Abstract
β1A integrin subunits with point mutations of the cytoplasmic domain were expressed in fibroblasts derived from β1-null stem cells. β1A in which one or both of the tyrosines of the two NPXY motifs (Y783, Y795) were changed to phenylalanines formed active α5β1 and α6β1 integrins that mediated cell adhesion and supported assembly of fibronectin. Mutation of the proline in either motif (P781, P793) to an alanine or of a threonine in the inter-motif sequence (T788) to a proline resulted in poorly expressed, inactive β1A. Y783,795F cells developed numerous fine focal contacts and exhibited motility on a surface. When compared with cells expressing wild-type β1A or β1A with the D759A activating mutation of a conserved membrane–proximal aspartate, Y783,795F cells had impaired ability to transverse filters in chemotaxis assays. Analysis of cells expressing β1A with single Tyr to Phe substitutions indicated that both Y783 and Y795 are important for directed migration. Actin-containing microfilaments of Y783,795F cells were shorter and more peripheral than microfilaments of cells expressing wild-type β1A. These results indicate that change of the phenol side chains in the NPXY motifs to phenyl groups (which cannot be phosphorylated) has major effects on the organization of focal contacts and cytoskeleton and on directed cell motility.
Integrins are transmembrane αβ heterodimers that mediate organization of focal contacts, actin-containing cytoskeleton, and extracellular matrix (1, 11, 24, 35, 56, 70). Intracellular signals cause changes in integrin extracellular domains and thereby regulate affinity for extracellular ligands (22, 30, 33, 35, 38, 59). Regulation of the interaction of integrins with extracellular ligands allows control of cell adhesion and migration. Adhesion and migration, in turn, are important determinants of cell proliferation and differentiation (21, 24, 27, 34, 35, 39). Integrins are involved in a number of pathological processes (1, 30, 35, 56, 64). Therefore, a detailed understanding is needed of the complex “inside-out” and “outside-in” signal transduction pathways underlying and provoked by the association of integrins with extracellular ligands and intracellular effector molecules and how these pathways control cellular behavior.
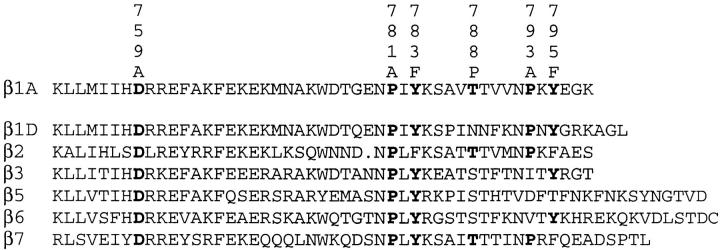
Three regions of the A splice form of β1 subunit (β1A) cytoplasmic domains have been implicated in the function of β1A integrins (see Fig. 1). The Asp of the membrane-proximal sequence KLLXXXXD likely forms a salt bridge with a conserved Arg of the α subunit, thus stabilizing a default inactive conformation (32). The other regions are comprised of two NPXY motifs (54). These motifs are found in the cytoplasmic domains of β1A and β1D, β2, β3, β5, β6, and β7 (see Fig. 1) (25, 49). In all except β5, the motifs are separated by an intervening sequence of eight residues. Substitution of a Pro for a Ser in the intervening sequence of β3 is associated with Glanzmann thrombasthenia, a bleeding disorder caused by abnormal function of platelet αIIbβ3 (16, 17). Mutagenesis of the Asn or Pro residues of the NPXY motifs results in β subunits that do not localize to focal contacts (19, 54). Such changes likely alter the tight β-turn predicted for such sequences (3, 28). When the cDNA for β1A was first sequenced, the tyrosine residues of the NPXY motifs were identified as potential sites of phosphorylation (62). Subsequent studies demonstrated tyrosine phosphorylation of β1 in transformed cells and of β3 in activated and aggregated platelets and in αvβ3-expressing K562 cells (8, 9, 31, 40, 63). Tyrosine phosphorylated β1 of src-transformed cells, detected by anti-phosphopeptide antibodies, localizes to podosomes rather than focal contacts (37). Transfected β1As with one or both Tyr mutated to Glu, Ala, or Ser localize less well than wild-type β1A to focal contacts (19, 54). Transfected βlA with conservative mutations of Tyr to Phe, which cannot be phosphorylated, localizes normally to focal contacts (19, 29, 48, 54, 65).
Figure 1.
Deduced amino acid sequence (in single letter code) of the intracellular domain of murine β1A and comparable domains of β1D, β2, β3, β5, β6, and β7. The sequences are aligned with lysine (K) at position 752 of mature β1A. The residues mutated in β1A and homologous residues in other β subunits are shown in bold. The mutations are depicted above the β1A sequence.
