Abstract
The sodium-chloride cotransporter (NCC) is the principal salt-absorptive pathway in the mammalian distal convoluted tubule (DCT) and is the site of action of one of the most effective classes of antihypertensive medications, thiazide diuretics. We developed a cell model system to assess NCC function in a mammalian cell line that natively expresses NCC, the mouse DCT (mDCT) cell line. We used this system to study the complex regulation of NCC by the phorbol ester (PE) 12-O-tetradecanoylphorbol-13-acetate (TPA), a diacylglycerol (DAG) analog. It has generally been thought that PEs mediate their effects on transporters through the activation of PKC. However, there are at least five other DAG/PE targets. Here we describe how one of those alternate targets of DAG/PE effects, Ras guanyl-releasing protein 1 (RasGRP1), mediates the PE-induced suppression of function and the surface expression of NCC. Functional assessment of NCC by using thiazide-sensitive 22Na+ uptakes revealed that TPA completely suppresses NCC function. Biotinylation experiments demonstrated that this result was primarily because of decreased surface expression of NCC. Although inhibitors of PKC had no effect on this suppression, MAPK inhibitors completely prevented the TPA effect. RasGRP1 activates the MAPK pathway through activation of the small G protein Ras. Gene silencing of RasGRP1 prevented the PE-mediated suppression of NCC activity, the activation of the H-Ras isoform of Ras, and the activation of ERK1/2 MAPK. This finding confirmed the critical role of RasGRP1 in mediating the PE-induced suppression of NCC activity through the stimulation of the MAPK pathway.
Keywords: diacylglycerol, distal convoluted tubule, NaCl cotransporter
The thiazide-sensitive sodium-chloride cotransporter (NCC) is the salt-absorptive pathway localized to the apical membrane of the mammalian distal convoluted tubule (DCT), which is responsible for reabsorbing 5–10% of the filtered load of sodium (1). Now, 50 years after the introduction of the first thiazide diuretic, pharmacological inhibition of NCC by thiazide diuretics is recommended as first-line treatment for essential hypertension (2). NCC also has been shown to play a role in genetic disorders of hypo- and hypertension (3–6). Despite the importance of this cotransporter in human disease, the mechanisms underlying regulation of this cotransporter in the mammalian kidney remain largely unknown. The relative difficulty in isolating the DCT for microperfusion studies and the lack of a mammalian DCT cell line that is amenable to physiological studies have been the primary hindrances to investigating the regulation of this cotransporter. Using the gold standard for assessment of NCC activity, thiazide-sensitive 22Na+ uptake, we developed a method for investigating the regulation of this cotransporter in a mammalian cell line that natively expresses it.
This system allowed us to assess the complex regulation of NCC by phorbol esters (PEs), the functional analogs of diacylglycerol (DAG). For many years, DAG and PEs have been assumed to mediate their effects strictly through the stimulation of PKC. The classical pathway of a G protein-coupled receptor (GPCR) stimulating phospholipase C (PLC), resulting in DAG release and stimulation of PKC, was the accepted pathway for DAG-dependent regulation. However, over the last few years, it has become apparent that PEs (and DAG) can bind and activate at least five other families of DAG targets, such as Munc 13s, DAG kinases, chimaerins, protein kinase Ds (PKDs), and Ras guanyl nucleotide-releasing proteins (RasGRPs) (7). All of these families of proteins, as well as PKCs, have been shown to bind DAG/PEs through a structurally similar DAG-binding domain, the C1 domain (7). However, in the physiology literature, the impact of these other DAG/PE targets has been largely unappreciated. In fact, to our knowledge, there has not been a single study that has investigated whether DAG/PE effects on transporters or channels were mediated by targets other than PKC or PKD (which was originally called PKCμ). The effect and impact of these other binding partners have been further masked by the use of older, nonspecific PKC inhibitors, such as Calphostin C (CalC), which inhibits all of the binding partners of DAG. Given the large body of literature in transport physiology that has ascribed DAG/PE effects to PKC, the implications of these findings may require us to reexamine a whole body of historical literature on the physiology of PKC regulation of channels and transporters. Here we describe that RasGRP1, not PKC, is the DAG/PE target that mediates the suppression of NCC.
Results
mDCT Cells Natively Exhibit NCC Activity.
