Abstract
The mechanisms underlying CNS arousal in response to homeostatic pressures are not known. In this study, we pitted two forces for CNS arousal against each other (circadian influences vs. restricted food availability) and measured the neuronal activation that occurs in a behaviorally defined group of animals that exhibited increased arousal in anticipation of feeding restricted to their normal sleeping time. The number of c-FOS+ neurons was significantly increased only in the ventromedial nucleus of the hypothalamus (VMH) in these mice, compared with control animals whose feeding was restricted to their normal active and feeding time (P < 0.01). Because the activation of VMH neurons coincides with the earliest signs of behavioral arousal preceding a change in meal time, we infer that VMH activation is involved in the increased arousal in anticipation of food.
Keywords: c-FOS, homeostatic, ventromedial hypothalamus, metabolic, sleep
The activation of behavior is driven by homeostatically regulated variables, such as hunger and circadian rhythms. The problem of exactly how these two types of influences interact to modulate behavior has been stated (1) but not solved. Mechanisms for changes in CNS arousal have remained controversial.
We pitted two forces for CNS arousal against each other (food availability vs. circadian influences) and searched for the first neuronal activation that occurs in animals as they began to change their activation of behavior from a circadian light-driven rhythm to one dictated by restricted food availability.
The present study (i) takes into account the individual differences in food anticipatory activity and links those to neuronal activation; (ii) pits the homeostatic drive for feeding against the circadian drive to rest during the light period, thus enabling a cell-by-cell dissection of these two pathways; (iii) has a control group that is exposed to the same restricted feeding paradigm as the test group, for the same number of days, with the difference that control animals receive their daily meal during their behaviorally active period; (iv) conceptualizes the problem as one of generalized CNS arousal; and (v) examines animals' brains as close to the development of the food anticipatory activity as possible. This design was intended to identify the earliest neuronal changes, and therefore the most likely to be causing these behavioral changes.
Results
Shifted animals were significantly more active in the 3-h period preceding the shifted food presentation time, compared with controls. In fact, running wheel revolutions were increased from 314 ± 151 in nonshifted animals to 1,768 ± 398 in shifted animals (P < 0.01) (Figs. 1 and 2 and Table 1).
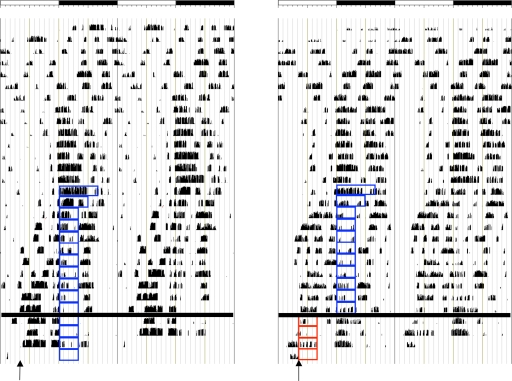
Fig. 1.
Double-plotted actograms of mice exhibiting food anticipatory activity to restricted feeding during the active period (nonshifted animal) (Left) or inactive period (shifted animal) (Right). Each panel represents 48 h. Open bar, light period; filled bar, dark period; blue boxes, restricted feeding during active period; red boxes, shifted restricted feeding during inactive period. Arrows denote time of killing.
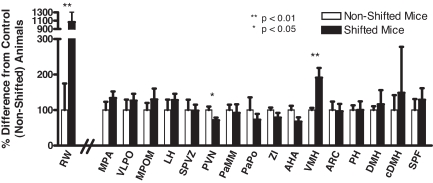
Fig. 2.
Normalized running wheel activity (RW) (first pair of histograms) and c-FOS-positive neurons (remaining histograms) in MPA, VLPO, MPOM, LH, sPVZ, PVN, PaMM, PaPo, ZI, AHA, VMH, Arc, PH, DMH, cDMH, and SPF of nonshifted and shifted mice. Empty bars, nonshifted mice; filled bars, shifted mice. Running wheel revolutions or number of c-FOS-immunoreactive nuclei were arbitrarily set at 100 for the nonshifted group, and values for shifted mice were normalized to nonshifted values. Data are presented as mean ± SE. Statistically significant difference between nonshifted and shifted groups: *, P < 0.05; **, P < 0.01.
Table 1.