We have studied the effects of the Tyr to Phe substitutions in the NPXY motifs on cell behavior. Mutated β1A was expressed in mouse GD25 cells, which lack β1 integrin heterodimers because of targeted knockout of the β1 gene (23, 67). The Tyr to Phe mutations resulted in β1As that were active in GD25 cells as assessed by antibody binding and fibronectin matrix assembly assays. However, the Tyr to Phe mutations were associated with loss of ability of cells to undergo directed migration.
Materials and Methods
Mutation of β1A
Mutant β1A constructs were generated from pBSβ1A encoding full-length mouse β1A integrin subunit (67). The β1A was cloned into pGEM7Zf by the unique XbaI and Acc65I restriction sites. Mutants were generated by oligonucleotide-primed DNA synthesis using the U.S.E. mutagenesis kit (Pharmacia Biotech Sevrage, Uppsala, Sweden). Regions spanning the Pm1I and Acc65I sites were analyzed by DNA sequence analysis. Mutagenized PmlI–Acc65I fragments were isolated and ligated into PmlI–Acc65I-digested pBSβ1A to generate cDNAs encoding full-length β1A polypeptides containing the individual point mutations in the cytoplasmic tail. The plasmid was linearized with XbaI and transfected into β1-deficient GD25 cells by electroporation. After 72 h, selection with 5 μg/ml puromycin was started. Surviving clones were isolated and expanded. Clones stably expressing β1A were identified for each mutation. Clones were monitored for expression of β1A by flow cytometry. If necessary, the population of higher expressing cells was selected by fluorescence detected cell sorting.
Flow Cytometry
Cells were harvested and suspended in PBS containing 3% (wt/vol) BSA. Approximately 1.0 × 106 cells were incubated with primary antibody, and then treated with FITC-conjugated secondary antibody at 4°C. We confirmed by titration that the dilutions of antibodies used were saturating and gave maximal specific signals. Cells (8,000 per sample) were analyzed in a FACScan® flow cytometer (Becton and Dickinson Co., Mountain View, CA). Monoclonal antibodies 9EG7 to mouse β1 (4, 42), MFR5 to mouse α5, H9.2B8 to mouse αv, and 2C9.G2 to mouse β3 were all from PharMingen (San Diego, CA). Antibody MB1.2 to mouse β1 and GoH3 to α6 were provided by Dr. B. Chan (University of Western Ontario, Ontario, Canada) and Dr. A. Sonnenberg (The Netherlands Cancer Institute, Amsterdam, Netherlands), respectively. Possible upregulation of the 9EG7 epitope by Mn2+ or recombinant adhesive modules III7-10 of fibronectin (41) was assessed by flow cytometry (4).
Metabolic Labeling and Immunoprecipitation
The cells were given methionine-free DMEM, then l-[35S]methionine (100 μCi/ml medium) (Dupont NEN, Boston, MA) and incubated for various time periods. After incubation, conditioned media were collected, and cells were solubilized with nonionic detergent buffer (1% [vol/vol] Triton X-100, 0.05% [vol/vol] Tween 20, 150 mM NaCl, 2 mM PMSF, 5 μg/ml leupeptin, 0.1 μg/ml pepstatin A, 0.4 mM pefabloc SC, and 20 mM Tris-HCl, pH 7.4). Extracellular matrix proteins were immunoprecipitated with rabbit antibodies from metabolically labeled, conditioned media as previously described (58). β1A integrins were immunoprecipitated from cellular lysates with MB1.2 or 9EG7 by an established protocol (60). The amounts of antibodies used were sufficient to precipitate all of the target antigen.
Cell Adhesion Assay
Cell adhesion assays were performed as previously described (15). Vitronectin (7), fibronectin (43), or laminin-1 (Sigma Chemical Co., St. Louis, MO) were coated at 2–10 μg/ml onto wells of a 96-well plate, and the wells were blocked with 2% BSA in PBS containing Ca2+ and Mg2+. The cells were then allowed to attach to wells for 60 min. After adhesion, nonadhered cells were removed by washing, and adhered cells were quantified by colorimetric detection at 595 nm after staining with 1% bromphenol blue.
Binding Assays
Binding of iodinated or FITC-labeled human plasma fibronectin or the 70-kD NH2-terminal fragment were assayed as described (43, 72).
