Abstract
Many factors influence the assembly of fibronectin into an insoluble fibrillar extracellular matrix. Previous work demonstrated that one component in serum that promotes the assembly of fibronectin is lysophosphatidic acid (Zhang, Q., W.J. Checovich, D.M. Peters, R.M. Albrecht, and D.F. Mosher. 1994. J. Cell Biol. 127:1447–1459). Here we show that C3 transferase, an inhibitor of the low molecular weight GTP-binding protein Rho, blocks the binding of fibronectin and the 70-kD NH2-terminal fibronectin fragment to cells and blocks the assembly of fibronectin into matrix induced by serum or lysophosphatidic acid. Microinjection of recombinant, constitutively active Rho into quiescent Swiss 3T3 cells promotes fibronectin matrix assembly by the injected cells. Investigating the mechanism by which Rho promotes fibronectin polymerization, we have used C3 to determine whether integrin activation is involved. Under conditions where C3 decreases fibronectin assembly we have only detected small changes in the state of integrin activation. However, several inhibitors of cellular contractility, that differ in their mode of action, inhibit cell binding of fibronectin and the 70-kD NH2-terminal fibronectin fragment, decrease fibronectin incorporation into the deoxycholate insoluble matrix, and prevent fibronectin's assembly into fibrils on the cell surface. Because Rho stimulates contractility, these results suggest that Rho-mediated contractility promotes assembly of fibronectin into a fibrillar matrix. One mechanism by which contractility could enhance fibronectin assembly is by tension exposing cryptic self-assembly sites within fibronectin that is being stretched. Exploring this possibility, we have found a monoclonal antibody, L8, that stains fibronectin matrices differentially depending on the state of cell contractility. L8 was previously shown to inhibit fibronectin matrix assembly (Chernousov, M.A., A.I. Faerman, M.G. Frid, O.Y. Printseva, and V.E. Koteliansky. 1987. FEBS (Fed. Eur. Biochem. Soc.) Lett. 217:124–128). When it is used to stain normal cultures that are developing tension, it reveals a matrix indistinguishable from that revealed by polyclonal anti-fibronectin antibodies. However, the staining of fibronectin matrices by L8 is reduced relative to the polyclonal antibody when the contractility of cells is inhibited by C3. We have investigated the consequences of mechanically stretching fibronectin in the absence of cells. Applying a 30–35% stretch to immobilized fibronectin induced binding of soluble fibronectin, 70-kD fibronectin fragment, and L8 monoclonal antibody. Together, these results provide evidence that self-assembly sites within fibronectin are exposed by tension.
Fibronectin (FN)1 is a large, multi-module extracellular matrix (ECM) protein that exists in two major states, either circulating in plasma as a soluble dimeric protein or found within ECMs as an insoluble component associated with cells and other ECM components. The structure of FN and its many functions have been reviewed (31, 48). FN plays a major role in cell adhesion, migration, differentiation, and growth regulation. FN has been implicated in normal wound healing and in embryonic development. Disruption of the FN gene in mice results in an embryonic lethal phenotype, confirming the importance of FN in development (23). Loss of FN from the cell surface is a characteristic of many transformed and tumorigenic cells. Restoration of a FN matrix often suppresses the transformed phenotype (24, 31). Many of the effects of FN on cells are exerted by FN when it is in the form of a fibrillar matrix. Although much has been learned about the assembly of FN into a matrix, this process is not fully understood (for reviews see references 49 and 51).
One factor that affects the assembly of the FN matrix is the state of the actin cytoskeleton. It has long been known that disruption of actin filaments with cytochalasin inhibits matrix assembly (2, 13, 67). Newly assembled FN fibrils coalign with bundles of actin filaments (27, 32), and with focal adhesions or cytoskeletal structures that contain many focal adhesion proteins (3, 7, 8, 10, 61). In addition, fluorescent fragments of FN involved in matrix assembly target to focal adhesions when added to cells (13, 18, 29, 63). The reason why an intact cytoskeleton is necessary for matrix assembly has not been established.
Another factor well known to promote FN matrix assembly is serum (40, 50). In serum a potent component promoting FN assembly was identified as lysophosphatidic acid (LPA) (9, 71). LPA is a bioactive lipid that triggers several signaling pathways, including mobilization of intracellular calcium, activation of phospholipase C, activation of protein kinase C, and activation of the GTP-binding protein, Rho (43). Rho itself triggers multiple signaling pathways (56). A prominent pathway stimulates assembly of large bundles of actin filaments (stress fibers) and focal adhesions (57). This effect on stress fiber and focal adhesion formation is due to Rho-stimulating contractility (15), which occurs via a kinase/phosphatase cascade that elevates myosin light chain phosphorylation (6, 35). Light chain phosphorylation stimulates myosin ATPase activity and myosin filament formation (6).
Here we have investigated whether the effect of LPA on FN matrix assembly is mediated via Rho, and in particular, by Rho-stimulated contractility. Inhibiting Rho blocks FN matrix assembly, whereas introducing recombinant, constitutively active Rho into quiescent Swiss 3T3 cells promotes matrix assembly by these cells. Inhibitors of cellular contractility antagonize the effect of LPA on matrix assembly, indicating that Rho-generated tension has a critical role in the assembly of the FN matrix. While this work was under review, similar results indicating that LPA stimulates FN matrix assembly via Rho and contractility were published (70). It was suggested by the authors of that study that contractility may stretch the uncharacterized molecules of large apparent molecular mass that they have suggested serve as FN assembly sites, thereby exposing cryptic binding sites for FN. However, previous work has identified cryptic self-association sites within FN (28, 29, 33) and it has also been proposed that these may be exposed by tension (28, 29, 67). Consistent with this idea, we have identified an mAb, previously shown to block assembly, that demonstrates enhanced binding to FN matrices when cells are contractile. In addition, we have detected increased binding of this antibody to FN that has been mechanically stretched in the absence of cells. Similarly, compared with unstretched FN, stretched FN binds more exogenous FN and more of the NH2-terminal, 70-kD FN fragment. These observations support the idea that tension generated by cells exposes self-assembly sites within FN. A model for how tension may promote the incorporation of FN molecules into a fibrillar matrix is proposed.
Materials and Methods
Cell Culture
Swiss 3T3 fibroblasts (American Type Culture Collection, Rockville, MD), were grown in DME (Life Technologies, Inc., Gaithersburg, MD), with 100 units/ml penicillin, 100 μg/ml streptomycin, and 250 ng/ml amphotericin B as fungizone, supplemented with 10% bovine calf serum (BCS) (JRH Biosciences, Lenexa, KS). Quiescence was obtained as described before (14). Briefly, in each experiment cells were seeded at identical density in 100-mm tissue culture dishes and maintained in the culture medium with serum, without feeding. The cells were used 7–10 d after they reached confluence, at which time they were washed and starved in serum-free culture medium for 16–24 h. Quiescent, serum-starved cells were stimulated with culture medium containing 0.5% FBS (JRH Biosciences) or 2 μg/ml LPA (1-oleoyl-2-hydroxy-sn-glycero-3-phosphate, monosodium salt in H2O) (Avanti Polar Lipids, Alabaster, AL) for the indicated periods of time. A time course of the response to LPA treatment revealed maximal stress fiber induction at 2–4 h and FN matrix assembly at 4–6 h as judged by immunofluorescence microscopy. To maintain a maximal stimulation in experiments measuring incorporation of FN into the detergent-insoluble matrix, we replenished LPA at 4–5-h intervals.
Ras-transformed human breast epithelial cells (R-MCF10A) (36) were grown in DME and Ham's F12 medium (1:1 ratio) containing 5% horse serum, 20 ng/ml EGF, 10 μg/ml insulin, and 0.5 μg/ml hydrocortisone.
In some experiments an inhibitor of actin–myosin interactions, 2,3- butanedione 2-monoxime (BDM; Sigma Chemical Co., St. Louis, MO) was used at a final concentration of 2.5–20 mM. Because of BDM's instability in aqueous solutions (16), for long-term experiments measuring the effect of BDM on the assembly of FN into the detergent-insoluble matrix, we added BDM in four aliquots of 5 mM each, 30 min before the addition of LPA at each 5-h interval. Another inhibitor of contractility, 1-(5-isoquinolinylsulfonyl)-2-methylpiperazine (H7; Sigma Chemical Co.) was added at 150–300 μM final concentration. 1-(5-chloronaphthalenesulfonyl)-1H-hexahydro-1,4-diazepine, (ML-7; BIOMOL, Plymouth Meeting, PA) was used at a final concentration of 2.5–25 μM.
Immunofluorescence Microscopy
Immunofluorescence was performed as described previously (15). Actin was stained with FITC–phalloidin (Molecular Probes, Inc., Eugene, OR). In some experiments FN was stained with a polyclonal rabbit anti–human FN antibody provided by Dr. L.B. Chen (Dana-Farber Cancer Center, Boston, MA), or with an mAb (Transduction Labs, Lexington, Kentucky) followed by TRITC-labeled donkey anti–rabbit or donkey anti–mouse IgG (Chemicon International, Inc., Temecula, CA). The L8 and C6F10 mAbs were provided by Dr. M. Chernousov (Weiss Center for Research, Danville, PA) and have been characterized previously (11, 12, 17). L8 binds to an epitope in I9III1 FN domains. C6F10 binds to the FN III10 module close to the RGDS sequence. For double immunofluorescence, L8 and C6F10 were used at 50 μg/ml in combination with the polyclonal anti-FN. Cells microinjected with recombinant Rho were comicroinjected with a marker IgG (FITC-conjugated goat anti–human IgG fraction; Cappel, Organon-Technika, Durham, NC).
Purification and Microinjection of Recombinant Proteins
Recombinant Val14rhoA (a gift from Dr. A. Hall, University College, London, UK) and recombinant C3 transferase (a gift from Dr. L. Feig, Tufts University, Boston, MA) were expressed as a glutathione-S-transferase (GST) fusion protein in Escherichia coli. Proteins were purified on glutathione–agarose beads as described before (34, 57) and cleaved from GST with thrombin (Sigma Chemical Co.) in a buffer (50 mM TrisCl, pH 7.5, 50 mM NaCl, 5 mM MgCl2, 1 mM DTT, 1 mM PMSF; C3: 50 mM TrisCl, pH 8.0, 150 mM NaCl, 2.5 mM CaCl2, 5 mM DTT), which was then removed by three subsequent incubations with para-aminobenzamidine agarose (Sigma Chemical Co.). Rho was dialyzed against microinjection buffer (50 mM TrisCl, pH 7.5, 50 mM KCl, 5 mM MgCl2, 0.1 mM DTT) and concentrated to 2–3 mg/ml using a Centricon concentrator (Amicon Inc., Beverly, MA). Purified Rho was loaded by capillarity into needles pulled on a Brown-Flaming micropipette puller (Sutter Instrument Co., San Francisco, CA) and injected into cells as described previously (8). Cells were injected for 15–30 min and then incubated at 37°C in an atmosphere of 10% CO2 for 4–5 h in the presence of 100 μg/ml human plasma FN (GIBCO BRL, Gaithersburg, MD or Chemicon International, Inc., or purified as described in reference 31).
C3 was concentrated using a Centricon concentrator and, for Swiss 3T3 cells, was added to the culture media at 25 μg/ml during 16–24-h starvation and at the same concentration following stimulation of cells with LPA. To introduce C3 transferase into R-MCF10A cells, cells were incubated with the same concentration of C3 in serum-containing media for 48 h.