Wheel-running revolutions in the last 3-h period before sacrifice and number of c-FOS-immunoreactive nuclei in the MPA, VLPO, MPOM, LH, sPVZ, PVN, PaMM, PaPo, ZI, AHA, VMH, Arc, PH, DMH, cDMH, and SPF of nonshifted and shifted mice
| Variable | Nonshifted group | Shifted group |
|---|---|---|
| Wheel-running revolutions | 313.5 ± 151.0 | 1,767.5 ± 397.8** |
| MPA | 443.2 ± 102.8 | 593.2 ± 82.5 |
| VLPO | 89.5 ± 26.3 | 113.0 ± 17.4 |
| MPOM | 320.0 ± 64.6 | 415.0 ± 98.7 |
| LH | 1,977.5 ± 582.2 | 2,533.5 ± 347.5 |
| sPVZ | 174.5 ± 51.0 | 171.7 ± 29.3 |
| PVN | 1,012.7 ± 99.2 | 721.5 ± 74.1* |
| PaMM | 360.7 ± 55.0 | 332.3 ± 86.3 |
| PaPo | 147.2 ± 52.6 | 107.2 ± 24.0 |
| ZI | 832.0 ± 57.3 | 653.3 ± 112.9 |
| AHA | 1,765.0 ± 213.5 | 1,190.8 ± 212.4 |
| VMH | 151.2 ± 9.1 | 289.0 ± 41.5** |
| ARC | 298.3 ± 71.3 | 287.7 ± 61.9 |
| PH | 722.7 ± 95.0 | 723.8 ± 176.7 |
| DMH | 378.0 ± 40.8 | 438.7 ± 151.8 |
| cDMH | 46.7 ± 19.6 | 69.3 ± 60.4 |
| SPF | 109.2 ± 33.8 | 140.8 ± 35.6 |
Data are presented as mean ± SE. Statistically significant difference between nonshifted and shifted mice. *, P < 0.05; **, P < 0.01.
We examined neuronal activation, as measured by c-FOS expression, in every neuronal group that could be conceived, based on the literature (see Discussion), as mediating these changes in the timing of the activation of behavior [16 regions: medial preoptic area (MPA), ventrolateral preoptic nucleus (VLPO), medial part of the medial preoptic nucleus (MPOM), lateral hypothalamus (LH), subparaventricular zone (sPVZ), paraventricular nucleus (PVN), magnocellular part of medial paraventricular hypothalamus (PaMM), ventral part of paraventricular hypothalamic nucleus (PaPo), zona incerta (ZI), anterior hypothalamic area (AHA), VMH, arcuate (Arc), posterior hypothalamus area (PH), dorsal medial hypothalamus (DMH), compact dorsal medial hypothalamus (cDMH), and subparafascicular thalamic nucleus (SPF)].
Of the brain regions studied, the only nerve cell group that showed increased c-FOS immunoreactivity in anticipation of food was VMH. The number of c-FOS-immunoreactive neurons in VMH was significantly increased by 91% in animals anticipating the shifted food presentation time, compared with animals not anticipating the food (nonshifted, 151 ± 9; shifted, 289 ± 41.5) (P < 0.01) (Figs. 2 and 3 and Table 1). Animals anticipating food during the lights-on period (shifted) had a significantly lower number of c-FOS immunoreactive neurons in PVN, compared with animals anticipating food during lights off (nonshifted) (nonshifted, 1,013 ± 99; shifted, 721 ± 74) (P < 0.05) (Fig. 2 and Table 1).
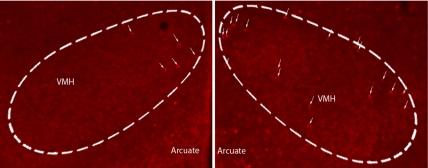
Fig. 3.
Photomicrographs of c-FOS-immunoreactive nuclei in VMH of a nonshifted (Left) and a shifted (Right) mouse. Area of VMH delineated by the dashed white line.
Discussion
The major goals of this study were to pit the contributions to CNS arousal of the light-entrainable clock versus the mechanisms detecting changes in food availability to determine the earliest brain regions to register these changes in resource availability. The current study uses behaviorally defined groups of animals to identify the earliest brain regions to become active, possibly causing arousal changes in anticipation of food. Based on the literature extant, the areas studied here were the most likely to lead to changes in behavior.