Immunofluorescence
Immunofluorescence studies were performed as described previously (57). Cells were seeded in serum-containing medium and grown on glass coverslips for 3 d. Alternatively, cells were seeded onto glass coverslips coated with vitronectin, fibronectin, or laminin-1 and incubated in DME containing 0.2% BSA for 4 h at 37°C. For double staining of fibronectin and β1 integrin, cells were incubated with FITC-labeled fibronectin in the presence of 1-oleoyl-lysophosphatidic acid (LPA)1 (Avanti Polar Lipids, Alabaster, AL) for an additional 1 h at 37°C. Cells were then fixed with 3.5% (wt/vol) paraformaldehyde in phosphate buffer, pH 7.4. Before staining of intracellular antigens, the cells were permeabilized with 0.2% Triton X-100 in TBS. After incubation with 3% BSA in TBS to block nonspecific protein binding, the fixed cells were incubated with rabbit antisera to FITC or fibronectin, and/or monoclonal antibody to paxillin, vinculin, or β1, and then treated with FITC anti–mouse IgG, FITC anti–rabbit IgG, lissamine rhodamine B sulfonyl chloride (LRSC)-labeled anti–rat IgG, or rhodamine-conjugated phalloidin (Molecular Probes, Inc., Eugene, OR). Monoclonal antibodies against human vinculin (clone hVIN-1) and chick paxillin were from Sigma Chemical Co. and Transduction Laboratories (Lexington, KY), respectively. β1 integrin was identified with antibody MB1.2. FITC- or LRSC-conjugated, affinity-purified secondary antibodies were from Jackson ImmunoResearch Labs. Inc. (West Grove, PA). Controls were done to insure the species specificity of the secondary antibodies. Photographs were taken with an Olympus BX-60 epifluorescence microscope using additional emission filters that allowed the FITC and LRSC or rhodamine fluorochromes to be visualized individually or simultaneously (Chroma Technology, Brattleboro, VT).
Cell Migration
Cell migration assays were performed in modified Boyden chambers containing Nucleopore polycarbonate membranes (5-μm pore size; Costar Corp., Cambridge, MA). The filters were soaked overnight in a 10 μg/ml solution of vitronectin, fibronectin, or laminin-1 or 100 μg/ml gelatin, briefly rinsed with PBS containing 0.2% BSA, air dried, and then placed in the chamber. EGF or PDGF at concentrations of 3–100 ng/ml in DME containing 0.2% BSA was added to the lower compartment of the chambers. Cells suspended in DME containing 0.2% BSA were introduced into the upper compartment of the chamber. The chambers were then incubated for 6 h at 37°C. The filters were fixed and stained, and the cells that had migrated to the lower surface were counted at 400× magnification. Two areas from each of two cells were counted. Values are the mean ± SD of cells per 0.16-mm2 field. EGF and PDGF were from Upstate Biotechnology (Lake Placid, NY) and R&D Systems, Inc. (Minneapolis, MN), respectively.
Cell Motility
The cell motility assay was adapted from an established method (50). Vitronectin, fibronectin or laminin-1, 10 μg/ml, was coated onto wells of a 96-well plate, and the wells were blocked with 2% BSA in PBS containing Ca2+ and Mg2+. Yellow polystyrene monodispersed particles (1.0-μm-diam polybeads; Polysciences, Inc., Warrington, PA) were suspended with DME, sonicated three times, and then added to wells. The plates were centrifuged at 1,400 rpm for 25 min and incubated overnight at 37°C. Cells (500 cells/well) suspended in DME containing 0.2% BSA in the absence or presence of motility factors, PDGF or EGF, 3–100 ng/ml, were introduced into wells and incubated for 16 h at 37°C. After incubation, the contents of the wells were fixed with 10% (vol/vol) glutaraldehyde, wells were washed, and clearing of beads by motile cells was assessed by phase microscopy.
Presentation of Results
Experiments were done on at least three separate occasions over an 8-mo period on clones of cells selected to give maximal expression in the case of the P781A, T788P, P793A, or P781,793A mutants or expression similar to GD25-β1A cells in the case of the D759A, Y783F, Y795F, or Y783,795F mutants. The cell migration assays and microscopic analysis of microfilament bundles and focal contacts were repeated on independently derived clones of D759A and Y783,795F cells. The experiments and photomicrographs shown were chosen as representative of differences constantly observed among cells expressing the different β1As.
Results
We characterized the effects of Tyr to Phe mutations in the NPXY motifs of β1A in mouse GD25 cells, which lack expression of β1 integrin heterodimers because of targeted knockout of the β1 gene (23, 67). We also created point mutations that would be expected, on the basis of previous studies described in the Introduction, to enhance or interfere with integrin activity (Fig. 1).
