging from 17.0 to 28.0 mA (average, 20.2 ± 2.3 mA; contralateral normal average, 23.7 ± 1.9 mA; index finger average, 36.7 ± 0.7 mA). Painful sensations were felt either simultaneously on her chest and projected to the missing hand, or they were felt on her chest only. As current levels increased, the surface area of the referred sensations increased.
Discussion
Before targeted reinnervation, the sensory pathways in these patients were dormant for >1 year; however, we found that robust sensations, projected to the missing limb, redeveloped in both patients after reinnervation. This discovery suggests that the peripheral and central sensory pathways endured and remained viable after prolonged periods of postamputation disuse, which is compelling in light of evidence suggesting that although the subcortical topography of the limb afferents is maintained after amputation, spinal cord and brainstem afferents from adjacent residual limb and face sprout into and strongly reactivate territory once occupied by the missing limb (7–10). Interestingly, although both patients examined in this work report the presence of a phantom limb, neither patient has ever felt any evoked phantom-limb sensations elicited from tactile stimulation of the face or trunk (11–13). The reactivation of sensation generated relatively extensive evoked sensations of the missing limb in both patients. When a single point on the chest was touched, the resulting referred sensations usually encompassed large regions of the hand or multiple disparate areas across the hand.
Although no electrophysiological recordings were made in these TR patients, it is interesting to note that the character of the referred-touch sensations are reflective of receptive fields described in nerve section and repair studies (14, 15). The large hand perceptions may result from the activation of anatomical connections between central pathways/cortical representations by a small number of randomly organized inputs from the skin surface (16–19). Another possibility is that these large or disparate percepts appear because when normally separate skin surfaces are activated simultaneously it may cause the formerly distinct representative regions within the cortex to expand and merge (20). Interestingly, even though the hand was not present, the patterns of sensation in these two patients are similar to patterns of paresthesias and multiple areas of sensation that have been reported in studies of sensory mislocalization after peripheral nerve transection and regeneration in the hand (21, 22).
An alternative possibility, involving peripheral mechanisms, is that the large, often multiple, referred sensations observed in BSD and STH are indicative of the character of the reinnervation occurring at the skin. For instance, there may be a concentration of somatotopically disorganized reinnervating sensory fibers stemming from the transferred nerves (5, 23–25). Thus, touching the skin could activate a large number of closely associated afferents from different areas of the missing hand.
A striking aspect of this work is that in both patients there were distinct areas of the chest where the regenerated arm sensation overlapped with the native-chest sensation (see Fig. 4, stars). The work of others provides evidence that when the inputs to the somatosensory cortex from two different cutaneous surfaces are stimulated simultaneously, the distinct topographical representations of those two surfaces become integrated (26–28). It might be possible that, after TR, the projected sensation to the hand would eventually be integrated into the native-chest sensation and lost. Alternatively, chest sensation might be lost after integration into the referred-hand sensation (17, 20); however, this reorganization does not appear to be evident in these patients. In the regions where there was overlap between the chest and projected sensation, the patients reported that they were being touched on both the missing hand and the chest. The resulting sensation was not confusing to either patient; they simply reported feeling as though they were being touched in two different areas at once. In both patients, this condition has been highly stable and has persisted since the reinnervation was established (>5 years in BSD and 18 months in STH). This evidence suggests that the perceptual identities of the two originally disparate skin regions have been maintained even though they share a common cutaneous surface, which may be the result of the physical limits of central reorganization from these highly disparate sensory surfaces (29–31).
The lowest reinnervated skin thresholds were in the reported range for normal hand and chest skin (0.6–1.4 gAF) (32–34). The reinnervated skin in our work had thresholds comparable with those for regenerating afferents in hand and chest in other examinations (22, 35–37). The average difference in thresholds between hand and chest was small within the low-threshold region and increased at the margins. We suggest that this difference is a reflection of the position of the regenerant afferents within the skin. Those afferents responsive to the lowest thresholds are likely mechanoreceptors residing at superficial positions within the skin, whereas afferents responding at higher thresholds are likely positioned much deeper in the skin or may even reside in the s.c. fat, breast, or muscle (38, 39).