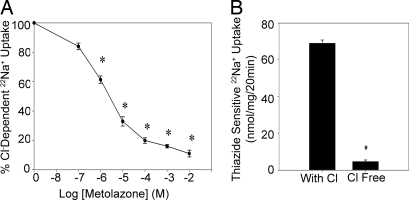
To assess the impact of DAG/PE stimulation on NCC activity, we used mouse DCT [mDCT, a gift of Peter Friedman (University of Pittsburgh, Pittsburgh, PA)] cells for our experiments. These cells demonstrate transport characteristics consistent with cells from the second portion of the mouse and human DCT, including NCC protein expression, amiloride-sensitive sodium uptake, and thiazide-sensitive sodium uptake (8, 9). However, the 22Na+ uptake protocols used in those studies involved determining radiotracer uptake while the mDCT cells were in suspension (9). To eliminate any artifact because of exposure to trypsin and to more closely mimic physiologic conditions, we developed a protocol to measure the NCC activity of mDCT cells grown in a monolayer. Using this protocol, the mDCT cells demonstrated significant thiazide-sensitive 22Na+ uptake given by the difference between 22Na+ uptake of mDCT cells exposed to thiazide-free and thiazide-containing media. To ensure that this thiazide-sensitive radiotracer uptake represented NCC activity, similar studies were done by using chloride-free and chloride-containing uptake media and showed significant chloride-dependent 22Na+ uptake, which could be inhibited by the addition of escalating concentrations of thiazide (Fig. 1A). This effect was significant at metolazone concentrations as low as 1 × 10−6 M. Similarly, thiazide-sensitive uptake depended on the presence of chloride in the uptake media (Fig. 1B). Furthermore, the addition of amiloride, benzamil, and bumetanide to inhibit sodium uptake from the epithelial sodium channel (ENaC), Na+/Ca+ exchanger (NCX1), and Na+K+2Cl− cotransporter (NKCC1), respectively, did not significantly alter chloride-dependent sodium radiotracer uptake (data not shown). These studies indicate that the mDCT cells demonstrate 22Na+ uptake that is both thiazide-sensitive and chloride-dependent, the hallmarks of NCC activity. Because this activity is native to the mDCT cells, these cells provided an opportunity to study the DAG/PE effect in a system with intrinsic NCC function and regulatory mechanisms.
Fig. 1.
Chloride-dependent and thiazide-sensitive 22Na+ uptake in mDCT cells. (A) mDCT cells were preincubated in chloride-free medium and then incubated for 20 min in either chloride-free or chloride-containing uptake medium and increasing concentrations of metolazone (n = 8). *, P < 0.01, compared with vehicle. (B) mDCT cells preincubated in chloride-free medium were incubated in chloride-free (Cl-free) or chloride-containing (with Cl) uptake medium and either vehicle or 1 mM metolazone. Thiazide-sensitive uptake was calculated as the difference in radiotracer uptake between the metolazone-containing and metolazone-free groups (n = 8). *, P < 0.01, compared with the chloride-containing group.
12-O-tetradecanoylphorbol-13-acetate (TPA) Decreases NCC Function.
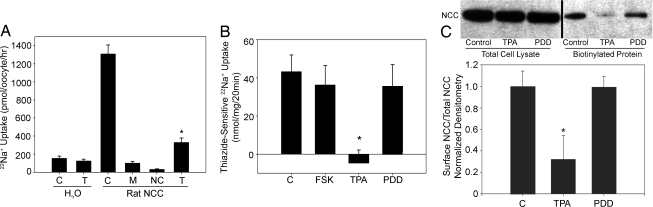
The initial studies assessing the DAG/PE effect on NCC function were performed by using the Xenopus laevis oocyte expression system. 22Na+ uptakes performed on oocytes expressing NCC showed a reduction in NCC activity when treated with the PE TPA, compared with vehicle (Fig. 2A). To assess TPA's effect on our mammalian cell system, 22Na+ uptakes on mDCT cells were performed in the presence of forskolin (an activator of PKA), TPA, or the functionally inactive phorbol ester 4-α-phorbol 12, 13-didecanoate (4α-PDD). The addition of 100 nM TPA for 15 min eliminated thiazide-sensitive uptake, reducing uptakes to statistically no different from zero for TPA versus 43.4 ± 8.68 nmol/mg for vehicle (P < 0.01 by ANOVA) (Fig. 2B). Treating the cells with 4α-PDD or forskolin did not significantly affect NCC function, compared with control. These data indicate that PEs act as potent suppressors of NCC function.
Fig. 2.
Inhibition of NCC Activity by TPA. (A) 22Na+ uptake of X. laevis oocytes injected with either water (H2O) or cRNA for rat NCC (rat NCC) and treated with 100 μM metolazone (M), 1 μM TPA (T), or DMSO vehicle (C). Data from uptakes performed by using an uptake medium with chloride (NC) are included as a negative control (n = 38). *, P < 0.01, compared with control rNCC. (B) 22Na+ uptake of mDCT cells treated with 150 nM forskolin (FSK), 100 nM TPA, 100 nM 4α-PDD (PDD), or control (DMSO vehicle, C) before being placed in uptake medium (n = 12). *, P < 0.01, compared with DMSO. (C) Surface proteins of mDCT cells treated as in B were biotinylated. Immunoblotting and densitometry for total lysate NCC protein and surface biotinylated NCC in each group is shown (n = 10). NCC band appears at 110 kDa. *, P < 0.001, compared with control and PDD groups.
TPA Reduces NCC Surface Expression.
The activity of NCC can be regulated by a change in either its surface expression or the intrinsic function of the cotransporter. To explore the role of the former in our cell system, cell-surface biotinylation experiments were performed on mDCT cells incubated with vehicle, TPA, or 4α-PDD. The amount of total NCC protein in the cell lysate was similar in each group (Fig. 2C). TPA significantly reduced the expression of NCC on the mDCT cell surface, whereas 4α-PDD had no significant effect on NCC surface expression. This ≈68% reduction in surface expression suggests that the majority of the decrease in activity seen with TPA exposure is mediated by changes in surface expression.