Analysis of Integrins and Cell Attachment
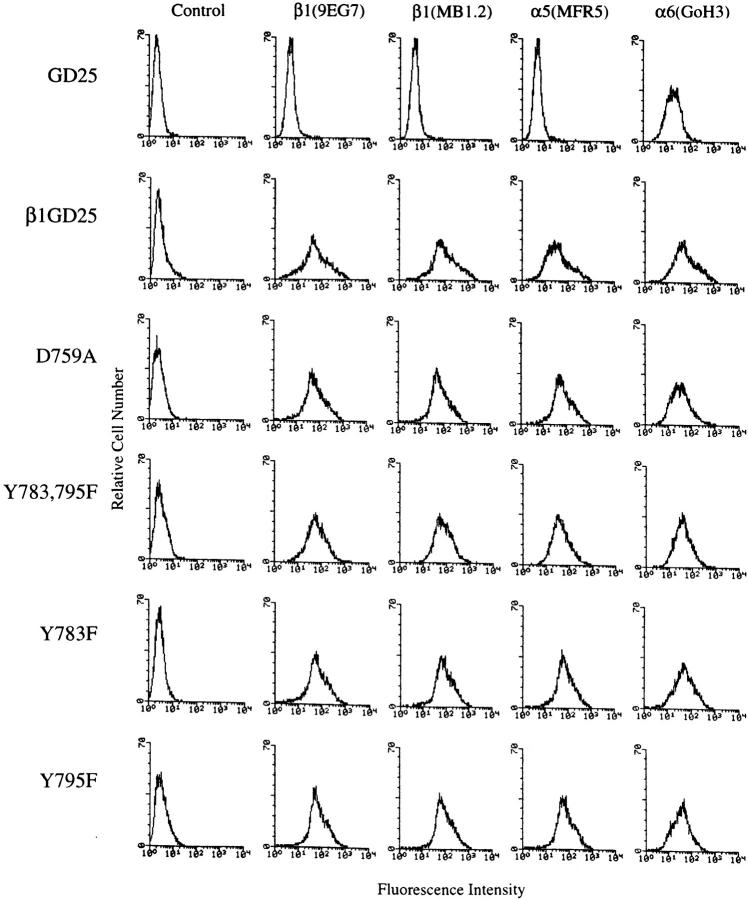
Expression of cell surface β1A and associated α5 and α6 subunits was analyzed by flow cytometry (Fig. 2). Transfection of β1A mutants resulted in appearance of cell surface α5 and upregulation of cell surface α6, as previously reported for GD25-β1A cells expressing wild-type β1A (23, 67). Binding of monoclonal antibody 9EG7, which recognizes an extracellular epitope of β1 that can be upregulated by Mn2+ or ligand, was compared with the binding of a second anti-β1 monoclonal antibody, MB1.2, as an index for conformationally active integrins. For GD25-β1A cells and cells expressing D759A, Y783F, Y795F, or double Y783,795F mutant β1A, fluorescence resulting from binding of 9EG7 was 80–100% of that due to MB1.2 binding. Attempts to upregulate the 9EG7 epitope further with Mn2+ or recombinant adhesive modules III-7-10 of fibronectin failed (not shown). For each of the four mutants, cell surface β1A was expressed at a level comparable to that of GD25-β1A cells (67) in at least 6 of the ∼50 clones tested. Metabolic labeling and immunoprecipitation analysis confirmed that the production level of the D759A or Y783,795F mutant β1A was not different from production of wild-type β1A in the GD25-β1A cells (not shown).
Figure 2.
Expression of β1A and associated α5 and α6 subunits in β1-deficient GD25, wild-type, and mutant β1A cells by flow cytometry. Control, no primary antibody; β1(9EG7), and β1(MB1.2), antibodies to β1; α5(MFR5), antibody to α5; α6(GoH3), antibody to α6; GD25, β1-deficient cells; β1GD25, GD25 cells expressing wild-type β1A; other cells are designated by mutation(s).
Binding of 9EG7 to cells expressing P781A, T788P, P793A, or double P781,793A mutant β1As, in contrast, was <50% of MB1.2 binding (not shown). In addition, we were unable to find cells expressing P781A, T788P, P793A or double P781,793A mutant β1As that bound MB1.2 at >30% of the expression level on GD25-β1A cells despite testing ∼50 (P781A and P793A) or ∼100 (T788P and P781,793A) clones. The flow cytometry results, therefore, indicate that wild-type β1A and the D759A, Y783F, Y795F, and double Y783,795F mutant β1As are all expressed in an active state by GD25 cells. In contrast, the P781A, T788P, P793A, and double P781,793A mutant β1As are expressed less well and in a less active state.
Transfection and selection of D759A or double Y783,795F mutant β1A resulted in variable downregulation of cell surface αv and β3 when compared with the parent GD25 cells (not shown). Similar results were obtained previously using surface iodination of GD25 and GD25 β1A cells (67).
Cell adhesion studies were carried out to characterize further the activity of the mutant integrins. Cells transfected with any of the set of β1As that resulted in higher ratios of 9EG7/MB1.2 binding adhered vigorously to laminin-1, whereas untransfected GD25 cells or GD25 cells transfected with the P781A, T788P, P793A, or P781,793A mutant attached and spread poorly on cell culture plastic coated with laminin-1 (Fig. 3). Adherence to laminin-1 was blocked by antibody GoH3 to α6β1 (not shown). GD25 cells or each of the transfectants attached and spread on vitronectin or fibronectin (Fig. 3), consistent with previous studies showing that adhesion of GD25 cells to fibronectin can be mediated by either the αvβ3 vitronectin receptor or the α5β1 fibronectin receptor (23, 67). These results indicate that substitution of Ala for Pro in the NPXY motifs or the thrombasthenia-like Thr-to-Pro substitution in the sequence between the two motifs results in poorly expressed integrins with little or no α6β1-mediated cell adhesive activity for laminin-1. Integrins formed by β1A carrying the D759A mutations or substitution of Phe for Tyr in the NPXY motifs, in contrast, were at least as active as those formed by wild-type β1A.