Under normal conditions, with no overriding homeostatic challenges, behavioral arousal is regulated predominantly by the environmental light/dark cycle. Light signals are communicated to the suprachiasmatic nucleus (SCN) from the retina by the retinohypothalamic tract. The major direct projections from the SCN are to the MPOA, sPVZ, and DMH (3). In fact, it is through these projections that the SCN is hypothesized to modulate arousal. The MPOA relays circadian information by direct projections to the median preoptic nucleus, a key brain region involved in motor activity, sleep, and thermoregulation, to the sleep-active VLPO, and several arousal-promoting brain areas, including histaminergic tuberomammillary nucleus (TMN), locus coeruleus (LC), LH/PeF, and substantia inominata (3). Another key nucleus involved in relaying circadian signals is DMH. DMH has the most extensive projections to arousal-promoting brain regions, including the dorsal raphe nucleus, TMN, LC, LH/PeF, and laterodorsal tegmental nucleus. In addition, DMH also has direct projections to the sleep-active VLPO and MnPO (3, 4). A third major projection from the SCN is to sPVZ. Together these three relay nuclei are responsible for transducing the majority of signals from the SCN to the rest of the brain, including rhythms of arousal, sleep, thermoregulation, locomotion, and feeding-mediating brain regions. Lesions of these relay nuclei have been shown to disrupt or compromise circadian entrainment of other brain regions (e.g., DMH lesions abolish circadian changes in spontaneous LC firing patterns) (5). Furthermore, lesions of sPVZ greatly dampen or abolish circadian rhythms of body temperature, sleep, and/or locomotor activity (6).
Properly regulated arousal responses to a restricted feeding schedule require fine coordination between the gut and brain. The present study indicates that when food becomes temporally restricted, VMH is the first brain nucleus, among the 16 brain nuclei studied, to express increased c-FOS immunoreactivity after a shift in mealtime. VMH is an ideal candidate for sensing changes in energy status, whether they are conveyed to glucose-sensing VMH neurons (7) or by binding of gastrointestinal hormones, such as leptin (8, 9), insulin (10), neuropeptide Y (11), or ghrelin (12). VMH neurons become active under restricted feeding conditions (13). VMH has long been thought to be the satiety center of the brain, whose activation inhibits feeding. Lesioning VMH, or disruption of estrogen receptor-α gene expression in VMH, leads to metabolic syndrome and obesity (14, 15). VMH has long been hypothesized to be a food-entrainable oscillator (16–18). VMH lesions abolish or compromise the ability of rodents to anticipate a food-restricted meal. However, these responses recover within 14–21 weeks (16). VMH also has been shown to be critical in coupling feeding time and energy intake to adrenocortical activity (13). Similar to our findings, Choi et al. (13) reported increases in c-FOS immunoreactivity in VMH of restricted-fed animals (after 16 days of restricted feeding), which also were accompanied by increases in c-FOS-immunoreactive neurons in the mPOA, magnoPVN, parvoPVN, and paraventricular thalamus. When VMH was disrupted by using intra-VMH colchicine applications, c-FOS expression in VMH, parvoPVN, magnoPVN, and paraventricular thamalus were abolished, strongly suggesting that VMH regulates the activity of other brain nuclei under restricted feeding conditions and the shift in adrenocortical activity (13).