Fibronectin and 70-kD Fibronectin Fragment Binding
The 70-kD FN fragment was purified as previously described (42). FN and the 70-kD FN fragment were labeled with 125I, using the Iodogen method (21). The specific activities were 2.5–4 × 106 cpm/μg for FN and 3.8 × 106– 1.9 × 107 cpm/μg for the 70-kD FN fragment. To measure the initial binding of FN and 70-kD FN fragment to cells, Swiss 3T3 or R-MCF10A cells were plated in 24-well plates. For Swiss 3T3 cells, after they had reached confluence, they were maintained for several days before being starved for 24 h and in some cases being incubated with C3 for the same period of time. Some starved cells were pretreated for 1 h with contractility inhibitors (either 20 mM BDM or 300 μM H7) and kept in the presence of contractility inhibitors during the incubation with 125I-FN or 125I-labeled 70-kD FN fragment. Cultures were then stimulated with 2 μg/ml LPA for 30 min, followed by the addition of the 125I-FN or 125I-labeled 70-kD FN fragment. Cells were incubated with 125I-FN (300 ng; 7.5 × 105 cpm/well) or 125I-labeled 70-kD FN fragment (300 ng; 1.1 × 106 cpm/well) in DME containing 2 mg/ml of delipidated BSA for 2 h at 37°C. For time course experiments, 125I-FN was incubated with either serum-starved or LPA-stimulated cells for the indicated periods of time. Nonspecific binding was determined by coincubation the cells with 400 μg/ml of unlabeled FN or 100 μg/ml of unlabeled 70-kD FN fragment. For recovery experiments, starved cells treated with either BDM or H7 for 1 h, were allowed to recover in regular growth medium for 2 h before addition of 125I-FN for 30 min. After incubations, the cells were washed three times with DME, cell layers were lysed in 1 N NaOH, and radioactivity was measured in a gamma counter. Duplicate measurements were performed for each experimental condition. For R-MCF10A cells, cells were pretreated with contractility inhibitors for 2 h before adding 125I-labeled 70-kD FN fragment.
To measure the incorporation of FN into the cross-linked matrix, Swiss 3T3 cells were plated in six-well dishes at identical density in 10% bovine calf serum in DME, grown to confluency, washed, and then starved as described above. Confluent R-MCF10A cells were transferred to the medium containing 0.5% horse serum before adding 125I-FN. For Swiss 3T3 cells, 125I-FN (18 μg/ml; 4.5 × 107 cpm/dish) was added and the cells were stimulated with LPA in the presence or absence of inhibitors, as described above. The medium was then removed and cells were washed three times in PBS. The deoxycholate (DOC)-insoluble fraction (cross-linked FN) was harvested as described before (42). Briefly, the dishes were washed for 1 min with 1 ml each of ice-cold buffer A (3% Triton X-100 in PBS, 10 mM EDTA, 1 mM PMSF, 1 mM benzamidine, 10 μg/ml aprotinin, and 10 μg/ml leupeptin), buffer B (50 mM Tris-HCl, pH 7.4, 1 M NaCl, 10 mM MnCl2, 100 μg/ml DNase I, 1 mM benzamidine, 10 μg/ml aprotinin, and 10 μg/ml leupeptin), and buffer C (2% sodium deoxycholate in 50 mM Tris-HCl, pH 8.8, 10 mM EDTA, 1 mM benzamidine, 10 μg/ml aprotinin, and 10 μg/ml leupeptin). The DOC-insoluble fraction was then rinsed with PBS and scraped into SDS sample buffer and boiled. 125I-FN incorporation by cells under different experimental conditions was measured using a gamma counter. Aliquots of the DOC-insoluble fraction were run on a 7.5% SDS-PAGE gel. Gels were dried and processed for autoradiography to visualize FN bands. Each assay was performed in duplicate. Similar procedures were used to measure the incorporation of FN into cross-linked matrix on the surface of R-MCF10A cells in the presence or absence of contractility inhibitors.
Silicone Rubber Substrates
To visualize cell contractility, flexible rubber substrates have been generated as previously described (15).
Integrin Activation Assays
The level of β1 integrin activation was measured using the antibody 12G10 (Serotec Ltd., Kidlington, Oxford, UK). This antibody binds to a ligand-induced epitope on human β1 integrins (52, 53). Parallel wells of cells under various conditions were incubated with 0.5 μg/ml 125I-12G10 (specific activity: 1.75 × 105 cpm/μg) or with 125I-TS2/16 (specific activity: 3.8 × 105 cpm/μg) for 1 h at 37°C. To induce a high affinity state of the integrin, cells were incubated with 100 μM MnCl2 (22) during the incubation with primary antibody. Nonspecific binding was determined by competition of binding with an excess of unlabeled antibody and this was substracted from the values obtained under the different experimental conditions. Cell number was equalized by TS2/16 binding and the percentage of integrin activation calculated by γ0/γ × 100, where γ0 was the cpm without Mn2+ and γ was the cpm obtained in the presence of 100 μM Mn2+.
Binding of 125I-Labeled mAbs L8 and C6F10 to FN Matrices Assembled by Cultured Cells
Purified mAbs L8 and C6F10 were labeled with 125I using the Iodogen method (21). The specific activities of both antibodies were 2.5 × 106 cpm/μg. To measure the binding of mAbs to FN matrices in culture, Swiss 3T3 cells were grown to 50–80% confluence in 24-well plates in the presence of 80 μg/ml human plasma FN. Cells were changed to serum-free medium, with or without C3 transferase at 25 μg/ml for 24 h. Under these conditions, the cells in the absence of C3 continued to display normal stress fibers, whereas those with C3 had lost them (data not shown). Cells growing in the presence or absence of C3 were washed three times with serum-free medium and then incubated for 1 h at 37°C with 3 μg of 125I-labeled L8 or C6F10 in DME containing 2 mg/ml delipidated BSA. Nonspecific binding was determined in the presence of excess unlabeled mAbs. After the incubations, the cultures were washed three times with DME, and then dissolved in 1 N NaOH. Bound radioactivity was measured in a gamma counter. The ratio of the amount of bound L8 to that of bound C6F10 was determined for both untreated and C3-treated cells.
Mechanical Stretching of FN
To apply tension to FN in the absence of cells, purified FN (10 μg/ml) was covalently coupled to chemically activated, flexible rubber “Bioflex” culture dishes (FlexCell International Corp., McKeesport, PA) (provided by Dr. A. Banes, University of North Carolina, Chapel Hill, NC). Three adjacent circles were marked in the centers of each well using the top of a cell cryotube (Nunc Inc., Naperville, IL) coated with silicon vacuum grease. All coupling reactions and binding experiments were performed within these restricted areas. Coupling of FN to the rubber was performed in PBS for 1 h at room temperature. Reactive groups were blocked with 100 mM ethanolamine, pH 7.5, for 40 min at room temperature. The amount of FN coupled to the surface was calculated by including a fraction of 125I-FN in the coupling reaction. Before addition of ligands, sites for nonspecific adsorption of proteins were blocked by preincubation of the rubber both before and after stretching with 2 mg/ml BSA in Tris-buffered saline (150 mM NaCl, 50 mM TrisHCl, pH 7.6, 0.1% NaN3) for 45 min. A sustained stretch of the FN-coated rubber was achieved by placing the culture dishes over vaseline-coated, plastic soda bottle lids and applying a large uniform weight on top of the dishes. This caused the rubber surfaces to be stretched over the bottle lids. The vaseline coating minimized the friction between the lid and the rubber, resulting in a uniform stretch of the rubber over the top of the lid. This procedure resulted in a linear stretch of between 30 and 35%. For binding experiments, stretched and unstretched wells were incubated for 1 h at room temperature with 30 μl of TBS containing 2 mg/ml BSA and 5 × 105 cpm 125I-FN (4 × 106 cpm/μg), 5 × 105 cpm 125I-labeled 70-kD fragment of FN (1.9 × 107 cpm/μg), and 106 cpm 125I-L8 (6 × 107 cpm/μg) or 125I-C6F10 (107 cpm/μg). After binding, the surfaces were washed multiple times with excess of TBS. For the FN-binding experiments, one wash occurred overnight. In each case, values for nonspecific binding to stretched and unstretched surfaces were calculated and deducted by measuring the binding in the presence of excess of unlabeled reagent (1 mg/ml for FN and 70-kD FN fragment, 0.5 mg/ml for the labeled antibodies).
Results
Rho Is Involved in the Stimulation of FN Matrix Assembly by Serum or LPA
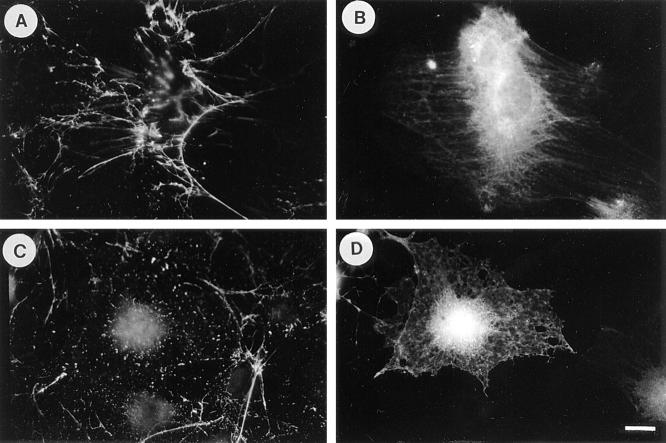
Previous work has demonstrated that serum and LPA stimulate FN assembly (71). Swiss 3T3 cells are a cell type well suited to examining whether the effects of serum and LPA are mediated via the GTP-binding protein, Rho. In the absence of serum, these cells become quiescent, lose stress fibers, and focal adhesions, but can be stimulated by serum or LPA to reassemble these structures in a Rho- dependent mechanism (57). We have examined FN matrix assembly by these cells, under conditions of quiescence or in response to stimulation by serum or LPA (Fig. 1). For most of these experiments, we have added exogenous FN because Swiss 3T3 cells only synthesize low levels of FN. When the cells are rendered quiescent by overnight serum deprivation, few stress fibers (Fig. 1 A) and little FN matrix (Fig. 1 B) are detected. In the presence of serum, the cells reveal prominent stress fibers (Fig. 1 C) and a fibrillar FN matrix (Fig. 1 D). Stimulation of quiescent cells with LPA similarly promoted organized stress fibers (Fig. 2 A) and FN matrix (Fig. 2 B).
Figure 1.
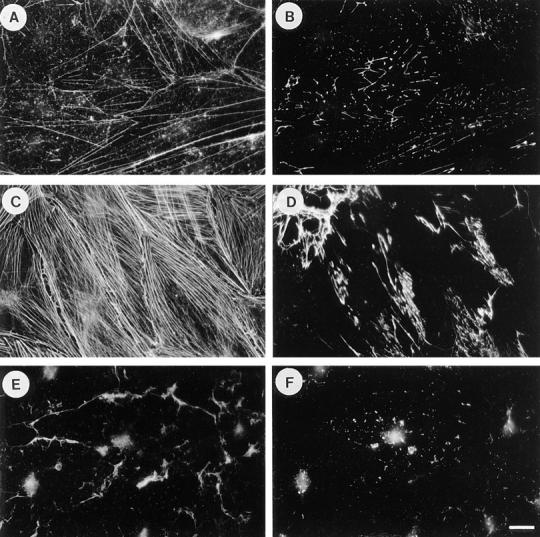
Serum induces fibronectin assembly in a Rho-dependent manner. Exogenous FN was added to quiescent, serum-starved Swiss 3T3 fibroblasts for 24 h (A and B), or to cells that were stimulated with serum for 24 h in the absence (C and D) or presence of C3 transferase (E and F). Cells were stained for actin (A, C, and E) and FN (B, D, and F). Quiescent cells have poorly developed actin stress fibers (A), and there is little FN assembly (B). Stimulation with serum induces stress fibers (C) and FN fibril assembly (D). These effects of serum are blocked when cells are incubated with C3 (E and F). Bar, 20 μm.