Figure 3.
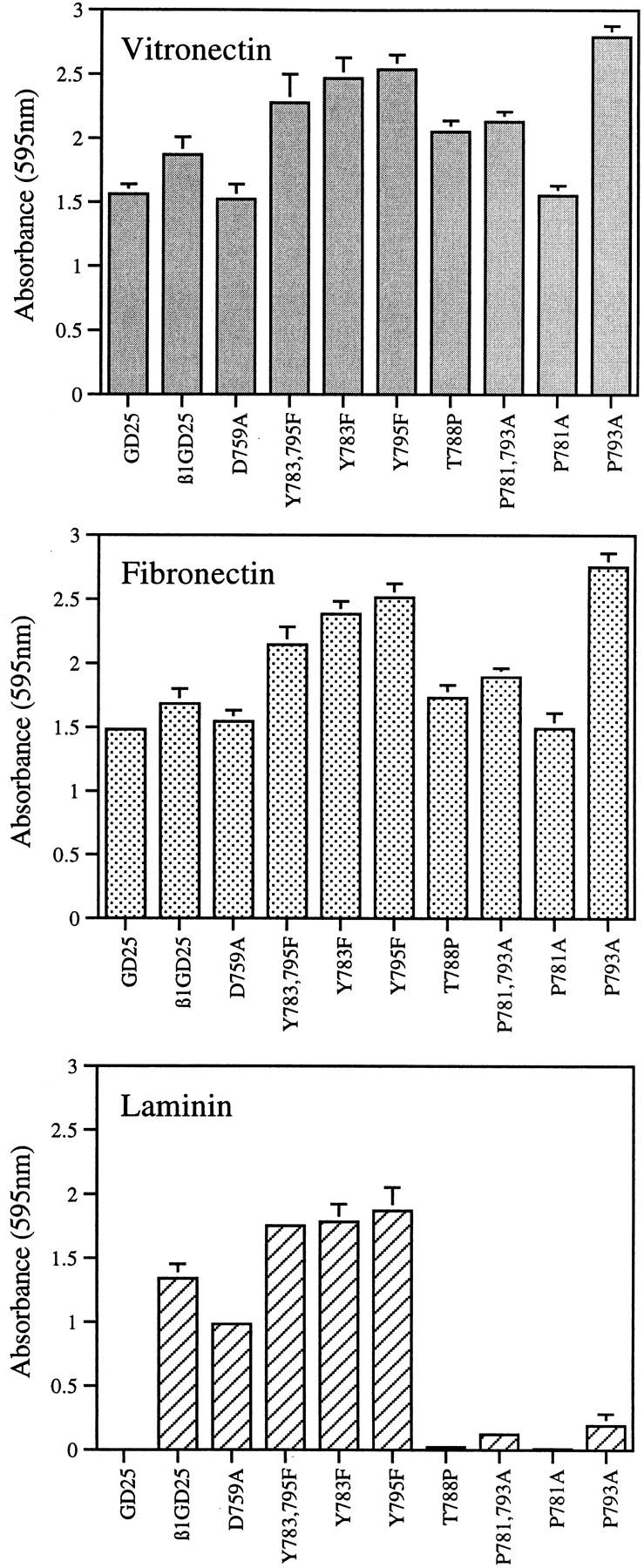
Attachment of wild-type and mutant β1A cells on vitronectin, fibronectin, and laminin-1. Bars represent the mean of attachment activity quantified by spectrophotometric analysis at OD = 595 nm after staining of adherent cells with bromphenol blue. Error bars represent ±SD of quadruplicate experiments. Absorbance resulting from nonspecific cell adhesion as measured on BSA-coated wells was ∼0.05, and has been subtracted. GD25, β1-deficient cells; β1GD25, GD25 cells expressing wild-type β1A; other cells are designated by mutation(s).
