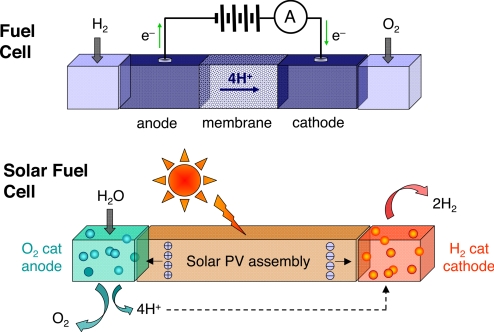
PERSPECTIVE. For the article “Powering the planet: Chemical challenges in solar energy utilization,” by Nathan S. Lewis and Daniel G. Nocera, which appeared in issue 43, October 24, 2006, of Proc Natl Acad Sci USA (103:15729–15735; first published October 16, 2006; 10.1073/pnas.0603395103), the authors note that in Fig. 1, the charges shown in the solar fuel cell are on the wrong sides of the cell. The holes should be at the anode, and the electrons should be at the cathode. This error does not affect the conclusions of the article. The corrected figure and its legend appear below.
Fig. 1.
H2 and O2 are combined in a fuel cell to generate a flow of electrons and protons across a membrane, producing electrical energy. The solar fuelcell uses light to run the electron and proton flow in reverse. Coupling the electrons and protons to catalysts breaks the bonds of water and makes the bonds H2 and O2 to effect solar fuel production.