TPA's Effect on NCC Activity Is Mediated by RasGRP1.
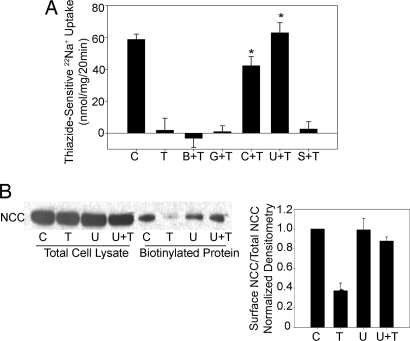
To determine the pathway by which TPA exerts its effect on NCC, 22Na+ uptakes were performed with TPA in the presence of various inhibitors. None of the inhibitors alone affected NCC function (data not shown). Treatment with bisindolylmaleimide (BIM), an inhibitor of classical and novel isoforms of PKC, did not abolish the effect of TPA on NCC activity in either mDCT cells or X. laevis oocytes [Fig. 3A and supporting information (SI) Fig. 8]. Similarly, Gö6976, a classical PKC inhibitor that also blocks the action of PKD, did not change TPA's effect on NCC activity. Taken together, these data suggest that TPA's effect was not because of the stimulation of an isoform of PKC or PKD and that TPA acted by binding to another mediator that shared the C1-binding domain. Therefore, the cells were treated with CalC, which blocks the C1 domain. As expected, the addition of CalC prevented any reduction in NCC activity in response to TPA (42.2 ± 5.7 nmol/mg for CalC with TPA vs. not different from zero for TPA alone) (Fig. 3A).
Fig. 3.
NCC activity and surface expression with exposure to TPA and various inhibitors. (A) 22Na+ uptake of mDCT cells treated with 100 nM TPA (T) and either 250 nM BIM (B+T), 200 nM CalC (C+T), 500 nM Gö6976 (G+T), 10 nM U0126 (U+T), 10 nM SB203408 (S+T), or control (DMSO vehicle, C) before being placed in uptake medium (n = 8). *, P < 0.005, compared with TPA alone. (B) Cells treated with 100 nM TPA (T) and 10 nM U0126 (U) were biotinylated, and immunoblotting for NCC was performed. NCC band appears at 110 kDa. *, P < 0.05, compared with control.
The RasGRP family of proteins is activated by DAG by the C1 domain and activates the small G protein Ras by removing GDP from inactive RasGDP and allowing binding of GTP to Ras, forming active RasGTP. RasGTP ultimately leads to MAPK activation, triggering Raf-1, MEK1/2 (MAP and ERK Kinase 1/2), and finally ERK1/2 MAPK (extracellular signal-regulated kinases 1 and 2) activation. Given this signaling pathway and the fact that RasGRP1 is expressed in the distal tubule of the mammalian kidney (10), we speculated that RasGRP1 was the binding partner of TPA. To test this hypothesis, we blocked the downstream targets of Ras activation and found that treatment with both TPA and 1,4-diamino-2,3-dicyano-1,4-bis [2-aminophenylthio] butadiene (U0126), a highly selective inhibitor of MEK1/2, negated the effect of TPA (62.9 ± 6.3 nmol/mg for U0126 and TPA vs. not statistically different from zero for TPA alone) (Fig. 3A). This effect was specific to MEK1/2 inactivation because SB203580, a p38 MAPK kinase inhibitor, had no significant effect on TPA-induced suppression of NCC activity. The dependence of MEK1/2 on the TPA effect also was seen with NCC surface expression because biotinylation experiments showed that the addition of U0126 abrogated the reduction in surface expression seen with TPA alone (Fig. 3B).
Because blockade of MEK1/2, and therefore ERK1/2 MAPK, prevented the DAG/PE effect, we directly investigated the role of RasGRP1 in this process. As expected, RasGRP1 is clearly expressed in mDCT cells, as seen by immunoprecipitation and subsequent Western blotting and by RT-PCR of mRNA collected from mDCT cells (SI Fig. 9).
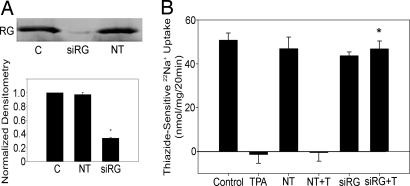
To assess the role of RasGRP1 in TPA's effect on NCC, mDCT cells were transfected with siRNA for RasGRP1. Gene silencing was confirmed by Western blotting and demonstrated 66% reduced expression, compared with control (Fig. 4A). 22Na+ uptakes were performed in the presence of TPA in a parallel fashion. Cells transfected with nontargeting siRNA did not differ from non-siRNA-treated controls with respect to mean NCC activity and response to TPA (Fig. 4B). Gene silencing for RasGRP1 completely prevented the effect of TPA on sodium uptake because these cells demonstrated similar levels of NCC activity in the presence or absence of TPA (43.7 ± 1.7 vs. 46.8 ± 3.6 nmol/mg) (Fig. 4B). The NCC activity of cells transfected with siRNA for RasGRP1 when treated with TPA was significantly greater than similarly treated control and nontargeting groups (46.8 ± 3.6 nmol/mg vs. statistically no different from zero for the control and nontargeting groups) (Fig. 4B). This finding indicates that the DAG/PE effect is mediated by RasGRP1.