Figure 2.
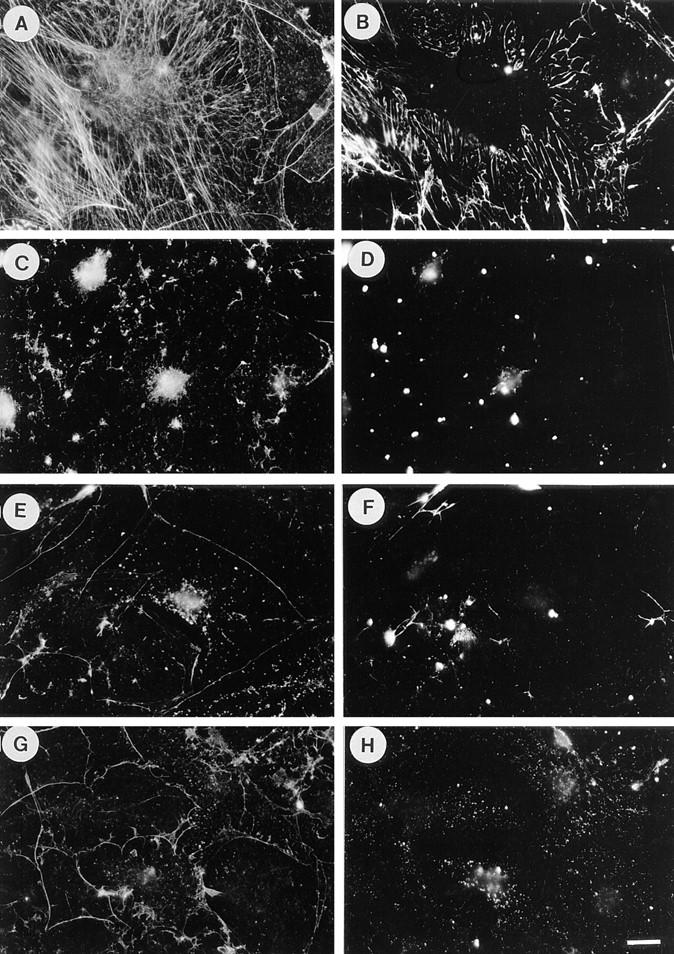
LPA-induced fibronectin assembly is blocked by inhibitors of Rho and contractility. Quiescent cells stimulated with LPA for 24 h form stress fibers (A) and assemble exogenous FN into matrix (B). Pretreatment of cells with C3 before and during LPA stimulation blocks both these effects (C and D). Addition of BDM (E and F) or H7 (G and H) during LPA stimulation blocks the formation of stress fibers (E and G) and FN matrix (F and H). Cells were stained for actin (A, C, E, and G) and FN (B, D, F, and H). Bar, 20 μm.
To determine whether the assembly of FN matrix in response to serum or LPA was dependent on Rho, we have used the Rho inhibitor C3 transferase. C3 is taken up by some cells slowly over time, so that its addition to the medium is sufficient to inhibit Rho activity (68). Quiescent Swiss 3T3 cells were pretreated with C3 before and during addition of serum and FN (Fig. 1, E and F). The C3 treatment abolished assembly of stress fibers (Fig. 1 E) and the formation of a FN matrix (Fig. 1 F). Similarly, C3 treatment blocked stress fiber and FN assembly in response to LPA stimulation of quiescent Swiss 3T3 cells (Fig. 2, C and D).
Activated Rho Stimulates FN Matrix Assembly
The inhibition of FN matrix assembly by C3 transferase provides strong evidence that Rho is involved in this process. To obtain further evidence for the involvement of Rho, we used microinjection to introduce recombinant, constitutively active Rho into quiescent Swiss 3T3 cells cultured in the presence of exogenous FN. Microinjection of active Rho stimulated matrix assembly by the injected cells (Fig. 3, A and B). In contrast, injection of only the marker protein used to identify the injected cells did not stimulate matrix assembly over the level that was detected in noninjected cells (Fig. 3, C and D).
Figure 3.
Recombinant, constitutively active Rho induces fibronectin assembly. Quiescent cells were microinjected with purified, recombinant active Rho (GST-Val14rho) and marker IgG (A and B) or with marker IgG alone (C and D) and exposed to exogenous FN for 5 h. FN (A and C) is assembled into fibrils only by cells injected with Rho (A), but not with IgG alone (C). A and C show the organization of FN, B and D show the injected cells. Bar, 20 μm.
Inhibitors of Contractility Block the Assembly of FN Matrix Induced by LPA
Previous work has shown that Rho-mediated assembly of stress fibers and focal adhesions involves increased contractility and can be blocked by inhibitors of actin–myosin activation or force generation (15). We have explored the effects of inhibitors of contractility on matrix assembly induced by LPA. Both BDM and H7 have been used previously to inhibit cell contraction (15, 65). These agents caused a complete block to stress fiber assembly (Fig. 2, E and G) and FN matrix assembly (Fig. 2, F and H), consistent with contractility contributing to both events.
For reasons described below, a second cell type had to be used to determine whether inhibiting Rho affected the activation state of integrins. It was important, therefore, to first explore the effects of inhibiting Rho and contractility on the deposition of a FN matrix by these cells. R-MCF10A cells, although transformed, exhibit many of the characteristics of a normal fibroblastic phenotype, including prominent stress fibers and focal adhesions (36). Furthermore, we have recently shown that inhibiting Rho in these cells leads to disassembly of stress fibers and focal adhesions (72). R-MCF10A cells develop an extensive FN matrix when grown routinely in the presence of serum (Fig. 4 A). The assembly of a FN matrix was inhibited by the myosin light chain kinase inhibitor ML-7 (Fig. 4 B) and by BDM (Fig. 4 C). Both of these inhibitors were found to decrease the contractility of these cells as judged by their ability to wrinkle a flexible rubber substratum upon which they were growing (72). We also examined the effect of inactivating Rho in these cells. Treatment of R-MCF10A cells with C3 abolished FN matrix assembly (Fig. 4 D) and this effect correlated with a decrease in contractility indicated by decreased wrinkling of rubber substrata (Fig. 4, E and F).
Figure 4.
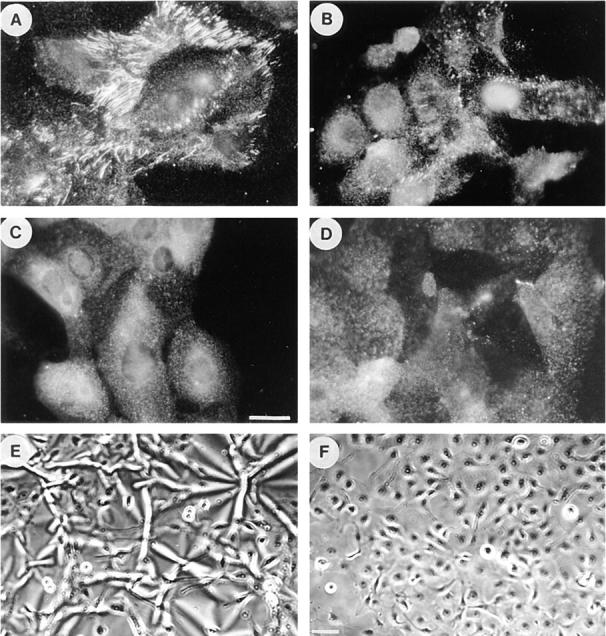
Effects of inhibitors of contractility on fibronectin matrix assembly by R-MCF10A cells. Immunofluorescence micrographs of R-MCF10A cells stained for FN after overnight treatment with serum alone (A) or with serum plus 2.5 μM ML-7 (B), 2.4 mM BDM (C), or for 2 d in culture medium plus 25 μg/ml C3 (D). FN matrix was visualized by staining with an antibody to FN. In E and F, phase micrographs are shown of the cells cultured on silicone rubber substrata in the absence (E) or presence (F) of C3. Bar: (A–D) 20 μm; (E and F) 200 μm.
Quantitation of FN and 70-kD FN Fragment Binding to Cells and Incorporation into Matrix
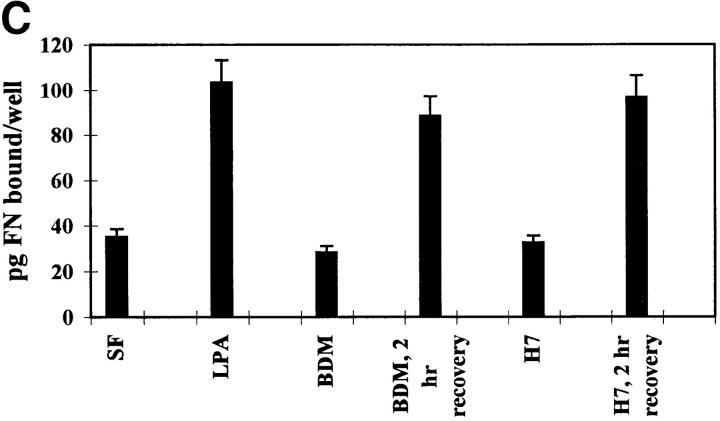
We have performed several types of experiment to measure the effects of Rho activation and cell contractility on the binding of FN and its 70-kD fragment to cells. A time course analysis of the effects of LPA on FN binding to cells showed that stimulation was detectable as early as 15 min and was maximal at 2 h after treatment (Fig. 5 A). We also examined the initial binding of 125I-labeled FN and 70-kD FN fragment to quiescent Swiss 3T3 cells stimulated with LPA, in the presence or absence of C3 and various inhibitors of contractility (Fig. 5 B). Binding of the 125I-FN to confluent LPA-stimulated Swiss 3T3 cells was significantly decreased by C3, BDM, and H7 (Fig. 5 B). Numerous studies have demonstrated that the 70-kD NH2-terminal fragment of FN is responsible for binding of FN to cells (42). Therefore, we examined the effects of these contractility inhibitors on the binding of the 125I-labeled 70-kD FN fragment to Swiss 3T3 cells. Binding of the 70-kD fragment to quiescent cells was stimulated by LPA, and this stimulation was inhibited by C3, H7 and BDM (Fig. 5 B). To determine whether the effects of the contractility inhibitors were reversible and not because of cell toxicity, the medium containing BDM or H7 was replaced with normal medium containing serum. This led to restoration of FN binding within 2 h (Fig. 5 C). The reversibility of the effect of these agents upon their removal indicated that they were not adversely affecting the cells and thus binding of FN in some permanent way.
Figure 5.