Fibronectin Matrix Assembly by Cells Expressing Mutant β1A
Fibronectin assembly is enhanced by experimental manipulations that activate β1 or β3 integrins, including activating antibodies, truncation of the α subunit cytoplasmic domain, and mutation of the membrane-proximal Asp to disrupt the interaction between α and β cytoplasmic domains (32, 69). To learn whether expression of β1As with Tyr to Phe mutations in the NPXY motifs also results in hyperactive integrins, we studied assembly of fibronectin by monolayers of transfected cells. After 3 d in 10% FBS, fibronectin was deposited in a fibrillar pattern around most cells expressing wild-type β1A or D759A or double Y783,795F mutant β1A (Fig. 4). Fibronectin deposition was more patchy in cultures of GD25 cells in that foci of cells had copious matrix whereas nearby cells had none (Fig. 4). No differences were found between GD25 cells and GD25 cells expressing wild-type β1A or among cells expressing wild-type or mutant β1As in the secretion of fibronectin, or of laminin-1 or tenascin-C, when media of cells incubated with [35S]methionine were analyzed by immunoprecipitation (not shown).
Figure 4.

Immunofluorescence of fibronectin matrix after 3 d of culture in serum-containing medium. GD25, β1-deficient cells; β1GD25, GD25 cells expressing wild-type β1A; other cells are designated by mutation(s). Bar, 60 μm.
We also analyzed fibronectin matrix deposition in short-term experiments. Fig. 5 shows fluorescence due to deposition over a 1-h period of FITC-labeled plasma fibronectin added to cells in plates coated with vitronectin or laminin-1. The vitronectin coating supported extensive network of assembled fibronectin formed around cells expressing wild-type β1A or D759A, Y783F, Y795F, or Y783,795F mutant β1A (Fig. 5). In contrast, untransfected GD25 (Fig. 5) cells or cells expressing inactive T788P or P781,793A mutant β1A (not shown) did not assemble fibronectin over this time period when cultured on vitronectin. Thus, assembly of a matrix by GD25 cells cultured short-term on vitronectin requires active β1 integrins. Fibronectin deposition around cells expressing β1A or the D759A mutation or the Phe for Tyr substitutions, whether cultured on vitronectin or laminin-1, consistently stained at least as intensely as cells expressing wild-type β1A (Fig. 5).
Figure 5.
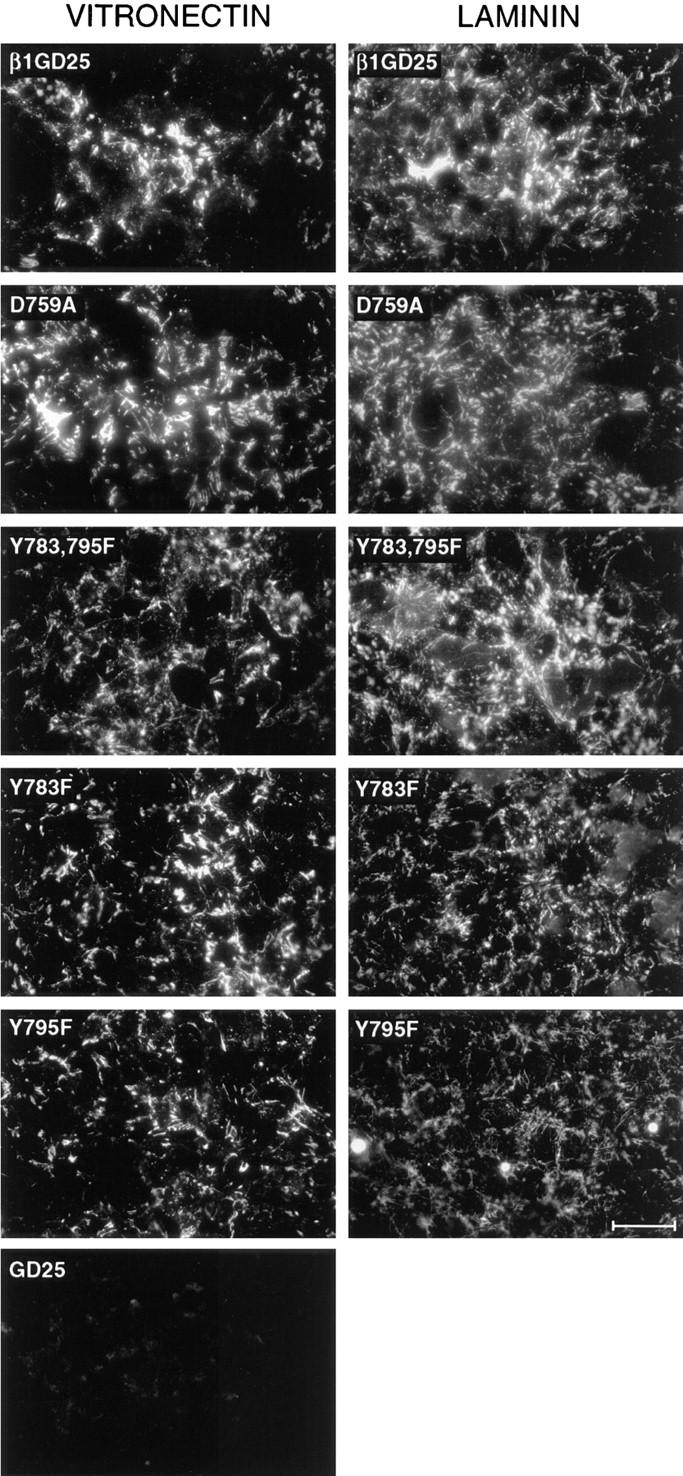
Assembly of exogenous fibronectin in short-term assay. Assembly of FITC–fibronectin during a 1-h period began 4 h after seeding of cells on surfaces coated with vitronectin or laminin-1. The cells used for analysis are indicated in each figure. Bar, 60 μm.