Fig. 4.
NCC activity after gene silencing of RasGRP1. (A) mDCT cells were transfected with siRNA for RasGRP1 (siRG), nontargeting siRNA (NT), or empty vector (C). Cells were lysed 48 h after transfection, and immunoprecipitation and immunoblotting with antiRasGRP1 antibody were performed (n = 5 for accompanying densitometry). *, P < 0.01, compared with C and NT. (B) 22Na+ uptake experiments were performed on similarly transfected cells treated with 100 nM TPA (T) or vehicle (DMSO) (n = 12). *, P < 0.05, compared with C+T or NT+T.
TPA-Induced Stimulation of RasGRP1 Activates H-Ras.
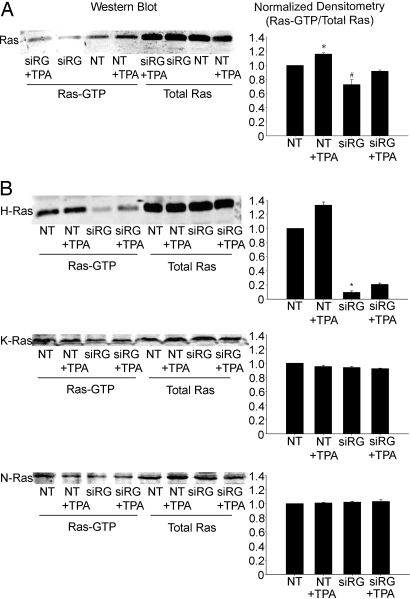
To confirm this finding, we examined the effect of TPA on RasGRP1's site of action, Ras. Studies were conducted by using an activated Ras pull-down assay on lysates of mDCT cells treated with either nontargeting siRNA or siRNA specific for RasGRP1. TPA was found to increase total Ras-GTP by 16.0 ± 1.5% (P < 0.05) (Fig. 5A). Total RasGTP in the RasGRP1-silenced mDCT cells was significantly reduced by 27.3 ± 6.9%, compared with control (P < 0.05). Notably, there are three major isoforms of Ras (H-Ras, K-Ras, and N-Ras), and preferential activation of specific isoforms by various effectors has been described (11). Given the disparity between the magnitude of the increase in RasGTP levels and the dramatic reduction in NCC activity, we therefore examined the Ras activation patterns of each of these isoforms of Ras. The increase in RasGTP with TPA was entirely because of increased H-Ras-GTP because activated Ras pull-down assays performed by using isotype-specific antibodies showed a 32.7 ± 4.5% increase (P < 0.01) in H-Ras-GTP with exposure to TPA (Fig. 5B). RasGRP1 silencing with siRNA significantly reduced H-Ras-GTP levels by 90.5 ± 2.1%, compared with control (P < 0.01) (Fig. 5B). No significant change was observed in H-Ras-GTP levels in RasGRP1-silenced mDCT cells treated with TPA. N-Ras and K-Ras showed no alterations in activation with TPA or gene silencing (Fig. 5B). These experiments demonstrate that stimulation of RasGRP1 by TPA in this system specifically and significantly activates H-Ras.
Fig. 5.
Ras activation in mDCT cells after gene silencing of RasGRP1. mDCT cells transfected with siRNA for RasGRP1 (siRG) or nontargeting siRNA (NT) were preincubated in chloride-free medium and then treated for an additional 15 min with 100 nM TPA or vehicle (DMSO). Cells were then lysed, and a RasGTP pull-down was performed, followed by immunoblotting for Ras (21 kDa). Shown are representative immunoblots and densitometries. (A) Ras (all isoforms) (n = 4). * and #, P < 0.01 as compared to NT. (B) H-, K-, and N-Ras (n = 4). *, P < 0.01 as compared to NT.
RasGRP1 Mediates Activation of ERK1/2 by TPA.
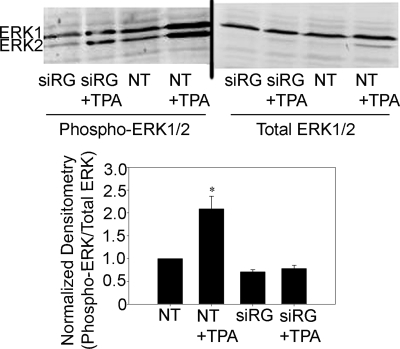
To assess the impact of TPA-mediated RasGRP1 stimulation on ERK1/2 MAPK activation, we examined levels of activated ERK1/2 by using an antibody specific for active, phosphorylated ERK1/2. Cells were again treated with either nontargeting siRNA or siRNA specific for RasGRP1 before lysis and immunoblotting. The addition of TPA significantly increased phosphorylated ERK1/2 by 209 ± 27%, compared with controls (P < 0.005) (Fig. 6). Notably, levels of ERK1/2 phosphorylation in mDCT cells treated with siRNA for RasGRP1 were not significantly different from control, suggesting that basal levels of ERK1/2 phosphorylation are maintained by other mechanisms. The addition of TPA in these RasGRP1 knockdown cells did not significantly affect phosphorylated ERK1/2 levels, compared with control. Taken together, these data indicate that PE-induced RasGRP1/H-Ras activation is responsible for the increased ERK1/2 MAPK activation that mediates reduced NCC cell-surface expression and decreased NCC function.