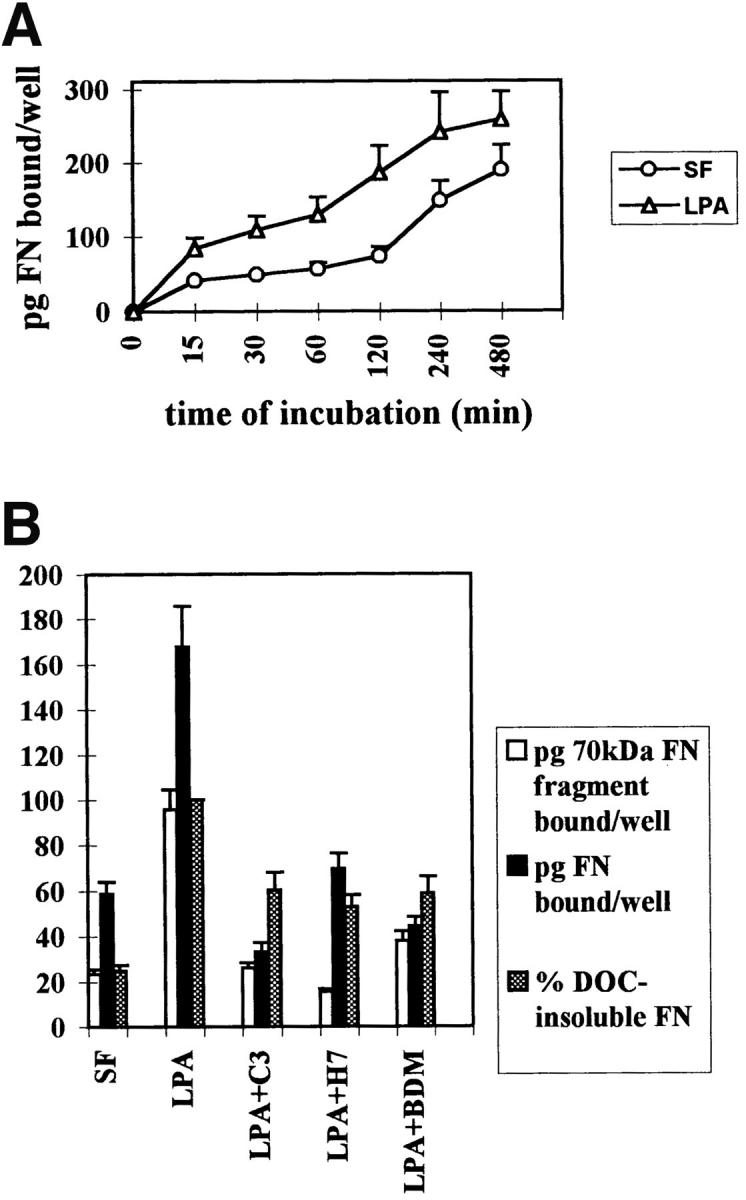
Binding of fibronectin and the 70-kD fibronectin fragment to Swiss 3T3 cells is blocked by inhibitors of Rho and contractility. (A) Time course of fibronectin binding to cells in the presence or absence of LPA. Starved (○) or LPA-treated (▵) cell monolayers were incubated with 125I-FN for increasing lengths of time. (B) Effects of inhibitors of Rho and contractility on the binding of FN (black bars) or the 70-kD FN fragment (open bars) to cells, and on the incorporation of FN into the DOC-insoluble matrix (shaded bars). Serum-free (SF) and LPA-stimulated (LPA) cells without inhibitors or pretreated with and maintained in the presence of C3, H7, or BDM were incubated for 2 h with 125I-FN or 125I-labeled 70-kD FN fragment. The amount of 125I-FN incorporated into DOC-insoluble matrix was determined as described in Materials and Methods, after incubating cells with 125I-FN for 22 h with or without inhibitors. Samples were assayed in duplicate and standard errors are indicated. (C) Reversibility of inhibition of FN binding to cells by contractility inhibitors BDM and H7. BDM- or H7-treated cells were allowed to recover in regular serum-containing medium for 2 h before a 30-min incubation with 125I-FN. Each point in A, B, and C represents the average of duplicate determinations. Nonspecific binding in the presence of unlabeled FN (400 μg/ml) or 70-kD FN fragment (100 μg/ml) was subtracted.
After the initial binding to cells, FN is incorporated into growing fibrils and becomes progressively more insoluble. This is because of disulfide bond formation and finally other covalent cross-linking events (49). To explore the effects of Rho-stimulation by LPA, Rho inhibition or inhibition of contractility on the incorporation of FN into the insoluble matrix, binding experiments were performed on Swiss 3T3 cells over a much longer time course. The incorporation of 125I-labeled FN into the DOC-insoluble matrix of Swiss 3T3 cells was stimulated by LPA and was significantly inhibited by C3, BDM, and H7 (Fig. 5 B).
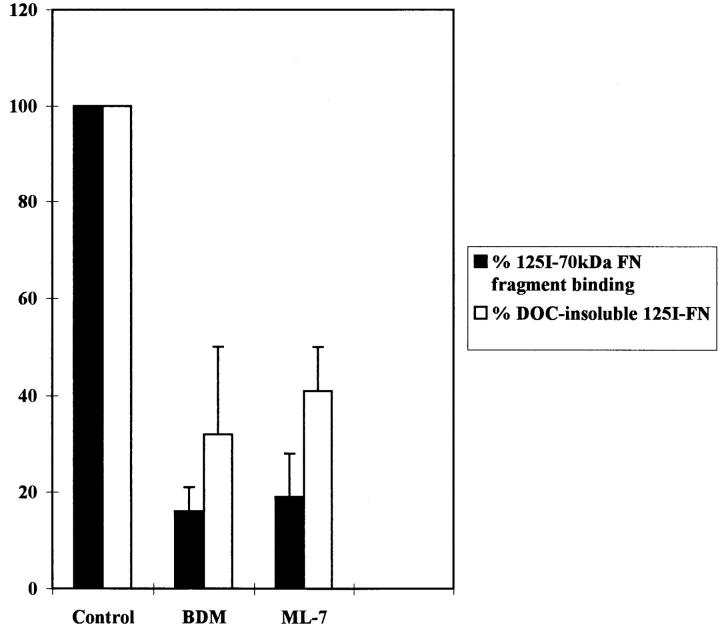
Binding experiments were also performed using the R-MCF10A cells. Inhibitors of contractility, such as BDM and ML-7, decreased the binding of the 70-kD FN fragment to cells as compared with the binding to untreated cells (Fig. 6). Similarly, the incorporation of 125I-FN into DOC-insoluble matrix by R-MCF10A cells was also diminished by BDM and ML-7 (Fig. 6).
Figure 6.
Binding of the 70-kD FN fragment to R-MCF10A cells and incorporation of FN into the cross-linked matrix is decreased by inhibitors of contractility. R-MCF10A cells were untreated (Control) or treated for 2 h with 20 mM BDM, or 25 μM ML-7, followed by incubation with 125I-labeled 70-kD FN fragment for another 1.5 h (black bars). Alternatively, R-MCF10A cells were incubated with 125I-FN for 18 h in low serum, either in the absence (Control) or presence of 2.4 mM BDM or 2.5 μM ML-7. Incorporation of FN into the DOC-insoluble fraction is shown (white bars). Results were from three independent experiments.
Integrin Activation and Matrix Assembly
Many factors contribute to the assembly of a FN matrix. Several studies have demonstrated the importance of the integrin α5β1, the major FN receptor, in the assembly of FN matrix (20, 41, 54). Integrins can exist in low affinity or high affinity (activated) states with respect to binding to extracellular ligands (58). Wu and coworkers demonstrated that integrin activation was necessary for FN matrix assembly (67). Several studies have implicated Rho in the regulation of integrin activation and so we wished to determine whether the Rho-dependent assembly of FN matrix that we were observing was due to integrin activation. Most frequently integrin activation is measured by specific mAbs that bind to epitopes that are only exposed in activated forms of the integrin. No such mAbs are available for use with mouse cells such as Swiss 3T3 fibroblasts. The mAb 12G10 has been used to quantitate the activation state of β1 integrins in human cells (52, 53). The R-MCF10A cells afforded us the opportunity to determine whether conditions that inhibit FN matrix assembly coordinately decrease integrin activation.
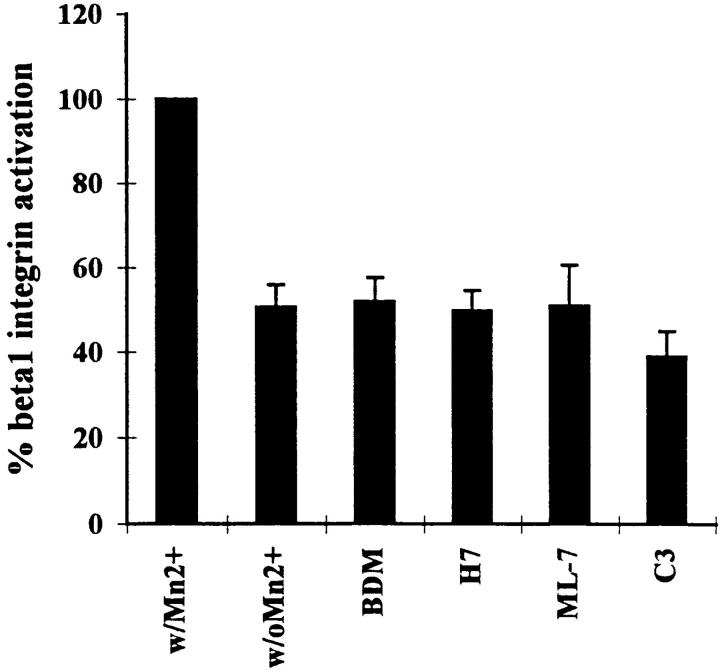
We wanted to determine whether the inhibitors of contractility used above might act by affecting the activation state of α5β1. We examined the binding of 12G10 to cells treated with or without these inhibitors. Except for a slight decrease in integrin activation following treatment of cells with C3, none of the inhibitors tested exhibited any effect on the state of integrin activation in these cells (Fig. 7). The C3 effect was small and only of marginal statistical significance (P < 0.025). As a positive control, the cells were exposed to Mn2+, which has been shown to stimulate the activated state of integrins (22). Treatment of the R-MCF10A cells with Mn2+ caused a marked shift in the amount of 12G10 bound by these cells (Fig. 7) confirming that a change in activation state of the α5β1 in these cells could be detected.
Figure 7.
Effects of C3 and inhibitors of contractility on the activation state of β1 integrins. β1 integrin activation was measured by the binding of 125I-labeled 12G10 antibody to R-MCF10A monolayers in the absence of any agents (control) or in the presence of 20 mM BDM, 150 μM H7, 25 μM ML-7, or 25 μg/ml C3. Cells were exposed to these agents for 2 h, except for C3 which was included in the culture medium for 2 d. Full activation of β1 integrins was achieved by treating control cells with 100 μM MnCl2. Experiments were performed in triplicate. Results are represented as the percentage of the value obtained with MnCl2.