Consistent with this notion, the present study's results reveal VMH as the earliest brain region (among the 16 studied) to show increases in c-FOS immunoreactivity in anticipation of a timed meal. Eventually, other brain regions are recruited that collectively contribute to increases in behavioral arousal. Brain regions that are activated by VMH, and are likely involved in arousal-activating pathways, include DMH and LH, which have both been extensively studied in the context of mediating arousal in the anticipation of food (19–24). DMH receives direct projections from SCN (25) and is likely a relay nucleus involved in mediating the arousal responses of the light-entrainable oscillator. In addition, when energy homeostasis is low, activation of DMH is uncoupled from that of the SCN, and the overall activity is mandated by the temporal availability of a timed meal (26). When food availability is restricted, expression of circadian genes (e.g., mPer 1 mRNA) in SCN and sPVZ continues to oscillate with environmental light/dark conditions. However, most other brain nuclei and peripheral tissues shift their expression profiles to match the shifted food presentation time (13, 27–31). Both DMH and LH neurons become activated in food-restricted animals 1 h before food presentation. However, the majority of this activation occurs postprandially (26). In the studies of Angeles-Castellanos et al. (26), the animals were kept under restricted feeding conditions for 3 weeks before the neuronal activation studies. In contrast, in the current study, we examined the c-FOS induction after the earliest behavioral change (3 days after shift). Mieda et al. (23) also demonstrated that the expression of mPer1 and mPer2 mRNA in cDMH is shifted to match the food presentation time, and its expression exhibits circadian rhythmicity under restricted feeding conditions. The effects of lesioning DMH in anticipation of food also were recently investigated. However, discrepant results were seen. Landry et al. (22) found that animals with radiofrequency lesions of the DMH still displayed proper food-directed anticipatory responses, whereas Gooley et al. (24) reported that DMH excitotoxic lesions abolished increases in total activity in the anticipation of food. Equally puzzling is the involvement of the orexigenic LH in food anticipation. Ablation of orexin-ergic neurons by using orexin-saporin injections in the LH did not compromise the entrainment or expression of food anticipatory activity (19). However, transgenic mice lacking the orexin gene (orexin/ataxin-3) exhibited reduced anticipatory responses to a time-restricted meal (20).
The recent studies of Gooley et al. (22) and Landry et al. (24) are particularly relevant to the current study because some DMH-lesioned animals also had moderate levels of damage to VMH, which could contribute to the behavioral phenotypes observed. Important differences between our studies are that we were looking for the earliest behavioral changes in animals anticipating a meal, as opposed to a food-entrainable oscillator. As such, our animals were killed 3 days after the food presentation time was shifted, not 14 days as in Gooley et al. (24) or 30 days—behavior was analyzed during the last week of food restriction (22). All of the animals used in our analysis were behaviorally defined (see Materials and Methods), and our control group was subjected to the same restricted feeding paradigm as the test group, with the exception that the food presentation time was later in the day (food availability: shifted group, 0400–0800 hours; nonshifted group, 1200–1600 hours), as opposed to an ad libitum-fed control group (24).
Based on the current study and extensive physiological and neuroanatomical evidence (32–36), we hypothesize that peripheral food timing signals are first perceived by VMH, which, together with DMH and LH, form a metabolic integration center of the brain. These signals are then communicated to the classical arousal-activating pathways (37) from reticular formation and brainstem monoaminergic groups to increase arousal. In addition, these metabolic integrator regions also have extensive projections to the anterior hypothalamus and preoptic locomotor region, which likely are involved in the increases in locomotion that precede meal presentation time. The metabolic integrator nuclei have direct and indirect (by sPVZ) projections from the SCN, thus mediating the switch between a light-driven arousal pathway and a homeostatic—in this case, a food-driven—pathway. Because proper anticipatory responses for a timed meal are critically important to animals' survival, it is highly unlikely that they would be mediated by a single neuronal group (hence the inability to abolish food anticipatory activity despite numerous lesions studies). Instead, we propose that arousal in anticipation of a timed meal is mediated by this multiply redundant metabolic integrator network. Thus, damaging one component of this network may attenuate or dampen the intensity of the output arousal behavior, but certainly not abolish it, as demonstrated by many lesion studies. In addition, the present studies clearly indicate that c-FOS immunoreactivity in VMH is increased during the time when motor activity is first increased in anticipation of a timed meal.
Although the days when humans required the proper behavioral arousal responses to temporally restricted environmental resources of food are long gone, these same mechanisms and brain regions could still be useful in targeting certain present-day health concerns such as obesity and diabetes. In terms of CNS arousal, with the increased number of jobs requiring high levels of sustained arousal (e.g., military, air-traffic controllers, and physicians), it becomes important to understand the neural mechanisms and molecules involved in mediating arousal to optimize performance. A thorough understanding of how different environmental and homeostatic stimuli feed into these centralized arousal mechanisms is of critical importance.
Technical Considerations.
Although c-FOS immunoreactivity is commonly used as an index of neuronal activation, some caution is warranted given that thresholds for immediate early gene immunoreactivity are not necessarily the same as those necessary for electrophysiological and behavioral activation. It is possible that changes in neuronal activation leading to the increased behavioral arousal occur significantly earlier than we can detect with this method. Another potential pitfall of using c-FOS immunoreactivity as a sign of neuronal activation is the reduced ability of detecting cells with a net inhibitory synaptic or transcriptional drive.