In addition to specific touch sensations, STH also had discrete areas that corresponded to feelings of joint position, skin stretch, and edge sensation. In contrast, BSD had no identifiable feelings of joint position or skin stretch. STH's single and strong proprioceptive feeling of the fourth finger being extended is suggestive of the reestablishment of some type of innervation involved with sensing joint position (40, 41). This proprioceptive sensation was described by STH as being entirely different in character from the feelings of skin pull (a tugging sensation) that were evident at other locations and may be representative of the difference between reestablished cutaneous stretch receptors (possibly SAII) and joint receptors (possibly capsular receptors) (42–44).
In BSD, the sensory nerve fibers from the transferred nerves appeared to regenerate through the muscle to the skin. In STH, we expected that the thick layer of s.c. fat and breast tissue, between the muscle and skin, would prevent reinnervation arising from the nerve to muscle transfer. Because we were unwilling to disfigure STH by removing tissue, as we had done in BSD, we attempted to provide STH with referred sensation in her chest by a different route. We created sensory nerve conduits to the ulnar nerve to guide and stimulate nerve fiber reinnervation (37, 45, 46). Surprisingly, we found that touching most points on her chest elicited sensation referred to parts of her hand served by the median nerve. It appears that the median-sensory nerve fibers were able to traverse through the chest muscle, breast tissue, and fat to reinnervate the skin. Sensations referred to regions of the hand formerly served by the ulnar nerve were present, but they were displaced to an area far from the nerve-to-muscle transfer site. This pattern was quite different from that for the innervation apparently arising from the median afferents regenerating through target muscle to overlying skin. The nerve conduit for the ulnar nerve appeared to provide no advantage over the median-sensory nerve fibers regenerating without the benefit of a direct neural bridge. Spontaneous growth of sensory fibers through multiple layers of tissue has been reported; however, it generally results in poor sensory reinnervation (47).
Thermal thresholds recorded within hand-only areas in both patients were reflective of those reported for normals at various locations on the hand (cold sensation, 28.3–30.6°C; warm sensation, 33.5–36.1°C; cold pain, 12.3–19.4°C; heat pain, 42.3–44.6°C) (48, 49). Thermal thresholds for the reinnervated skin were also similar to those collected on the normal control sites of the amputees. In both patients, percepts of cold sensation, warm sensation, cold pain, and heat pain were primarily referred to the missing limb. Neither patient reported paradoxical sensations such as cold perception with increasing thermal stimulus or warm perception with decreasing thermal stimulus (48). Comparison of thresholds between reinnervated skin and normal skin in these patients and with the work of others (48) is suggestive that Aδ and C fibers and consequently thermal sensation have returned to the reinnervated chest skin. However, it is important to note that because of the large size of the peltier thermal element, with respect to the reinnervation region, influence on the thermal thresholds by surrounding native innervation cannot be expressly ruled out.
Threshold-level electrical stimulation of reinnervated chest skin, in both patients, elicited primarily referred-hand sensation, suggesting that the regenerated afferents include mechanoreceptors served by large Aβ fibers. Referred-hand sensations from electrical stimulation, in both patients, correlated fairly well with mechanical stimulation sensations elicited at the same testing points. When the cutaneous touch threshold levels are compared with the electrical stimulation threshold levels, an interesting comparison becomes evident. In both patients the native-chest mechanosensory thresholds are lower than those of the regenerant afferents. However, this trend is reversed for electrical-stimulation sensation thresholds where the referred sensation primarily occurs at lower levels than native-chest sensation, perhaps because of sensory hyperreinnervation from the transfer of large nerves. Many afferent fibers may have regenerated into or near the skin but not reinnervated functional end organs (25). Electrical stimulation bypasses the receptor terminal to activate the axon directly. Electrical stimulation may have activated a larger number of axons than mechanical stimulation.
Generally, as electrical stimulation intensity increased to painful levels, the surface area of the referred sensations increased as would be expected from increased current spread. Pain sensations were felt in the chest but were also projected to the missing limb. The referred electrical stimulation percepts also correlated fairly well with the mechanical stimulation percepts. At painful electrical stimulation intensities, activation of Aδ and C fiber axons occurs (50, 51). The painful sensations referred to the missing limb suggest that Aδ and C fiber afferents have regenerated from the transferred peripheral nerves, which is consistent with the thermal perception findings noted above.
Our results illustrate a method for creating a portal to the sensory pathways of a lost limb. This work offers the possibility that an amputee may one day be able to feel with an artificial limb as although it was his own. Sensors could be placed in a prosthetic hand to measure contact forces and temperature, while a device could press or thermally stimulate the reinnervated skin to provide sensory feedback that appropriately correlates to hand perception.