Cell Tension Exposes a Cryptic Epitope in FN
One possible explanation for the observation that cell contractility promotes FN matrix assembly is that tension on FN molecules stretches particular domains (17) to expose new binding sites (see Discussion). We wished to explore this possibility and rationalized that if cryptic sites are involved in assembly, then some antibodies that inhibit assembly may do so by binding to and blocking these sites. The L8 mAb binds to a site within the adjacent I9 and III1 modules in FN, and L8 blocks FN assembly into matrix (10, 11). The staining properties of L8 were explored on FN matrix assembled by Swiss 3T3 cells using exogenous human plasma FN under conditions where cell contractility was modulated by the presence or absence of C3 transferase (Fig. 8). Cells grown to 50–80% confluence in the presence of exogenous human FN were changed to serum-free medium, with or without C3. After 24 h, cells were stained for FN using double labeling with both polyclonal and mAbs (Fig. 8). (It should be noted that under these subconfluent conditions, the Swiss 3T3 cells did not become quiescent when cultured in the absence of serum for 24 h, as judged by the presence of stress fibers.) In the absence of C3, L8 stained a fibrillar FN matrix indistinguishable from that revealed by double labeling with a polyclonal anti-FN antibody (Fig. 8, A and B). However, compared with the staining obtained with the polyclonal antibody, the staining by L8 was decreased when cells were incubated with C3 (Fig. 8, C and D). Similarly, the staining with L8, but not the polyclonal antibody, was also diminished in cultures treated with inhibitors of contractility such as H7 (data not shown). Other mAbs, such as C6F10, did not show this differential staining pattern (Fig. 8, E–H), indicating that this was not a general characteristic of mAbs directed against FN.
Figure 8.
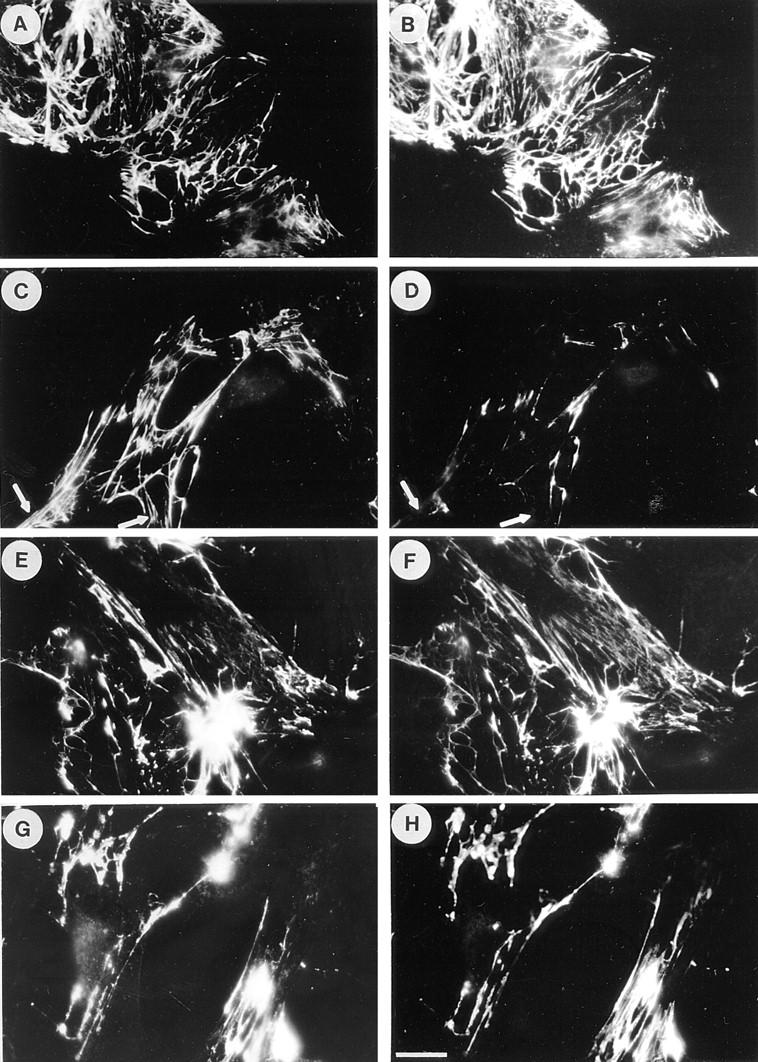
Inhibition of Rho decreases selectively the staining of FN matrices by the L8 mAb. Swiss 3T3 cells were grown to 50–80% subconfluence in media containing serum supplemented with 80 μg/ml human plasma FN. The medium was replaced with serum-free media with or without 25 μg/ml C3 and the cells cultured for a further 24 h. The FN matrix was visualized by double-labeling with polyclonal antibodies to FN (A, C, E, and G) or with mAbs, L8 (B and D) and C6F10 (F and H). Untreated cells are shown in A, B, E, and F. Cells treated with C3 are shown in C, D, G, and H. Note that the mAbs give an essentially identical staining pattern to the polyclonal antibody with the exception of reduced L8 staining in the presence of C3. Arrows in C and D indicate regions of differential staining between the polyclonal antibody and L8. Bar, 20 μm.
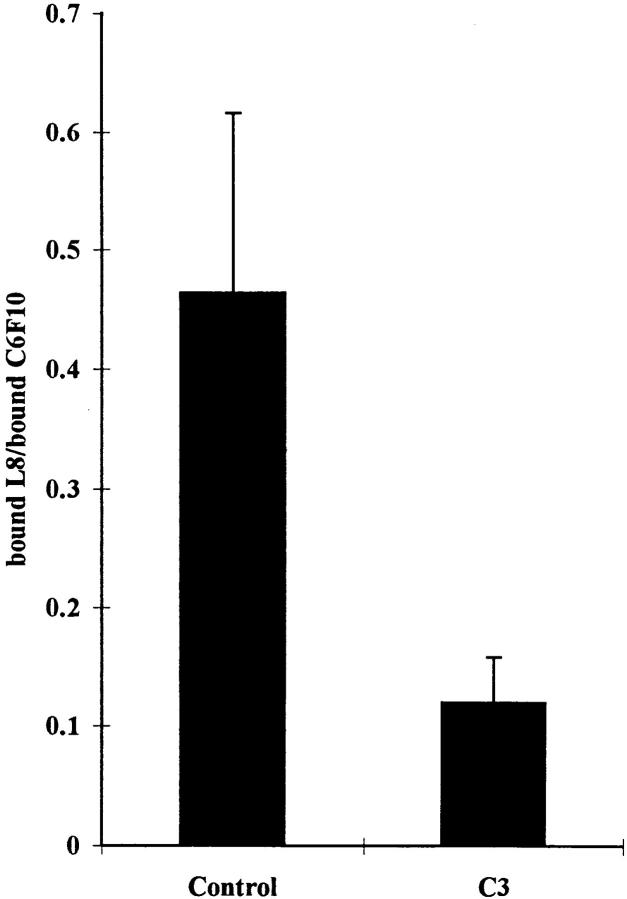
Radiolabeled antibodies were used to quantitate the effects of C3 on the binding of L8 and C6F10 to FN matrices. Parallel cultures were incubated with either 125I-labeled L8 or C6F10 and the ratio of bound counts determined (Fig. 9). The ratio of L8 to C6F10 binding to cultures decreased by approximately threefold when cultures were treated with C3.
Figure 9.
Inhibition of binding of L8 mAb to FN matrix by the Rho inhibitor C3. Parallel dishes of Swiss 3T3 cells supplemented with 80 μg/ml of human plasma FN were changed to serum-free media with or without C3 for 24 h. Binding of 125I-labeled mAb L8 or C6F10 was determined. The level of nonspecific binding was calculated by competition with excess unlabeled antibody and these values were used to calculate the levels of specific binding. The ratio of specific binding of L8 to C6F10 was determined for untreated cells (Control) and cells treated with C3 (C3). Three independent experiments were performed.
Mechanical Stretching of FN Exposes Binding Sites and Promotes Self-Assembly
We wished to determine whether mechanically stretching FN in the absence of cells exposed sites for self-assembly and, in particular, the L8 epitope. To stretch FN we took advantage of “Bioflex” tissue culture plates that were designed to allow cultured cells to be exposed to cyclic stretch (4). FN (10 μg/ml) was coupled covalently to the rubber surface of these plates, generating a FN density of ∼3.5 ng/mm2. Using a molecular mass for the FN dimer of 500 kD, we calculated that this corresponds to ∼4 × 103 molecules/μm2. If all the molecules were spread out flat, based on the calculations of Lightner and Erickson (38), this should more than saturate the surface. Because increased binding of FN to the surface was obtained by extending the coupling reaction, we conclude that some of the molecules were not spread out flat and must have extended up from the surface.
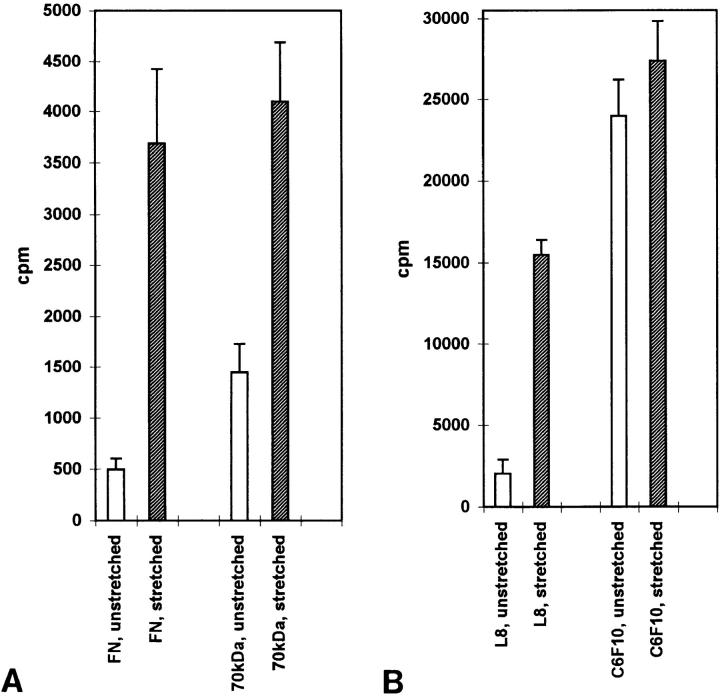
The binding of 125I-FN and 125I-labeled 70-kD FN fragment to the FN-coated surfaces was measured with and without stretching for 1 h (Fig. 10 A; Table I). In both cases, specific binding to unstretched FN was low and close to the value for nonspecific binding, defined as the amount bound in the presence of 1 mg/ml of unlabeled ligand. Specific binding was significantly increased when the surfaces were stretched (30–35% in a linear dimension) (Fig. 10 A; Table I).
Figure 10.
Mechanical stretching of purified fibronectin stimulates binding of fibronectin, the 70-kD fibronectin fragment and the L8 mAb. FN covalently coupled to rubber surfaces was left unstretched or stretched as described in the Materials and Methods. The surfaces were incubated with 125I-labeled FN, 70-kD FN fragment or mAbs C6F10 and L8. Specific binding was calculated as described in the Materials and Methods. Open columns represent the binding to unstretched surfaces, shaded columns represent the binding to stretched surfaces. Each column represents the mean of six measurements (FN and 70-kD FN fragment binding) or triplicate measurements (C6F10 and L8). Error bars indicate the standard errors of the mean.
Table I.