In this study, we focused our efforts on examining changes in c-FOS immunoreactivity in portions of the hypothalamus that are output targets from the SCN, as well as on regions involved in food-intake mechanisms. However, we cannot exclude the possibility that other brain regions or peripheral tissues also lend contributing influences on the increased arousal preceding food presentation. Likewise, this type of study does not permit one to make firm conclusions about causality. In fact, it is conceivable that arousal responses in anticipation of food presentation time actually could be a source of the increases in c-FOS immunoreactivity in the VMH. Furthermore, it also is possible that other brain regions (including some analyzed here) could have been active before the time of death, and they could have played a role in the increased c-FOS expression in VMH. However, by the time the behavioral change was demonstrated, their activation already subsided. Killing animals at carefully timed intervals, even before the development of the behavioral changes, may prove informative in understanding the brain regions and pathways involved in the genesis of this critically important behavior.
Materials and Methods
Behavioral Analysis.
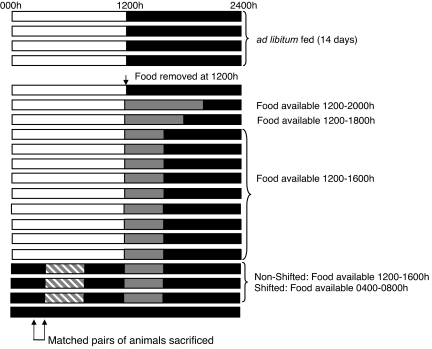
Male Swiss–Webster mice were housed in individual running wheel cages under a 12-h light/12-h dark cycle (light on at 0000 hours) for 2 weeks, with food and water available ad libitum. After this period, food was removed at dark onset (1200 hours), and food availability was gradually decreased in the following manner: day 1, food available from 1200 to 2000 hours; day 2, food available from 1200 to 1800 hours; days 3–11, food available from 1200 to 1600 hours. On day 12, mice were placed under constant dark conditions with a dim red light (<1 lux). One group of animals continued to receive food from 1200 to 1600 hours (nonshifted animals, n = 8; solid gray bars), whereas the other group had food from 0400 to 0800 hours (shifted group, n = 8; hatched gray bars) (Fig. 4).
Fig. 4.
Experimental design for eliciting food anticipatory activity. Animals were maintained under 12-h light/12-h dark conditions with food available ad libitum for 14 days. Food availability was gradually decreased from 8 to 6 to 4 h, starting at dark onset (1200 hours) on day 15. Mice were maintained on a 4-h restricted feeding schedule for 9 days, after which the animals were placed under constant dark conditions, and food presentation time was shifted to 0400–0800 hours for the shifted animals, whereas food presentation time for nonshifted animals was kept from 1200 to 1600 hours. All animals were killed on the third day after the food shift between 0300 and 0400 hours (arrows) (1 h before shifted food presentation time). Horizontal bars represent sequential days. Open bar, light period; filled bar, dark period; gray bar, restricted food availability time; cross-hatched bar, shifted restricted food availability time.
Running wheel activity was monitored daily, and matched pairs of animals were killed between 0300 and 0400 hours on the day after the shifted animal first displayed food anticipatory activity (food anticipatory activity was highest 1–2 h before food presentation). That is, two mice were killed side by side: one that was anticipating the presentation of food (shifted) and one that was not expecting food (nonshifted). All animals were behaviorally defined in addition to their experimental group assignment. An animal was classified as shifted once it met either of two criteria: (i) the total number of revolutions in the 3-h period preceding food presentation was >1,000 revolutions per 3 h, or (ii) the number of revolutions in this same 3-h period was at least double what it had been in the two most motorically active days in the week before food shifting. Nonshifted animals were excluded from the analysis if the number of running wheel revolutions exceeded 500 in the 3-h period before shifted food presentation or if the number of revolutions in this same period was higher than it had been in any day during the week before food shifting. The day before killing (when animal selection took place), behaviorally defined shifted animals (n = 6) had between 978 and 2,701 revolutions per 3 h before shifted food presentation, whereas behaviorally defined nonshifted animals (n = 4) had between 0 and 488 revolutions during this same time. Two mice, one from each group, died during the restricted feeding period. In addition, one shifted and two nonshifted mice were excluded from the analysis because their running wheel activities were outside the range dictated by our behavioral criteria. One additional nonshifted mouse was eliminated because of technical issues pertaining to the immunocytochemistry. Before killing, all mice were anesthetized under dim red light. Their heads were wrapped in two layers of aluminum foil to prevent exposure to light and transcardially perfused with 20 ml of 0.1 M phosphate buffer (pH 7.4) and 100 ml of 4% paraformaldehyde in 0.1 M phosphate buffer. Brains were removed, postfixed overnight, and transferred to 30% sucrose in 0.1 M phosphate buffer for storage. Brains were frozen, and coronal 30-μm sections were collected in four series.