Fig. 6.
ERK phosphorylation in mDCT cells after gene silencing of RasGRP1. mDCT cells transfected with siRNA for RasGRP1 (siRG) or nontargeting siRNA (NT) were treated for 15 min with 100 nM TPA or vehicle (DMSO). Cells were then lysed, and immunoblotting for phosphorylated or total ERK1/2 was performed. ERK1 appears at 44 kDa, and ERK2 appears at 42 kDa (n = 4). *, P < 0.01, compared with NT.
Discussion
The vast majority of work on the regulation of NCC has been carried out in heterologous expression systems. Using the X. laevis oocyte expression system, we found that PEs were potent suppressors of NCC activity (Fig. 2A). However, a study (12) investigating the regulation of NCC by using cells of collecting duct origin transfected with human NCC reported that PEs had no effect on the thiazide-sensitive 22Na+ uptake. We have now established a method for reliably measuring the function of NCC in a physiologically relevant cell system that natively expresses NCC. Investigation of the complex regulation of NCC by DAG/PE using this system demonstrated the suppression of NCC and revealed a new regulatory pathway for the physiological effects of DAG/PE on transport proteins. Given the well described heterogeneity of hormonal response, effectors, and second messengers between different nephron segments (13), the heterologous expression of NCC in cells of collecting duct origin may have played a role in the conflicting results.
Although DAG has been historically linked to the activation of PKC as the sole mediator of its effects, there are at least five other families of proteins (including RasGRP) that are bound and activated by DAG and PEs (7, 14). Using specific inhibitors of PKC and PKD, we were able to demonstrate that these DAG targets are not involved in the suppression of NCC by PE. Because one of the members of the RasGRP family of proteins (RasGRP1) is expressed in the distal tubule of the mammalian kidney (10), we investigated whether it was mediating the PE effect. The RasGRP family of small G proteins also includes RasGRP2, −3, and −4. RasGRP1 and −3 are high-affinity DAG binders (15, 16) that have been implicated in the control of cell differentiation and proliferation, particularly in T and B cells (17, 18). They also appear to play key regulatory roles in keratinocytes and neuroendocrine cells (19, 20). The pathway of DAG/PE activation of RasGRPs is complex and cell-specific. In some cells, the activation of RasGRPs by PE appears to be mediated through PKC (21, 22). In other cells, DAG/PE stimulation of RasGRPs is PKC-independent (19, 20). After activation by DAG, RasGRP1 activates the small G protein Ras by causing release of GDP from Ras. Ras is then free to bind and be activated by GTP (Fig. 7). There are three isoforms of Ras (H-Ras, K-Ras, and N-Ras). Ras activates Raf, the first kinase in the ERK1/2 MAPK pathway. Raf then phosphorylates and activates MEK1/2, which phosphorylates and activates ERK1/2. This Ras/Raf/MEK/ERK cascade of kinases has been well characterized and plays a role in numerous cellular processes. The presence of RasGRP1 in the mammalian distal tubule and mDCT cells, as well as the complete prevention of PE suppression of NCC by ERK1/2 MAPK blockade (Fig. 3), suggested that RasGRP1 could be the mediator of the PE effect. The specificity of the ERK1/2 MAPK pathway for the inhibition of NCC is emphasized by the complete lack of effect of inhibitors of PKC, PKD, and p38 MAPK (Fig. 3). The only other inhibitor that blocked this effect was CalC, which inhibits all of the DAG targets. Confirmation of the critical role of RasGRP1 was demonstrated by the elimination of the PE suppression of NCC with knockdown of RasGRP1.
Fig. 7.
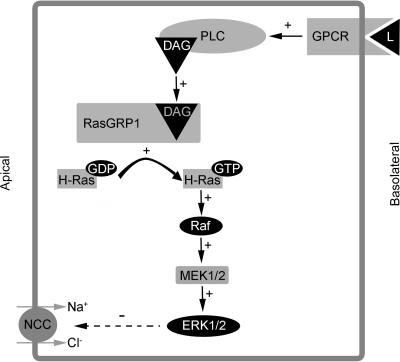
Proposed mechanism for the DAG/PE effect on NCC in mDCT cells. GPCR, G protein-coupled receptor; L, ligand; PLC, phospholipase C; DAG, diacylglycerol; RasGRP1, Ras guanyl-releasing protein 1; MEK, MAP and ERK kinase 1/2; ERK, extracellular signal-regulated kinases 1 and 2.