The qualitative fluorescence microscopic observations in Fig. 5 were buttressed by assays of binding of 125I-labeled, 70-kD NH2-terminal fragment of fibronectin (Fig. 6). This fragment is a suitable probe to quantify the ability of cells to assemble fibronectin, inasmuch as the fragment and intact fibronectin bind to cell layers with the same affinities, cross-compete for binding, and can be cross-linked to the same large apparent molecular mass target in cell layers (46, 71). Cells expressing D759A or Y783,795F mutant β1A bound 1.5- to 2.5-fold more 70-kD fragment than cells expressing wild-type β1A. Binding to cells expressing β1A with the single Tyr mutation was intermediate between binding to cells expressing β1A with the double Tyr mutations and cells expressing wild-type β1A. Increased binding was found regardless of whether cells were cultured on vitronectin, fibronectin, or laminin-1 and was enhanced by LPA, which causes a coordinated change in cell shape, and upregulation of binding of fibronectin or the 70-kD fragment (45, 72).
Figure 6.
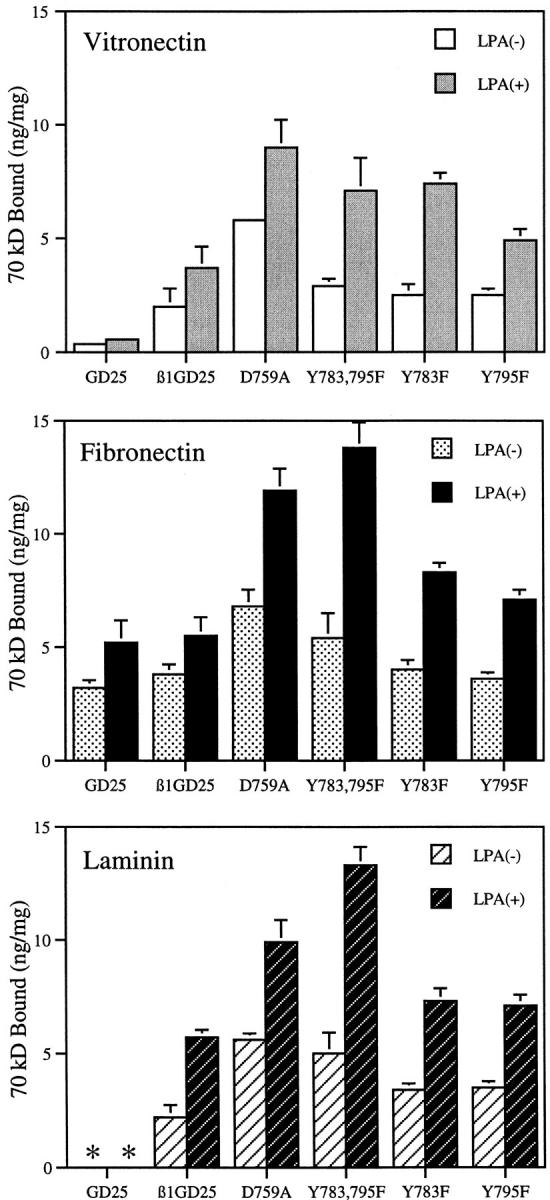
Binding of the 70-kD NH2-terminal fragment of fibronectin to cells seeded on surfaces coated with vitronectin, fibronectin, or laminin-1. Symbols represent the mean of specific binding. Bars represent mean ± SD of triplicate experiments with duplicate determinations in each experiment (n = 6). GD25, β1-deficient cells; β1GD25, GD25 cells expressing wild-type β1A; other cells are designated by mutation(s). *, β1-deficient GD25 cells could not be studied on laminin-1 coating because too few cells adhered (see Fig. 3).