These results suggest that spatial discrimination may be limited and that psychophysical studies are needed to assess the patients' ability to distinguish graded pressure, vibration, and perhaps more complex sensations such as tactile discrimination and skin shear. It will also be interesting to learn how the patients' perceptions may change with time and consistent use of a prosthesis providing cutaneous feedback. The contrasting results of these two subjects highlight that there could be high variability in outcomes. Methods need to be developed to control the reinnervation process for optimizing the results.
Materials and Methods
All experiments were performed with Institutional Review Board approval and the informed consent of all patients at the Rehabilitation Institute of Chicago. Reference points were laid over the reinnervated skin area on the chest for each experimental session. A grid of holes was placed in a thermoplastic test socket that fit intimately to the anatomy of the amputation site. Points were drawn on the chest with a permanent marker through these holes. Sensation diagrams were defined by indenting the skin at grid points with a cotton-tipped probe on the end of a size 6.65 Semmes–Weinstein monofilament (North Coast Medical, Inc.). BSD described where referred sensation was felt by using numbered positions on schematics of his missing limb. STH drew referred sensations on similar diagrams with her intact hand. Measurements of cutaneous sensibility were determined by applying Semmes–Weinstein monofilaments directly to selected grid points (52). Each patient was tested on three separate times to determine average thresholds for each grid point. In cases where one or two thresholds for a grid point exceeded the testing range of the monofilaments, the value of 500 gAF was averaged in with the within-range recorded values to reflect the intensity of the sensation in the border regions.
Temperature thresholds were determined by using a TSA II neurosensory analyzer (Medoc). The 3.2- × 3.2-cm thermal probe face was placed and secured with an elastic bandage over BSD's hand-only region (determined by monofilaments) and on intact skin on his contralateral chest. STH was tested on a hand-only region (as determined above), her contralateral chest, and the thenar eminence of her hand.
Electrical stimulation was applied at selected points on the reinnervated chest skin of each amputee. Control sites were the contralateral intact chest of BSD and STH and the left index finger of STH. A Compex2 stimulator (Compex) delivered constant current impulses (1-ms pulse durations at 1-Hz frequency). Pulse amplitude started at 0 mA and increased during each trial at a rate of 1 mA/s (or 1 mA per pulse) for sensory threshold trials and 5 mA/s for pain threshold trials. Electrodes (pediatric neurology electrodes; Ambu Neuroline) were placed 2 cm apart at each testing site. Three trials each of sensory and pain threshold detection were performed at each site. Trials were randomized by site, detection type, and trial number. Polarity of the electrode pairs was maintained throughout the trials. The subject was instructed on the type of trial (sensory or pain) and told the experimenter to “stop” when that perception was reached. Threshold-intensity levels were recorded, and the subjects reported stimulus quality and location on a limb schematic.
Acknowledgments
We thank T. Sukal and G. Wallach for assistance with experiments. We thank R. Johansson, P. Tsuruda, and D. Bautista for comments on the manuscript. This work was supported by National Institutes of Health Grants R01-HD-4-3137 and N01-HD-5-3402 and the Searle Funds at the Chicago Community Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Kuiken TA, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield KA. Prosthet Orthot Int. 2004;28:245–253. doi: 10.3109/03093640409167756. [DOI] [PubMed] [Google Scholar]