We also examined the effects on the immediate downstream effector of RasGRP1, Ras. The decrease in activation of Ras was consistent with the knockdown of RasGRP1 (Fig. 5). However, because the degree of knockdown of RasGRP1 appears greater than the decrease of activation of Ras, this finding could reflect a decrease in the activation of a specific Ras isoform or isoforms, whereas the activation state of other Ras isoforms is unchanged. There is some evidence that Ras isoforms can be preferentially activated under certain conditions. In Jurkat T cells, low-grade stimulation activates N-Ras preferentially, whereas more intense stimulation activates both K-Ras and N-Ras (Jurkat cells express little H-Ras endogenously) (11, 23). This effect has been attributed to differential cellular localization, with N-Ras and H-Ras capable of being activated at the Golgi and K-Ras only being activated at the plasma membrane (23, 24). However, other studies using different reporter systems and conditions failed to find this preferential localization (25, 26). Given the heterogeneity of Ras isoform-specific expression and stimulation, we tested whether RasGRP1 was preferentially activating specific Ras isoforms. This finding revealed that PEs appear to preferentially activate H-Ras, and that basal and PE-stimulated activation of H-Ras depends on RasGRP1 (Fig. 5). The other isoforms of Ras are not activated by PE and are not dependent on RasGRP1.
An examination of the effect of RasGRP1 silencing on the activation of ERK1/2 provides additional insight into this process. The expected significant increase in ERK1/2 activation with PE exposure is present, and the suppression of RasGRP1 expression clearly prevents this activation (Fig. 6). However, there is a basal level of ERK1/2 activation that is unaffected by the suppression of RasGRP1 expression. This RasGRP1-independent basal level of ERK1/2 activation correlates well with the RasGRP1-independent basal activation of K-Ras and N-Ras. Thus, N-Ras and K-Ras activation through a non-PE, non-RasGRP1 pathway could potentially mediate the basal ERK1/2 activation. There are a number of proteins that are not DAG/PE-stimulated proteins that activate Ras. One of these proteins could be mediating the basal activation of ERK through N-Ras and K-Ras, whereas RasGRP1 mediates the PE-stimulated activation of H-Ras and ERK that regulates surface expression of NCC.
The suppression of NCC function mediated by TPA appears to be primarily because of a decrease in surface expression (Figs. 2 and 3) that depends on MAPK. Acute changes in surface expression are a well established regulatory mechanism for transport proteins. Presently, the only established mechanism of regulating NCC surface expression is WNK4 inhibition of forward trafficking of NCC (27, 28). This process does not involve dynamin-dependent endocytosis and may involve alterations in lysosomal degradation before plasma membrane insertion. However, there are numerous examples of transport protein regulation through dynamin-dependent endocytosis. MAPKs also have been shown to mediate changes in surface expression of ion transport proteins (29–31). The RasGRP1/MAPK pathway could regulate the endocytotic pathway in NCC, providing a pathway for regulation of NCC surface expression distinct from WNK4 effects on NCC. Further elucidation of the mechanisms mediating these changes in surface expression is necessary.
Although the regulatory impact of DAG/PE on NCC has been demonstrated in two systems here, the question of what triggers the DAG release in the mammalian distal tubule cells remains unanswered. It is well established that certain heterotrimeric G proteins activate phospholipase C, which then releases DAG and inositol (1,4,5)-trisphosphate from the cell membrane (Fig. 7). However, the identity of a hormonal or physiological stimulus that activates a GPCR and regulates NCC has yet to be identified. There is some evidence that aldosterone regulates NCC (32, 33). However, the mineralocorticoid receptor is not a GPCR. Angiotensin II is a GPCR, and there is some evidence that angiotensin II increases the surface expression of NCC (34). However, changes in the function of NCC by angiotensin II have not been demonstrated, and an increase in surface expression is the opposite effect from what we see with activation of RasGRP1. There are numerous other GPCRs present in the DCT, and there are presently no definitive studies linking these receptors to the function of NCC. Future studies are necessary to define these upstream regulators of this important cotransporter.
We have established a reliable, reproducible system to study the regulation of NCC function in mammalian DCT cells and used this system to correlate function with surface expression. This system allowed us to study the regulation of NCC by DAG/PE, revealing that a regulator of transport proteins, RasGRP1, mediates the effects of DAG/PE in a PKC-independent manner. These findings suggest that we may need to reexamine the effects on transport proteins that have previously been attributed to PKC. Additionally, we have shown that PEs, acting through RasGRP1, appear to preferentially activate the H-Ras isoform of Ras in mDCT cells, resulting in ERK1/2 activation and suppression of NCC function and surface expression (Fig. 7).