Binding of FN, 70-kD FN Fragment, L8, and C6F10 mAbs to Unstretched and Stretched FN
| Specific binding | Nonspecific binding | Ratio stretched/ unstretched | p value | |||||
|---|---|---|---|---|---|---|---|---|
| cpm | cpm | t test | ||||||
| FN, unstretched | 498 (± 103) | 6,947 (± 499) | ||||||
| FN, stretched | 3,688 (± 735) | 6,948 (± 298) | 7.4 | 0.01 | ||||
| 70 kD, unstretched | 1,447 (± 283) | 4,469 (± 589) | ||||||
| 70 kD, stretched | 4,099 (± 588) | 5,464 (± 334) | 2.8 | 0.005 | ||||
| L8, unstretched | 2,052 (± 839) | 7,327 (± 92) | ||||||
| L8, stretched | 15,497 (± 897) | 10,905 (± 508) | 7.6 | 0.0004 | ||||
| C6F10, unstretched | 23,941 (± 2,255) | 3,239 (± 72) | ||||||
| C6F10, stretched | 27,382 (± 2,457) | 5,049 (± 241) | 1.1 | 0.36 |
Values for binding of 125I-labeled ligands and antibodies to unstretched and stretched FN are shown. Specific binding is the binding obtained after subtraction of the nonspecific binding. Nonspecific binding was the value obtained in the presence of 1 mg/ ml of unlabeled ligand (FN or 70-kD FN fragment) or 0.5 mg/ml of unlabeled antibody. For FN and 70-kD FN fragment, the means of six measurements are shown; for the antibodies, the means of triplicate measurements are shown. The standard errors of the means are indicated in parentheses. The ratio of counts bound to the stretched versus the unstretched FN (i.e., the fold increase in binding in response to stretching) is shown.
We also examined the binding of 125I-labeled L8 and C6F10 mAbs to FN in stretched and unstretched conditions (Fig. 10 B; Table I). The binding of 125I-L8 to unstretched FN was low and not much above the value for nonspecific binding, consistent with the L8 epitope being inaccessible in most unstretched FN molecules. In contrast, the binding of C6F10 to unstretched FN was relatively high and considerably above the value for nonspecific binding. A slight increase was observed in binding of C6F10 to stretched FN. This was not statistically significant, but was observed on multiple occasions, possibly reflecting an increased accessibility of the antibody to FN molecules that had been sterically blocked by other overlapping FN molecules on the unstretched surface. In comparison, the binding of L8 to stretched FN was significantly increased, indicating exposure of the epitope upon stretching (Fig. 10 B; Table I).
Discussion
In this work we have demonstrated that Rho-mediated contractility stimulates the assembly of a fibrillar FN matrix. In addition, we have shown that tension applied directly to FN in the absence of cells generates binding sites for additional FN molecules and, in particular, for the 70-kD NH2-terminal domain of FN, which is critical for matrix assembly (42). Our studies were motivated by an interest in the low molecular weight G protein Rho, and by the earlier observations that LPA stimulates FN matrix assembly (9, 71). Because Rho is one of several downstream effectors of LPA (43), and because the assembly of FN correlates with the development of stress fibers (31) that are regulated by Rho activity (57), it seemed likely to us that Rho may regulate FN matrix assembly. We have shown that this is indeed the case, blocking matrix assembly by a variety of inhibitors of contractility as well as by inhibiting Rho itself using the C3 transferase. Similar results have been reported recently by Mosher's group (70) while this work was under review.
The assembly of FN into a fibrillar matrix is a complex process involving multiple steps. Previous work has demonstrated a role for integrin activation in this process and several studies have suggested that integrin activation may be regulated by Rho (37, 45, 64). While investigating the effect of cell contractility on FN matrix assembly, we considered the possibility that agents that affect contractility might also be affecting integrin activation. To address this question, we used a cell type in which we could assay the state of β1 integrin activation with a mAb that recognizes an activation-dependent epitope. Treatment of cells with inhibitors of contractility at concentrations that blocked matrix assembly had no effect on integrin activation. However, with C3 a slight decrease in integrin activation was observed. Although Rho may exert some effect on matrix assembly by affecting the activation state of integrins, the predominant effect would appear to be via Rho-stimulated contractility. Consistent with this interpretation are the results with the FN 70-kD fragment. This NH2-terminal domain of FN does not interact with integrins, but its binding to cells is stimulated by LPA (71). Significantly, this interaction is decreased both by inhibiting Rho and by inhibiting contractility (see reference 70 in addition to these results). We conclude that, in cells already expressing activated integrins, Rho-stimulated contractility is critical for the assembly of a FN matrix. Our findings are consistent with the observations of Halliday and Tomasek, who showed that cells in a collagen gel assemble a FN matrix if they exert tension on the gel, but fail to assemble FN in gels without tension (26).
We can conceive of several ways by which tension/contractility could contribute to FN matrix assembly. One possible mechanism derives from previous work demonstrating that Rho induces integrin clustering (15, 30). In the case of integrins bound to FN, tension-induced clustering would juxtapose FN molecules possibly in a conformation favorable to promote self-assembly. The clustering resulting from tension would also be expected to aggregate small fibrils into larger fibrils typical of a mature ECM. Although clustering integrins would effectively concentrate FN molecules, this mechanism does not account for the observed increase in the number of binding sites for the 70-kD NH2-terminal fragment of FN that are induced by LPA (71).
An alternative mechanism is that tension applied directly to FN exposes self-assembly sites to which additional FN molecules may bind. Our results with FN stretched mechanically support this hypothesis. Tension on FN could expose self-assembly sites either by unfolding a large region of FN that is looped back on itself, or by partially unfolding FN type III domains. The existence of a loop structure in native FN is controversial but favored by some investigators. Evidence exists for several intramolecular associations, such as the binding of the I1–5 modules in the NH2-terminal 70-kD domain to the III1 module (1, 12, 46). Such an interaction within a single molecule would generate a loop. Schwarzbauer and colleagues have found that deleting the first seven type III modules accelerates the incorporation of FN into the insoluble FN matrix (59, 60). Accelerated incorporation suggests that the deleted construct has removed some constraint on assembly. This is consistent with a loop structure for the native FN, which has to be unfolded for incorporation of native FN into the assembling matrix. Morla and Ruoslahti demonstrated that a peptide from the III1 module interacted with intact FN and promoted assembly into an insoluble matrix in the absence of cells (47). This is also consistent with opening of a loop by the III1 peptide with consequent exposure of masked binding sites.
The FN constructs lacking the first seven type III modules, although they incorporate more rapidly than native FN, take much longer to generate a fibrillar matrix (60). However, the incorporation of these truncated constructs into a matrix implies that the I1–5 modules involved in matrix assembly must be binding to something on the cell surface other than the III1 domain. Support for this has been provided by Sottile and Mosher, who identified two distinct binding sites within I1–5 (63). Modules I4,5 were shown to interact with III1, but modules I1–3 inhibited binding of the NH2-terminal 70-kD fragment of FN to cells, indicating that these modules bind to another as yet unidentified site important in assembly. Whether this is elsewhere on FN or a distinct surface molecule has not been determined.
A second way by which tension may expose cryptic binding sites in FN is by causing some unfolding of FN type III modules. This idea was first suggested and considered theoretically by Erickson (19), both in the context of FN as well as the muscle structural protein titin, which contains many FN type III modules and that is subject to considerable stretching. The energy to unfold a type III module was calculated to be surprisingly small and in the same range as the force generated by one or a few myosin motors interacting with actin (19). Evidence that there are cryptic binding sites within FN type III modules has been provided by McKeown-Longo's group (28, 29). Cryptic sites have been found both within the III1 module for binding the NH2-terminal 70-kD FN fragment (28), and within the III10 module for binding the III1 module (29). Similarly, Ingham and coworkers have identified additional cryptic sites within III7 and III15 for III1 (33). The exposure of these or other sites involved in FN assembly by tension has been suggested previously (13, 28, 67), although direct evidence for this has been missing. Intrigued by the possibility that tension may expose cryptic sites in FN, we looked for a reagent that might reveal such a site. The L8 mAb, which binds to I9/III1 and blocks matrix assembly (11, 12), appears to be such a reagent. Under normal conditions of culture, this antibody labels FN matrices. However, when cell contractility is inhibited by C3, the binding of L8 to the FN matrix is reduced, consistent with an epitope that is exposed by tension exerted on the FN molecule.
We wanted to examine this possibility directly by stretching FN in the absence of cells. In addition, it seemed to us that if tension exposes self-assembly sites within FN, it should be possible to detect these in isolated FN molecules being stretched mechanically. We explored several potential ways of applying tension to FN before settling on a system that allowed covalent coupling of FN to rubber surfaces that could be stretched easily. We found that stretched FN revealed increased binding of intact FN and the 70-kD NH2-terminal FN fragment. Moreover, compared with a control mAb, L8 showed elevated binding to the stretched FN. These findings lead us to conclude that stretched FN does expose cryptic binding sites and can nucleate FN assembly.
It is relevant that FN matrix assembly is often observed at sites of tension, such as beneath cells (32) and, in particular, at focal adhesions, where tension is transmitted to the underlying substratum. A striking example of this is seen when cells are plated on fluorescent FN adsorbed to a glass coverslip (3, 25). In this situation, matrix assembly occurs as cells exert tension on the underlying FN and pull it off the coverslip. Similarly, FN fibrils are frequently detected stretching between adjacent cells in an alignment that suggests tension. For tension to be exerted on a FN dimer, forces have to be applied either in opposing directions to two sites in the dimer (an unlikely situation for a single cell), or force has to be applied to one site in a dimer that is tethered in some way to a relatively immobile structure. Tethering of FN to tissue culture surfaces is a frequent occurrence because FN adsorbs well to glass and plastic. However, tethering may also occur to adjacent cells or to other ECM components.
Any model for FN assembly into fibrils must satisfy multiple experimental observations, including the need for a functional actin cytoskeleton and the need for tension demonstrated here and previously (26, 70). Several critical sites in FN have been identified. The cell-binding site (module III10), which interacts with integrins (particularly α5β1) and the adjacent synergy site (module III9), are both required as evidenced by the inhibition of assembly by antibodies that bind to these regions (20, 41, 54) and by the need for integrin activation (67). The 70-kD NH2-terminal region of FN (including the I1–5 modules) is also required (42). This region of FN binds to cells, but not through integrins. Cell surface receptors for this domain have been elusive, although several candidates have been identified (5, 39, 44, 69). However, this region of FN also associates with the III1 module within FN (1, 28, 55) and has been implicated in generating the loop structure at the NH2 terminus of FN, discussed above. Antibodies against the III1 module block matrix assembly (11, 12). Models of assembly must account for the observation that deleting the first seven type III modules accelerates incorporation of FN into the DOC-insoluble matrix (i.e,. the cross-linked polymer) (59, 60). These findings indicate that other binding sites for the 70-kD region of FN exist beside the III1 module and evidence for such sites has been obtained (63).