- 2.Hijjawi JB, Kuiken TA, Lipschutz RD, Miller LA, Stubblefield KA, Dumanian GA. Plastic Reconstr Surg. 2006;118:1573–1578. doi: 10.1097/01.prs.0000242487.62487.fb. [DOI] [PubMed] [Google Scholar]
- 3.Kuiken TA, Miller LA, Lipschutz RD, Lock BA, Stubblefield K, Marasco PD, Zhou P, Dumanian GA. Lancet. 2007;369:371–380. doi: 10.1016/S0140-6736(07)60193-7. [DOI] [PubMed] [Google Scholar]
- 4.Hoffer JA, Loeb GE. Ann Biomed Eng. 1980;8:351–360. doi: 10.1007/BF02363438. [DOI] [PubMed] [Google Scholar]
- 5.Kuiken TA, Childress DS, Rymer WZ. Brain Res. 1995;676:113–123. doi: 10.1016/0006-8993(95)00102-v. [DOI] [PubMed] [Google Scholar]
- 6.Kuiken TA. J Technol Disabil. 2003;15:105–111. [Google Scholar]
- 7.Florence SL, Kaas JH. J Neurosci. 1995;15:8083–8095. doi: 10.1523/JNEUROSCI.15-12-08083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu CW, Kaas JH. Somatosens Mot Res. 2002;19:153–163. doi: 10.1080/08990220220133261. [DOI] [PubMed] [Google Scholar]
- 9.Jones EG, Pons TP. Science. 1998;282:1121–1125. doi: 10.1126/science.282.5391.1121. [DOI] [PubMed] [Google Scholar]
- 10.Kiss ZH, Dostrovsky JO, Tasker RR. Stereotact Funct Neurosurg. 1994;62:153–163. doi: 10.1159/000098612. [DOI] [PubMed] [Google Scholar]
- 11.Halligan PW, Marshall JC, Wade DT, Davey J, Morrison D. Neuroreport. 1993;4:233–236. doi: 10.1097/00001756-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Ramachandran VS. Proc Natl Acad Sci USA. 1993;90:10413–10420. doi: 10.1073/pnas.90.22.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doetsch GS. Somatosens Mot Res. 1997;14:6–16. doi: 10.1080/08990229771178. [DOI] [PubMed] [Google Scholar]
- 14.Paul RL, Goodman H, Merzenich M. Brain Res. 1972;39:1–19. doi: 10.1016/0006-8993(72)90782-2. [DOI] [PubMed] [Google Scholar]
- 15.Mackel R, Brink EE, Wittkowsky G. Brain Res. 1985;329:49–69. doi: 10.1016/0006-8993(85)90511-6. [DOI] [PubMed] [Google Scholar]
- 16.Wall JT, Kaas JH, Sur M, Nelson RJ, Felleman DJ, Merzenich MM. J Neurosci. 1986;6:218–233. doi: 10.1523/JNEUROSCI.06-01-00218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merzenich MM, Recanzone GH, Jenkins WM, Nudo RJ. In: Natural and Artificial Parallel Computation. Arbib M, Robinson JA, editors. Cambridge, MA: MIT Press; 1990. pp. 177–210. [Google Scholar]
- 18.Merzenich MM, Jenkins WM. J Hand Ther. 1993;6:89–104. doi: 10.1016/s0894-1130(12)80290-0. [DOI] [PubMed] [Google Scholar]
- 19.Hansson T, Brismar T. J Neurosurg. 2003;99:100–105. doi: 10.3171/jns.2003.99.1.0100. [DOI] [PubMed] [Google Scholar]
- 20.Merzenich MM, Recanzone G, Jenkins WM, Allard TT, Nudo RJ. In: Neurobiology of Neocortex. Rakic P, Singer W, editors. New York: Wiley; 1988. pp. 41–67. [Google Scholar]
- 21.Hallin RG, Wiesenfeld Z, Lindblom U. Exp Neurol. 1981;73:90–106. doi: 10.1016/0014-4886(81)90047-9. [DOI] [PubMed] [Google Scholar]
- 22.Mackel R, Kunesch E, Waldhor F, Struppler A. Brain Res. 1983;268:49–65. doi: 10.1016/0006-8993(83)90389-x. [DOI] [PubMed] [Google Scholar]
- 23.Horch K. J Neurophysiol. 1979;42:1437–1449. doi: 10.1152/jn.1979.42.5.1437. [DOI] [PubMed] [Google Scholar]
- 24.Horch KW, Burgess PR. In: Nerve Repair and Regeneration: Its Clinical and Experimental Basis. Jewett DL, McCarroll HR, editors. St Louis: Mosby; 1980. pp. 105–114. [Google Scholar]
- 25.Manger PR, Woods TM, Jones EG. Proc Biol Sci. 1996;263:933–939. doi: 10.1098/rspb.1996.0138. [DOI] [PubMed] [Google Scholar]