Materials and Methods
Assessment of NCC Function in mDCT Cells.
mDCT cells grown to 90% confluence in 12-well plates were incubated at 37°C in an isotonic, Cl−-free medium [130 mM Na gluconate, 2 mM/kg gluconate, 1.0 mM Ca gluconate, 1 mM/mg gluconate, 5 mM HEPES/Tris (pH 7.4), 1 mM amiloride, 0.1 mM bumetanide) for 30 min to increase the driving force for radiotracer uptake. During this time period, the cells were treated with the various agents being studied or vehicle (DMSO). The medium was then changed to a 22Na+-containing medium [140 mM NaCl, 1 mM CaCl, 1 mM MgCl, 5 mM HEPES/Tris (pH 7.4), 1 mM amiloride, 0.1 mM bumetanide, 0.1 mM benzamil, 1 mM ouabain, and 1μCi/ml of 22Na+Cl−] with or without 0.1 mM metolazone and incubated for 20 min. Tracer uptake was then stopped by washes with ice-cold PBS. Cells were subsequently lysed with 0.1% (wt/vol) SDS. Radioactivity was measured by liquid scintillation, and protein concentrations of the lysates were determined [bicinchoninic acid (BCA) protein assay; Pierce]. Uptakes were normalized to nmol/mg. Thiazide-sensitive uptake was given by the difference of the uptakes with and without thiazide.
X. laevis Oocyte Preparation and Assessment of NCC Function.
The preparation and assessment of NCC function of X. laevis oocytes is described in detail in SI Materials and Methods.
Cell-Surface Biotinylation.
mDCT cells were incubated in Cl−-free medium for 15 min. Then 100 μm TPA, 4αPDD, or DMSO (vehicle) was added to the medium for 15 min. The Cl−-free medium was removed, and uptake medium (without 22Na+) was added for 30 min. The cells were washed with PBS, and cell-surface proteins were labeled, collected, and isolated by using the pinpoint cell-surface protein isolation kit (Pierce). Protein concentrations were determined by using a BCA protein assay kit. For SDS/PAGE analysis, 80 μg of total protein from cell lysates were loaded along with 30 μl of biotinylated protein from the DMSO control group and the proportionate volume from the rest of the biotinylated protein groups.
Activated Ras Assay.
mDCT cells grown to 80% confluence after transfection with siRNA were incubated for 30 min in Cl−-free medium. During this time period, the cells were treated with 100 nM TPA for 15 min. Cells were subsequently lysed and affinity-purified for activated Ras by using the EZ-Detect Ras Activation Kit (Pierce). Immunoblotting was then performed by using antibodies for Ras and its major isoforms.
ERK1/2 Phosphorylation Assay.
mDCT cells grown to 80% confluence after transfection with siRNA were incubated for 30 min in Cl−-free medium. During this time period, the cells were treated with 100 nM TPA for 15 min. Cells were subsequently lysed in 1 ml of lysis. The lysates were homogenized by sonication on ice. Samples were immunoblotted for phosphorylated ERK1/2 and total ERK1/2.
Other Methods.
The previous methods also used typical uses of cell culture, immunoprecipitation, immunoblotting, transfection of siRNA, and RT-PCR. For complete details regarding these methods, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Eugene Chang for advice and discussions. This work was supported by National Institutes of Health Grants K08 DK070668 (to R.S.H.), T32 DK007510–19 (to B.K.), DK38510 (to M.W.M.), DK42086 (to B.K, M.W.M., and R.S.H.), and DK64635 (to G.G.) and by a Leducq Foundation grant (to S.C.H. and G.G.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709506104/DC1.
References
- 1.Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee WS, Hediger MA, Hebert SC. J Biol Chem. 1994;269:17713–17722. [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. J Am Med Assoc. 2003;289:2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, et al. Nat Genet. 1996;12:24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 4.Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, et al. Nat Genet. 2006;38:1124–1132. doi: 10.1038/ng1877. [DOI] [PubMed] [Google Scholar]
- 5.Wilson FH, Kahle KT, Sabath E, Lalioti MD, Rapson AK, Hoover RS, Hebert SC, Gamba G, Lifton RP. Proc Natl Acad Sci USA. 2003;100:680–684. doi: 10.1073/pnas.242735399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang CL, Angell J, Mitchell R, Ellison DH. J Clin Invest. 2003;111:1039–1045. doi: 10.1172/JCI17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Springett GM, Kawasaki H, Spriggs DR. BioEssays. 2004;26:730–738. doi: 10.1002/bies.20057. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Nunez D, Morales-Ruiz M, Leivas A, Hebert SC, Poch E. Am J Physiol. 2004;286:F936–F944. doi: 10.1152/ajprenal.00070.2003. [DOI] [PubMed] [Google Scholar]