Immunocytochemistry.
Only behaviorally defined animals were used for immunocytochemical analysis (shifted group, n = 6; nonshifted group, n = 4). One of four series was processed for immunohistochemistry for c-FOS. Briefly, free-floating sections were blocked in 2% normal donkey serum in phosphate buffer with 0.1% Triton X-100 (PBST) for 1 h, followed by incubation with the anti-c-FOS antibody (1:10,000; Santa Cruz Biotechnology) in 0.1% PBST + 2% normal donkey serum at 4°C for 48 h. Sections were rinsed three times in 0.1 M PBST and then incubated with the fluorescently labeled secondary antibody Cy5-donkey-anti-rabbit IgG (1:200; Jackson ImmunoResearch) for 2 h at room temperature and then rinsed three times in 0.1 M PBST. Sections were mounted on gelatin-coated slides and cover-slipped with ProLong Gold (Invitrogen). Images were collected by using a Zeiss Axioskop microscope connected to an rgbVision camera. Image analysis and collection were done by using IPLab for Windows Software (Scanalytics). To quantify c-FOS expression in discrete brain nuclei, panels from the Paxinos Mouse Brain Atlas (2) were overlayed on top of the immunofluorescent photographs from a complete brain series by using Adobe Photoshop 11.0 (Adobe Systems). All immunoreactive cells were manually counted by one individual blind to the treatment conditions. For each brain nucleus, the number of c-FOS-immunoreactive neurons was summed from all of the brain slices containing that brain region. The number of slices per brain nuclei varied among brain region (from 6.7 ± 0.4 in LH to 1.6 ± 0.3 in MPOM), but was not significantly different between shifted and nonshifted groups [two-way ANOVA; F(1, 14) = 0.586, not significant].
Statistical Analysis.
Comparisons were made between behaviorally defined shifted and nonshifted animals by using two-way ANOVA, followed by paired t test with Bonferroni correction.
Footnotes
The authors declare no conflict of interest.
References
- 1.Borbely AA. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 2.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. New York: Academic; 1997. [Google Scholar]
- 3.Deurveilher S, Semba K. Neuroscience. 2005;130:165–183. doi: 10.1016/j.neuroscience.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 4.Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. J Neurosci. 2002;22:977–990. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. Nat Neurosci. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- 6.Lu J, Zhang YH, Chou TC, Gaus SE, Elmquist JK, Shiromani P, Saper CB. J Neurosci. 2001;21:4864–4874. doi: 10.1523/JNEUROSCI.21-13-04864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canabal DD, Song Z, Potian JG, Beuve A, McArdle JJ, Routh VH. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1418–R1428. doi: 10.1152/ajpregu.00216.2006. [DOI] [PubMed] [Google Scholar]
- 8.Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB. Proc Natl Acad Sci USA. 1998;95:741–746. doi: 10.1073/pnas.95.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funahashi H, Yada T, Muroya S, Takigawa M, Ryushi T, Horie S, Nakai Y, Shioda S. Neurosci Lett. 1999;264:117–120. doi: 10.1016/s0304-3940(99)00186-x. [DOI] [PubMed] [Google Scholar]
- 10.Levin BE, Routh VH. Am J Physiol. 1996;271:R491–R500. doi: 10.1152/ajpregu.1996.271.3.R491. [DOI] [PubMed] [Google Scholar]
- 11.Kishi T, Elmquist JK. Mol Psychiatry. 2005;10:132–146. doi: 10.1038/sj.mp.4001638. [DOI] [PubMed] [Google Scholar]