- 26.Clark SA, Allard T, Jenkins WM, Merzenich MM. Nature. 1988;332:444–445. doi: 10.1038/332444a0. [DOI] [PubMed] [Google Scholar]
- 27.Allard T, Clark SA, Jenkins WM, Merzenich MM. J Neurophysiol. 1991;66:1048–1058. doi: 10.1152/jn.1991.66.3.1048. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Merzenich MM, Sameshima K, Jenkins WM. Nature. 1995;378:71–75. doi: 10.1038/378071a0. [DOI] [PubMed] [Google Scholar]
- 29.Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. J Comp Neurol. 1984;224:591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- 30.Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M. Science. 1991;252:1857–1860. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- 31.Jain N, Florence SL, Kaas JH. J Neurophysiol. 1995;73:1537–1546. doi: 10.1152/jn.1995.73.4.1537. [DOI] [PubMed] [Google Scholar]
- 32.Semmes J, Weinstein S, Ghent L, Teuber H. Somatosensory Changes After Penetrating Brain Wounds in Man. Cambridge, MA: Harvard Univ Press; 1960. pp. 4–11. [Google Scholar]
- 33.Weinstein S. In: The Skin Senses. Kenshalo DR, editor. Springfield, IL: Thomas; 1968. pp. 195–222. [Google Scholar]
- 34.Voerman VF, van Egmond J, Crul BJP. Am J Phys Med Rehab. 1999;78:24–29. doi: 10.1097/00002060-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Terzis JK, Dykes RW. J Neurophysiol. 1980;44:1214–1225. doi: 10.1152/jn.1980.44.6.1214. [DOI] [PubMed] [Google Scholar]
- 36.Blondeel PN, Demuynck M, Mete D, Monstrey SJ, Van Landuyt K, Matton G, Vanderstraeten GG. Br J Plast Surg. 1999;52:37–44. doi: 10.1054/bjps.1998.3011. [DOI] [PubMed] [Google Scholar]
- 37.Ozkan T, Ozer K, Gulgonen A. J Hand Surg (Am) 2001;26:44–51. doi: 10.1053/jhsu.2001.20156. [DOI] [PubMed] [Google Scholar]
- 38.Gutmann E. J Anat. 1945;79:1–8-4. [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss P, Edds MV., Jr J Neurophysiol. 1945;8:173–193. [Google Scholar]
- 40.Edin BB, Abbs JH. J Neurophysiol. 1991;65:657–670. doi: 10.1152/jn.1991.65.3.657. [DOI] [PubMed] [Google Scholar]
- 41.Edin BB. J Neurophysiol. 1992;67:1105–1113. doi: 10.1152/jn.1992.67.5.1105. [DOI] [PubMed] [Google Scholar]
- 42.Grigg P, Greenspan BJ. J Neurophysiol. 1977;40:1–8. doi: 10.1152/jn.1977.40.1.1. [DOI] [PubMed] [Google Scholar]
- 43.Johansson RS. J Physiol. 1978;281:101–125. doi: 10.1113/jphysiol.1978.sp012411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hulliger M, Nordh E, Thelin AE, Vallbo AB. J Physiol. 1979;291:233–249. doi: 10.1113/jphysiol.1979.sp012809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viterbo F, Franciosi LF, Palhares A. Plast Reconstr Surg. 1995;96:494–495. doi: 10.1097/00006534-199508000-00054. [DOI] [PubMed] [Google Scholar]
- 46.Voche P, Ouattara D. Br J Plast Surg. 2005;58:239–244. doi: 10.1016/j.bjps.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Shaw WW, Orringer JS, Ko CY, Ratto LL, Mersmann CA. Plast Reconstr Surg. 1997;99:394–399. doi: 10.1097/00006534-199702000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Defrin R, Ohry A, Blumen N, Urca G. Brain. 2002;125:501–510. doi: 10.1093/brain/awf055. [DOI] [PubMed] [Google Scholar]
- 49.Verdugo R, Ochoa JL. Brain. 1992;115:893–913. doi: 10.1093/brain/115.3.893. [DOI] [PubMed] [Google Scholar]
- 50.McAllister RM, Urban LA, Dray A, Smith PJ. J Hand Surg (Br) 1995;20:437–443. doi: 10.1016/s0266-7681(05)80149-4. [DOI] [PubMed] [Google Scholar]
- 51.Hallin RG, Torebjork HE. Exp Brain Res. 1973;16:309–320. doi: 10.1007/BF00233333. [DOI] [PubMed] [Google Scholar]
- 52.Bell JA. In: Rehabilitation of the Hand. Hunter JM, Mackin EJ, Callahan AD, editors. St Louis: Mosby; 1995. pp. 109–128. [Google Scholar]
- 53.Netter FH. Atlas of Human Anatomy. Teterboro: ICON Learning Systems; 2001. [Google Scholar]