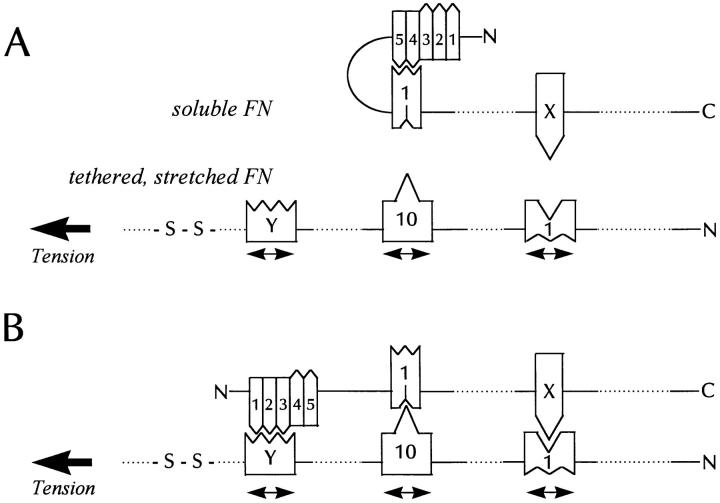
Combining these various observations with the requirement for cell contractility, we favor a model that is illustrated in Fig. 11. We envisage soluble FN protomers being folded in their NH2-terminal regions, so that the I4,5 modules interact with the III1 module (63). The incoming FN assembles onto FN that is under tension, with the tension exposing multiple cryptic binding sites within FN type III modules (three are indicated in Fig. 11). The transmission of tension from the cytoskeleton to FN accounts for the need both for an intact cytoskeleton as well as for functional (activated) integrins in FN fibril assembly. One of the exposed binding sites is the L8 epitope (within I9/III1). In Fig. 11, the domain on the incoming FN interacting with III1 is designated “X,” because its identity is uncertain. However, several modules have been found to bind III1 in vitro, including I4,5, III10, III7, and III15 (1, 28, 29, 33, 63). Further work will be required to determine which interactions are inhibited by L8. A second site that appears to be exposed by tension is within III10. This module has a conformation-dependent binding site for III1 (29). Interestingly, isolated III1 domains target to focal adhesions when cells are plated on cell-binding fragments of FN, some of which may be as small as the combined III10 module and the adjacent synergy site III9 (29). This is consistent with the incoming III1 module binding to stretched III10 modules within focal adhesions. The postulated loop structure in the incoming FN may be opened by the interaction of the I1–3 modules with a COOH-terminal, stretched type III module marked “Y” in Fig. 11. Alternatively, the interaction of the incoming FN III1 module with the stretched III10 may lower the affinity of III1 for the I4,5 modules in the 70-kD domain, thereby opening the loop. This is similar to the model proposed by Sechler and coworkers, who suggested that integrin binding to III10 affects the interactions of III1 with the NH2 terminus of FN causing the loop to open (60). Here we suggest that the conformational change induced by integrin binding may be due to the tension transmitted to FN rather than the binding itself, although binding and tension may both contribute. Upon opening of the NH2-terminal loop, the I1–3 modules bind to the type III module marked “Y” in Fig. 11. This postulated site should be critical in binding the 70-kD FN fragment to cells (the “matrix assembly site”) and is also regulated by tension, as evidenced by its exposure by LPA and its inhibition by inhibitors of contractility (62, 70, 71; and this paper). In future work, it will be important to look for this tension-regulated binding site, which we predict will be in a type III module COOH-terminal to III10.
Figure 11.
A model for tension-induced fibronectin assembly. (A) shows part of a soluble FN molecule that is going to interact with a tethered FN molecule under tension (for example, spanning between a cell and the substratum). (B) shows the interacting pair. The incoming FN in (A) has an intramolecular loop resulting from the III1 module binding to the I4,5 modules. The tension stretches and opens up some FN type III modules (three are shown) exposing cryptic binding sites for domains on the incoming FN molecule. One of the tension-exposed binding sites is in III1 and corresponds to the L8 epitope. This is involved in binding a domain in the incoming FN, marked “X” in the diagram. Another stretched domain is the III10 module, which exposes a site that binds the III1 module of the incoming FN. A third site is postulated in another type III module marked “Y.” We predict that this site will bind the I1–3 modules of the incoming FN. The binding of III10 to III1 may decrease the affinity of III1 for I4,5 and so trigger opening of the loop. Alternatively, the stretched III–Y module may exhibit a higher affinity for the I1–3 modules than the affinity of III1 for I4,5. This would also favor opening of the NH2-terminal loop. Because only one of the pair of interacting FN molecules is stretched, misalignment of the anti-parallel interacting molecules would be expected. Whether this is corrected with maturation of the fibril remains to be determined.
Recent work has indicated that the 70-kD NH2-terminal domain can be cross-linked to very high molecular mass complexes on the surfaces of cells (69). These have also been postulated to be opened by tension to expose cryptic 70-kD binding sites (70). Our data does not address the relative contribution of such sites to matrix assembly. However, our observations that purified, stretched FN binds the NH2-terminal 70-kD fragment as well as other FN molecules indicates that tension on FN alone may be sufficient to nucleate FN polymerization.
With ECM proteins like FN, it is important that assembly be tightly regulated so that it does not occur at inappropriate locations or times. One way to prevent spontaneous assembly is to have the self-assembly sites involved in polymerization cryptic within the molecule, such that they are only unmasked in response to physiological stimuli. Tension is a physiologically relevant signal and it is noteworthy that the generation of tension and FN matrix assembly are often coupled together. One example is the response to wounds, a situation where tension generated by cells and tissues contributes to wound closure and tissue remodelling. Our results suggest that cell-generated tension may be an important stimulus to the assembly and deposition of ECM that occurs during the wound repair process (66).
Acknowledgments
We thank Dr. A. Banes for valuable advice on the flexible rubber substrates that we used for stretching FN and for gifts of Bioflex plates. We are indebted to Dr. M. Chernousov for providing mAbs L8 and C6F10. We greatly appreciate the comments and advice of Drs. L. Petch and S. Sastry.
This work has been supported by National Institutes of Health grants GM29860 and HL45100.
Footnotes
Address all correspondence to Keith Burridge, Department of Cell Biology and Anatomy, 108 Taylor Hall, CB#7090, University of North Carolina, Chapel Hill, NC 27599. Tel.: (919) 966-5783. Fax: (919) 966-1856. E-mail: Kburridg@med.unc.edu
M. Chrzanowska-Wodnicka's present address is Becton Dickinson Research Center, Research Triangle Park, NC.
A.M. Belkin's present address is Department of Biochemistry, American Red Cross, Rockville, MD.
1. Abbreviations used in this paper: BDM, 2,3-butanedione 2-monoxime; DOC, deoxycholate; ECM, extracellular matrix; FN, fibronectin; GST, glutathione-S-transferase; H7, 1-(5-isoquinolinylsulfonyl)-2-methylpiperazine; LPA, lysophosphatidic acid; ML-7, 1-(5-chloronaphthalenesulfonyl)-1H-hexahydro-1,4-diazepine; R-MCF10A, Ras-transformed MCF10A breast epithelial cells.
References
- 1.Aguirre KM, McCormick RJ, Schwarzbauer JE. Fibronectin self-association is mediated by complementary sites within the amino-terminal one-third of the molecule. J Biol Chem. 1994;269:27863–27868. [PubMed] [Google Scholar]
- 2.Ali IU, Hynes RO. Effects of cytochalasin B and colchicine on attachment of a major surface protein of fibroblasts. Biochim Biophys Acta. 1977;471:16–24. doi: 10.1016/0005-2736(77)90388-1. [DOI] [PubMed] [Google Scholar]
- 3.Avnur Z, Geiger B. The removal of extracellular fibronectin from areas of cell–substrate contact. Cell. 1981;25:121–132. doi: 10.1016/0092-8674(81)90236-1. [DOI] [PubMed] [Google Scholar]
- 4.Banes AJ, Link GW, Gilbert JA, Tran R Son Tay, and O. Monbureau. Culturing cells in a mechanically active environment. Am Biotechnol Lab. 1990;8:12–22. [PubMed] [Google Scholar]
- 5.Blystone SD, Kaplan JE. Isolation of an amino-terminal fibronectin-binding protein on human U937 cells and rat peritoneal macrophages. J Biol Chem. 1992;267:3968–3975. [PubMed] [Google Scholar]
- 6.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility and signaling. Annu Rev Cell Dev Biol. 1996;12:463–519. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 7.Burridge K, Connell L. A new protein of adhesion plaques and ruffling membranes. J Cell Biol. 1983;97:359–367. doi: 10.1083/jcb.97.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burridge K, Feramisco JR. Microinjection and localization of a 130K protein in living fibroblasts: a relationship to actin and fibronectin. Cell. 1980;19:587–595. doi: 10.1016/s0092-8674(80)80035-3. [DOI] [PubMed] [Google Scholar]
- 9.Checovich WJ, Mosher DF. Lysophosphatidic acid enhances fibronectin binding to adherent cells. Arterioscler Thromb. 1993;13:1662–1667. doi: 10.1161/01.atv.13.11.1662. [DOI] [PubMed] [Google Scholar]
- 10.Chen WT, Singer SJ. Immunoelectron microscopic studies of the sites of cell–substratum and cell–cell contacts in cultured fibroblasts. J Cell Biol. 1982;95:205–222. doi: 10.1083/jcb.95.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chernousov MA, Faerman AI, Frid MG, Printseva OY, Koteliansky VE. Monoclonal antibody to fibronectin which inhibits extracellular matrix assembly. FEBS (Fed Eur Biochem Soc) Lett. 1987;217:124–128. doi: 10.1016/0014-5793(87)81255-3. [DOI] [PubMed] [Google Scholar]
- 12.Chernousov MA, Fogerty FJ, Koteliansky VE, Mosher DF. Role of the I–9 and III–1 modules of fibronectin in formation of an extracellular fibronectin matrix. J Biol Chem. 1991;266:10851–10858. [PubMed] [Google Scholar]
- 13.Christopher RA, Kowalczyk AP, McKeown–Longo PJ. Localization of fibronectin matrix assembly sites on fibroblasts and endothelial cells. J Cell Sci. 1997;110:569–581. doi: 10.1242/jcs.110.5.569. [DOI] [PubMed] [Google Scholar]
- 14.Chrzanowska-Wodnicka M, Burridge K. Tyrosine phosphorylation is involved in reorganization of the actin cytoskeleton in response to serum or LPA stimulation. J Cell Sci. 1994;107:3643–3654. doi: 10.1242/jcs.107.12.3643. [DOI] [PubMed] [Google Scholar]
- 15.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cramer LP, Mitchison TJ. Myosin is involved in postmitotic cell spreading. J Cell Biol. 1995;131:179–189. doi: 10.1083/jcb.131.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darribere T, Koteliansky VE, Chernousov MA, Akiyama SK, Yamada KM, Thiery JP, Boucaut JC. Distinct regions of human fibronectin are essential for fibril assembly in an in vivo developing system. Dev Dyn. 1992;194:63–70. doi: 10.1002/aja.1001940108. [DOI] [PubMed] [Google Scholar]
- 18.Dzamba BJ, Bultmann H, Akiyama SK, Peters DM. Substrate-specific binding of the amino terminus of fibronectin to an integrin complex in focal adhesions. J Biol Chem. 1994;269:19646–19652. [PubMed] [Google Scholar]
- 19.Erickson HP. Reversible unfolding of fibronectin type III and immunoglobulin domains provides the structural basis for stretch and elasticity of titin and fibronectin. Proc Natl Acad Sci USA. 1994;91:10114–10118. doi: 10.1073/pnas.91.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fogerty FJ, Akiyama SK, Yamada KM, Mosher DF. Inhibition of binding of fibronectin to matrix assembly sites by anti-integrin (α5 β1) antibodies. J Cell Biol. 1990;111:699–708. doi: 10.1083/jcb.111.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraker PJ, Speck JC. Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycouril. Biochem Biophys Res Commun. 1978;80:849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- 22.Gailit J, Ruoslahti E. Regulation of the fibronectin receptor affinity by divalent cations. J Biol Chem. 1988;263:12927–12932. [PubMed] [Google Scholar]
- 23.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development (Camb) 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 24.Giancotti FG, Ruoslahti E. Elevated levels of the α5β1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990;60:849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- 25.Grinnell F. Focal adhesion sites and the removal of substratum-bound fibronectin. J Cell Biol. 1986;103:2697–2706. doi: 10.1083/jcb.103.6.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halliday NL, Tomasek JJ. Mechanical properties of the extracellular matrix influence fibronectin fibril assembly in vitro. Exp Cell Res. 1995;217:109–117. doi: 10.1006/excr.1995.1069. [DOI] [PubMed] [Google Scholar]
- 27.Heggeness MH, Ash JF, Singer SJ. Transmembrane linkage of fibronectin to intracellular actin-containing filaments in cultured human fibroblasts. Ann NY Acad Sci. 1978;312:414–417. doi: 10.1111/j.1749-6632.1978.tb16822.x. [DOI] [PubMed] [Google Scholar]
- 28.Hocking DC, Sottile J, McKeown-Longo PJ. Fibronectin's III-1 module contains a conformation-dependent binding site for the amino-terminal region of fibronectin. J Biol Chem. 1994;269:19183–19187. [PubMed] [Google Scholar]
- 29.Hocking DC, Smith RK, McKeown-Longo PJ. A novel role for the integrin-binding III-10 module in fibronectin matrix assembly. J Cell Biol. 1996;133:431–444. doi: 10.1083/jcb.133.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hotchin NA, Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hynes, R.O. 1990. Fibronectins. 1st Ed. Springer-Verlag, New York. 546 pp.