- 9.Gesek FA, Friedman PA. Am J Physiol. 1995;268:F89–F98. doi: 10.1152/ajprenal.1995.268.1.F89. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita S, Mochizuki N, Ohba Y, Tobiume M, Okada Y, Sawa H, Nagashima K, Matsuda M. J Biol Chem. 2000;275:25488–25493. doi: 10.1074/jbc.M003414200. [DOI] [PubMed] [Google Scholar]
- 11.Perez de Castro I, Bivona TG, Philips MR, Pellicer A. Mol Cell Biol. 2004;24:3485–3496. doi: 10.1128/MCB.24.8.3485-3496.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Jong JC, Willems PH, Van Den Heuvel LP, Knoers NV, Bindels RJ. J Am Soc Nephrol. 2003;14:2428–2435. doi: 10.1097/01.asn.0000089832.52063.f5. [DOI] [PubMed] [Google Scholar]
- 13.Reilly RF, Ellison DH. Physiol Rev. 2000;80:277–313. doi: 10.1152/physrev.2000.80.1.277. [DOI] [PubMed] [Google Scholar]
- 14.Brose N, Betz A, Wegmeyer H. Curr Opin Neurobiol. 2004;14:328–340. doi: 10.1016/j.conb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Lorenzo PS, Beheshti M, Pettit GR, Stone JC, Blumberg PM. Mol Pharmacol. 2000;57:840–846. [PubMed] [Google Scholar]
- 16.Lorenzo PS, Kung JW, Bottorff DA, Garfield SH, Stone JC, Blumberg PM. Cancer Res. 2001;61:943–949. [PubMed] [Google Scholar]
- 17.Dower NA, Stang SL, Bottorff DA, Ebinu JO, Dickie P, Ostergaard HL, Stone JC. Nat Immunol. 2000;1:317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- 18.Coughlin JJ, Stang SL, Dower NA, Stone JC. J Immunol. 2005;175:7179–7184. doi: 10.4049/jimmunol.175.11.7179. [DOI] [PubMed] [Google Scholar]
- 19.Ozaki N, Miura Y, Yamada T, Kato Y, Oiso Y. Biochem Biophys Res Commun. 2005;329:765–771. doi: 10.1016/j.bbrc.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 20.Rambaratsingh RA, Stone JC, Blumberg PM, Lorenzo PS. J Biol Chem. 2003;278:52792–52801. doi: 10.1074/jbc.M308240200. [DOI] [PubMed] [Google Scholar]
- 21.Roose JP, Mollenauer M, Gupta VA, Stone J, Weiss A. Mol Cell Biol. 2005;25:4426–4441. doi: 10.1128/MCB.25.11.4426-4441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng Y, Liu H, Coughlin J, Zheng J, Li L, Stone JC. Blood. 2005;105:3648–3654. doi: 10.1182/blood-2004-10-3916. [DOI] [PubMed] [Google Scholar]
- 23.Mor A, Campi G, Du G, Zheng Y, Foster DA, Dustin ML, Philips MR. Nat Cell Biol. 2007;9:713–719. doi: 10.1038/ncb1592. [DOI] [PubMed] [Google Scholar]
- 24.Bivona TG, Perez de Castro I, Ahearn IM, Grana TM, Chiu VK, Lockyer PJ, Cullen PJ, Pellicer A, Cox AD, Philips MR. Nature. 2003;424:694–698. doi: 10.1038/nature01806. [DOI] [PubMed] [Google Scholar]
- 25.Ebinu JO, Stang SL, Teixeira C, Bottorff DA, Hooton J, Blumberg PM, Barry M, Bleakley RC, Ostergaard HL, Stone JC. Blood. 2000;95:3199–3203. [PubMed] [Google Scholar]
- 26.Augsten M, Pusch R, Biskup C, Rennert K, Wittig U, Beyer K, Blume A, Wetzker R, Friedrich K, Rubio I. EMBO Rep. 2006;7:46–51. doi: 10.1038/sj.embor.7400560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai H, Cebotaru V, Wang YH, Zhang XM, Cebotaru L, Guggino SE, Guggino WB. Kidney Int. 2006;69:2162–2170. doi: 10.1038/sj.ki.5000333. [DOI] [PubMed] [Google Scholar]
- 28.Golbang AP, Cope G, Hamad A, Murthy M, Liu CH, Cuthbert AW, O'Shaughnessy KM. Am J Physiol. 2006;291:F1369–F1376. doi: 10.1152/ajprenal.00468.2005. [DOI] [PubMed] [Google Scholar]
- 29.Soundararajan R, Zhang TT, Wang J, Vandewalle A, Pearce D. J Biol Chem. 2005;280:39970–39981. doi: 10.1074/jbc.M508658200. [DOI] [PubMed] [Google Scholar]
- 30.Li D, Wang Z, Sun P, Jin Y, Lin DH, Hebert SC, Giebisch G, Wang WH. Proc Natl Acad Sci USA. 2006;103:19569–19574. doi: 10.1073/pnas.0609555104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Babilonia E, Li D, Wang Z, Sun P, Lin DH, Jin Y, Wang WH. J Am Soc Nephrol. 2006;17:2687–2696. doi: 10.1681/ASN.2006050426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA. Proc Natl Acad Sci USA. 1998;95:14552–14557. doi: 10.1073/pnas.95.24.14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Velazquez H, Bartiss A, Bernstein P, Ellison DH. Am J Physiol. 1996;270:F211–F219. doi: 10.1152/ajprenal.1996.270.1.F211. [DOI] [PubMed] [Google Scholar]
- 34.Sandberg MB, Riquier ADM, Pihakaski-Maunsbach K, McDonough AA, Maunsbach AB. Am J Physiol. 2007;293:F662–F669. doi: 10.1152/ajprenal.00064.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.