- 12.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi S, Wong LS, Yamat C, Dallman MF. J Neurosci. 1998;18:3843–3852. doi: 10.1523/JNEUROSCI.18-10-03843.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weingarten HP, Chang PK, McDonald TJ. Brain Res Bull. 1985;14:551–559. doi: 10.1016/0361-9230(85)90104-2. [DOI] [PubMed] [Google Scholar]
- 15.Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S. Proc Natl Acad Sci USA. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mistlberger RE, Rechtschaffen A. Physiol Behav. 1984;33:227–235. doi: 10.1016/0031-9384(84)90104-5. [DOI] [PubMed] [Google Scholar]
- 17.Honma S, Honma K, Nagasaka T, Hiroshige T. Physiol Behav. 1987;39:211–215. doi: 10.1016/0031-9384(87)90011-4. [DOI] [PubMed] [Google Scholar]
- 18.Challet E, Pevet P, Lakhdar-Ghazal N, Malan A. Brain Res Bull. 1997;43:209–218. doi: 10.1016/s0361-9230(96)00439-x. [DOI] [PubMed] [Google Scholar]
- 19.Mistlberger RE, Antle MC, Kilduff TS, Jones M. Brain Res. 2003;980:161–168. doi: 10.1016/s0006-8993(03)02755-0. [DOI] [PubMed] [Google Scholar]
- 20.Akiyama M, Yuasa T, Hayasaka N, Horikawa K, Sakurai T, Shibata S. Eur J Neurosci. 2004;20:3054–3062. doi: 10.1111/j.1460-9568.2004.03749.x. [DOI] [PubMed] [Google Scholar]
- 21.Mieda M, Williams SC, Sinton CM, Richardson JA, Sakurai T, Yanagisawa M. J Neurosci. 2004;24:10493–10501. doi: 10.1523/JNEUROSCI.3171-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landry GJ, Simon MM, Webb IC, Mistlberger RE. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1527–R1534. doi: 10.1152/ajpregu.00874.2005. [DOI] [PubMed] [Google Scholar]
- 23.Mieda M, Williams SC, Richardson JA, Tanaka K, Yanagisawa M. Proc Natl Acad Sci USA. 2006;103:12150–12155. doi: 10.1073/pnas.0604189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gooley JJ, Schomer A, Saper CB. Nat Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- 25.Deurveilher S, Burns J, Semba K. Eur J Neurosci. 2002;16:1195–1213. doi: 10.1046/j.1460-9568.2002.02196.x. [DOI] [PubMed] [Google Scholar]
- 26.Angeles-Castellanos M, Aguilar-Roblero R, Escobar C. Am J Physiol Regul Integr Comp Physiol. 2004;286:R158–R165. doi: 10.1152/ajpregu.00216.2003. [DOI] [PubMed] [Google Scholar]
- 27.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 29.Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, Shibata S. Genes Cells. 2001;6:269–278. doi: 10.1046/j.1365-2443.2001.00419.x. [DOI] [PubMed] [Google Scholar]
- 30.Angeles-Castellanos M, Mendoza J, Escobar C. Neuroscience. 2007;144:344–355. doi: 10.1016/j.neuroscience.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 31.Wakamatsu H, Yoshinobu Y, Aida R, Moriya T, Akiyama M, Shibata S. Eur J Neurosci. 2001;13:1190–1196. doi: 10.1046/j.0953-816x.2001.01483.x. [DOI] [PubMed] [Google Scholar]
- 32.Saper CB, Swanson LW, Cowan WM. J Comp Neurol. 1976;169:409–442. doi: 10.1002/cne.901690403. [DOI] [PubMed] [Google Scholar]
- 33.Krieger MS, Conrad LC, Pfaff DW. J Comp Neurol. 1979;183:785–815. doi: 10.1002/cne.901830408. [DOI] [PubMed] [Google Scholar]
- 34.Ter Horst GJ, Luiten PG. Brain Res Bull. 1987;18:191–203. doi: 10.1016/0361-9230(87)90190-0. [DOI] [PubMed] [Google Scholar]
- 35.Fahrbach SE, Morrell JI, Pfaff DW. Exp Brain Res. 1989;77:221–233. doi: 10.1007/BF00274980. [DOI] [PubMed] [Google Scholar]
- 36.Canteras NS, Simerly RB, Swanson LW. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- 37.Pfaff DW. Brain Arousal and Information Theory: Neural and Genetic Mechanisms. Cambridge, MA: Harvard Univ Press; 2006. [Google Scholar]