- 32.Hynes RO, Destree AT. Relationships between fibronectin (LETS protein) and actin. Cell. 1978;15:875–886. doi: 10.1016/0092-8674(78)90272-6. [DOI] [PubMed] [Google Scholar]
- 33.Ingham KC, Brew SA, Huff S, Litvinovich SV. Cryptic self-association sites in type III modules of fibronectin. J Biol Chem. 1997;272:1718–1724. doi: 10.1074/jbc.272.3.1718. [DOI] [PubMed] [Google Scholar]
- 34.Jalink K, van Corven EJ, Hengeveld T, Morii N, Narumiya S, Moolenaar WH. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994;126:801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng JH, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho- associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 36.Kinch MS, Clark GJ, Der CJ, Burridge K. Tyrosine phosphorylation regulates the adhesions of ras-transformed breast epithelia. J Cell Biol. 1995;130:461–471. doi: 10.1083/jcb.130.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laudanna C, Campbell JJ, Butcher EC. Role of rho in chemoattractant-activated leukocyte adhesion through integrins. Science. 1996;271:981–983. doi: 10.1126/science.271.5251.981. [DOI] [PubMed] [Google Scholar]
- 38.Lightner VA, Erickson HP. Binding of hexabrachion (tenascin) to the extracellular matrix and substratum and its effects on cell adhesion. J Cell Sci. 1990;95:263–277. doi: 10.1242/jcs.95.2.263. [DOI] [PubMed] [Google Scholar]
- 39.Limper AH, Quade BJ, LaChance RM, Birkenmeier TM, Rangwala TS, McDonald JA. Cell surface molecules that bind fibronectin's matrix assembly domain. J Biol Chem. 1991;266:9697–9702. [PubMed] [Google Scholar]
- 40.Mautner V, Hynes RO. Surface distribution of LETS protein in relation to the cytoskeleton of normal and transformed cells. J Cell Biol. 1977;75:743–768. doi: 10.1083/jcb.75.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDonald JA, Quade BJ, Broekelmann TJ, LaChance R, Forsman K, Hasegawa E, Akiyama S. Fibronectin's cell-adhesive domain and an amino-terminal matrix assembly domain participate in its assembly into fibroblast pericellular matrix. J Biol Chem. 1987;262:2957–2967. [PubMed] [Google Scholar]
- 42.McKeown-Longo PJ, Mosher DF. Interaction of the 70,000-mol-wt amino-terminal fragment of fibronectin with the matrix-assembly receptor of fibroblasts. J Cell Biol. 1985;100:364–374. doi: 10.1083/jcb.100.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moolenaar WH. Lysophosphatidic acid signalling. Curr Opin Cell Biol. 1995;7:203–210. doi: 10.1016/0955-0674(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 44.Moon KY, Shin KS, Song WK, Chung CH, Ha DB, Kang MS. A candidate molecule for the matrix assembly receptor to the N-terminal 29-kDa fragment of fibronectin in chick myoblasts. J Biol Chem. 1994;269:7651–7657. [PubMed] [Google Scholar]
- 45.Morii N, Teru-uchi T, Tominaga T, Kumagai N, Kozaki S, Ushikubi F, Narumiya S. A rho gene product in human blood platelets. II. Effects of the ADP-ribosylation by botulinum C3 ADP-ribosyltransferase on platelet aggregation. J Biol Chem. 1992;267:20921–20926. [PubMed] [Google Scholar]
- 46.Morla A, Ruoslahti E. A fibronectin self-assembly site involved in fibronectin matrix assembly: reconstruction in a synthetic peptide. J Cell Biol. 1992;118:421–429. doi: 10.1083/jcb.118.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morla A, Zhang Z, Ruoslahti E. Superfibronectin is a functionally distinct form of fibronectin. Nature. 1994;367:193–196. doi: 10.1038/367193a0. [DOI] [PubMed] [Google Scholar]
- 48.Mosher, D.F. 1989. Fibronectin. Academic Press, San Diego. 474 pp.
- 49.Mosher DF. Organization of the provisional fibronectin matrix: control by products of blood coagulation. Thromb Haemostasis. 1995;74:529–533. [PubMed] [Google Scholar]
- 50.Mosher DF, Vaheri A. Thrombin stimulates the production and release of a major surface-associated glycoprotein (fibronectin) in cultures of human fibroblasts. Exp Cell Res. 1978;112:323–334. doi: 10.1016/0014-4827(78)90215-x. [DOI] [PubMed] [Google Scholar]
- 51.Mosher DF, Sottile J, Wu C, McDonald JA. Assembly of extracellular matrix. Curr Opin Cell Biol. 1992;4:810–818. doi: 10.1016/0955-0674(92)90104-k. [DOI] [PubMed] [Google Scholar]
- 52.Mould AP, Akiyama SK, Humphries MJ. Regulation of α5β1-fibronectin interactions by divalent cations. Evidence for distinct classes of binding sites for Mn2+, Mg2+, and Ca2+ . J Biol Chem. 1995;270:26270–26277. doi: 10.1074/jbc.270.44.26270. [DOI] [PubMed] [Google Scholar]
- 53.Mould AP, Garratt AN, Askari JA, Akiyama SK, Humphries MJ. Identification of a novel anti-integrin monoclonal antibody that recognizes a ligand-induced binding site epitope on the β1 subunit. FEBS (Fed Eur Biochem Soc) Lett. 1995;363:118–122. doi: 10.1016/0014-5793(95)00301-o. [DOI] [PubMed] [Google Scholar]
- 54.Nagai T, Yamakawa N, Aota S, Yamada SS, Akiyama SK, Olden K, Yamada KM. Monoclonal antibody characterization of two distant sites required for function of the central cell-binding domain of fibronectin in cell adhesion, cell migration, and matrix assembly. J Cell Biol. 1991;114:1295–1305. doi: 10.1083/jcb.114.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quade BJ, McDonald JA. Fibronectin's amino-terminal matrix assembly site is located within the 29-kDa amino-terminal domain containing five type I repeats. J Biol Chem. 1988;263:19602–19609. [PubMed] [Google Scholar]
- 56.Ridley AJ. Rho - theme and variations. Curr Biol. 1996;6:1256–1264. doi: 10.1016/s0960-9822(02)70711-2. [DOI] [PubMed] [Google Scholar]
- 57.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 58.Schwartz MA, Schaller MD, Ginsberg MH. INTEGRINS: Emerging paradigms of signal transduction. Annu Rev Cell Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 59.Schwarzbauer JE. Identification of the fibronectin sequences required for assembly of a fibrillar matrix. J Cell Biol. 1991;113:1463–1473. doi: 10.1083/jcb.113.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sechler JL, Takada Y, Schwarzbauer JE. Altered rate of fibronectin matrix assembly by deletion of the first type iii repeats. J Cell Biol. 1996;134:573–583. doi: 10.1083/jcb.134.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singer II, Paradiso PR. A transmembrane relationship between fibronectin and vinculin (130 kd protein): serum modulation in normal and transformed hamster fibroblasts. Cell. 1981;24:481–492. doi: 10.1016/0092-8674(81)90339-1. [DOI] [PubMed] [Google Scholar]
- 62.Somers CE, Mosher DF. Protein kinase C modulation of fibronectin matrix assembly. J Biol Chem. 1993;268:22277–22280. [PubMed] [Google Scholar]
- 63.Sottile J, Mosher DF. N-terminal type I modules required for fibronectin binding to fibroblasts and to fibronectins III1 module. Biochem J. 1997;323:51–60. doi: 10.1042/bj3230051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tominaga T, Sugie K, Hirata M, Morii N, Fukata J, Uchida A, Imura H, Narumiya S. Inhibition of PMA-induced, LFA-1–dependent lymphocyte aggregation by ADP ribosylation of the small molecular weight GTP binding protein, rho. J Cell Biol. 1993;120:1529–1537. doi: 10.1083/jcb.120.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Volberg T, Geiger B, Citi S, Bershadsky AD. Effect of protein kinase inhibitor H-7 on the contractility, integrity, and membrane anchorage of the microfilament system. Cell Motil Cytoskeleton. 1994;29:321–338. doi: 10.1002/cm.970290405. [DOI] [PubMed] [Google Scholar]
- 66.Welch MP, Odland GF, Clark RAF. Temporal relationships of F-actin bundle formation, collagen and fibronectin matrix assembly, and fibronectin receptor expression to wound contraction. J Cell Biol. 1990;110:133–145. doi: 10.1083/jcb.110.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu CY, Keivens VM, Otoole TE, McDonald JA, Ginsberg MH. Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell. 1995;83:715–724. doi: 10.1016/0092-8674(95)90184-1. [DOI] [PubMed] [Google Scholar]
- 68.Yamamoto M, Marui N, Sakai T, Morii N, Kozaki S, Ikai K, Imamura S, Narumiya S. ADP-ribosylation of the rhoA gene product by botulinum C3 exoenzyme causes Swiss 3T3 cells to accumulate in the G1 phase of the cell cycle. Oncogene. 1993;8:1449–1455. [PubMed] [Google Scholar]
- 69.Zhang Q, Mosher DF. Cross-linking of the NH2-terminal region of fibronectin to molecules of large apparent molecular mass. Characterization of fibronectin assembly sites induced by the treatment of fibroblasts with lysophosphatidic acid. J Biol Chem. 1996;271:33284–33292. doi: 10.1074/jbc.271.52.33284. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Q, Magnusson KE, Mosher DF. Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through rho-dependent actin stress fiber formation and cell contraction. Mol Biol Cell. 1997;8:1415–1425. doi: 10.1091/mbc.8.8.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Q, Checovich WJ, Peters DM, Albrecht RM, Mosher DF. Modulation of cell surface fibronectin assembly sites by lysophosphatidic acid. J Cell Biol. 1994;127:1447–1459. doi: 10.1083/jcb.127.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhong C, Kinch MS, Burridge K. Rho-stimulated contractility contributes to the fibroblastic phenotype of Ras-transformed epithelial cells. Mol Biol Cell. 1997;8:2329–2344. doi: 10.1091/mbc.8.11.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]