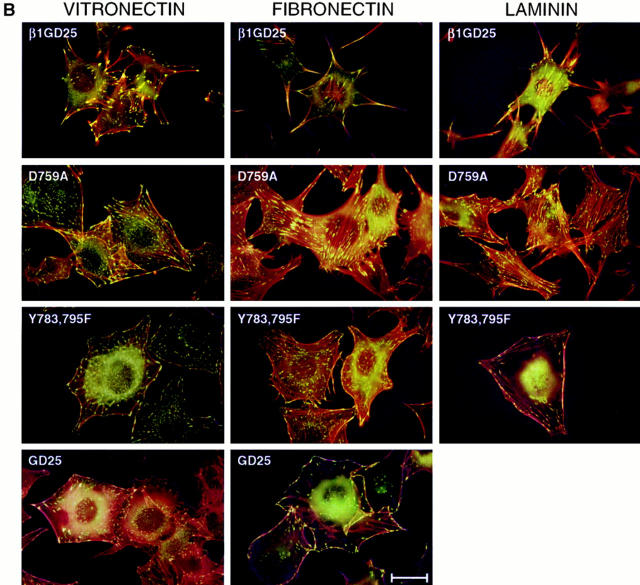
Cellular Localization of β1A in Relationship to Assembled Fibronectin
Activated integrins are required in the process of fibronectin matrix assembly (69). We therefore analyzed cellular localization of wild-type β1A or D759A or Y783,795F mutant β1A in relationship to assembled fibronectin by fluorescence microscopy of cells cultured on vitronectin, fibronectin, or laminin-1 and incubated with FITC–fibronectin before fixation and staining for β1 (Fig. 7). These three cell types in general behaved in the same manner on a given substrate. However, they showed different subcellular localization of β1A that depended upon the different substratum. In cells adherent to vitronectin, β1A did not localize to focal contacts but rather to assembled fibronectin. In cells adherent to fibronectin, some β1A localized to focal contacts of cells, and some was associated with assembled fibronectin, especially around cells expressing wild-type β1A and, to a lesser extent, around cells expressing D759A or Y783,795F mutant β1A. β1A localized to focal contacts of all three cell types when cultured on laminin-1. These focal contacts were linear and often associated with FITC–fibronectin.
Figure 7.
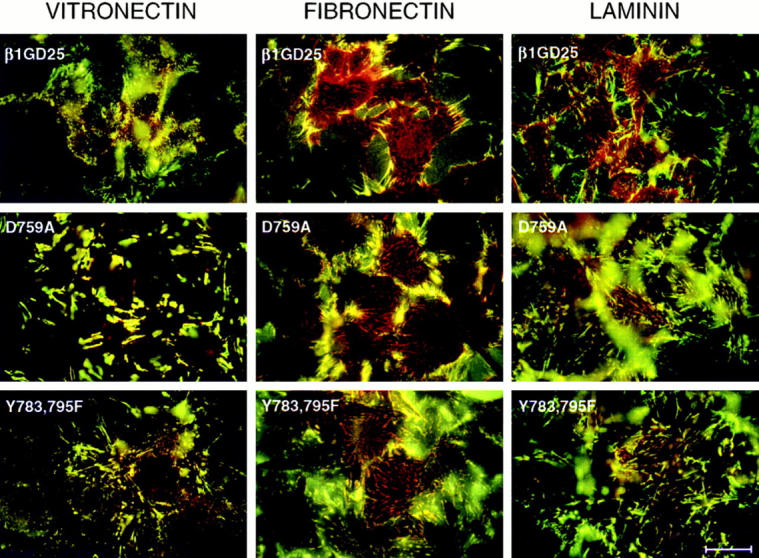
Double fluorescence of β1 integrin (red) and FITC-labeled fibronectin (green). Cells were incubated for 4 h on a fibronectin-, laminin-, or vitronectin-coated substratum and additional 1 h with FITC–fibronectin. After fixation with paraformaldehyde, staining for β1 integrin was performed using β1 antibody MB1.2 and LRSC-labeled anti–rat IgG. Samples were viewed with an emission filter that allowed visualization of both fluorochromes and photographed focusing on the interface of cells and substrate. GD25, β1-deficient cells; β1GD25, GD25 cells expressing wild-type β1A; other cells are designated by mutation(s). Bar, 25 μm.
Cell Motility and Migration of Cells Expressing Wild-Type β1A or Mutant β1As
Motility of GD25 cells and the various transfectants was assessed by an assay in which moving cells displace polystyrene beads layered onto surfaces coated with vitronectin, fibronectin, or laminin-1 (Fig. 8). GD25 cells cultured in the presence of PDGF (Fig. 8) or EGF (not shown) demonstrated little motility. GD25 cells expressing wild-type β1A or various mutants of β1A were not motile in the absence of PDGF or EGF (not shown). Addition of PDGF (Fig. 8) or EGF (not shown) caused greater motility of β1A-expressing cells, that is, a greater area around cells was cleared of beads. The areas cleared by cells expressing wild-type β1A or the D759A mutant tended to be asymmetrical and irregular whereas areas cleared by cells expressing the Phe for Tyr substitutions, especially Y795F, were rounder. These patterns were found regardless of which adhesive protein (vitronectin, fibronectin, or laminin-1) was present on the surface and which motility agent (EGF or PDGF) was present in the medium.
Figure 8.
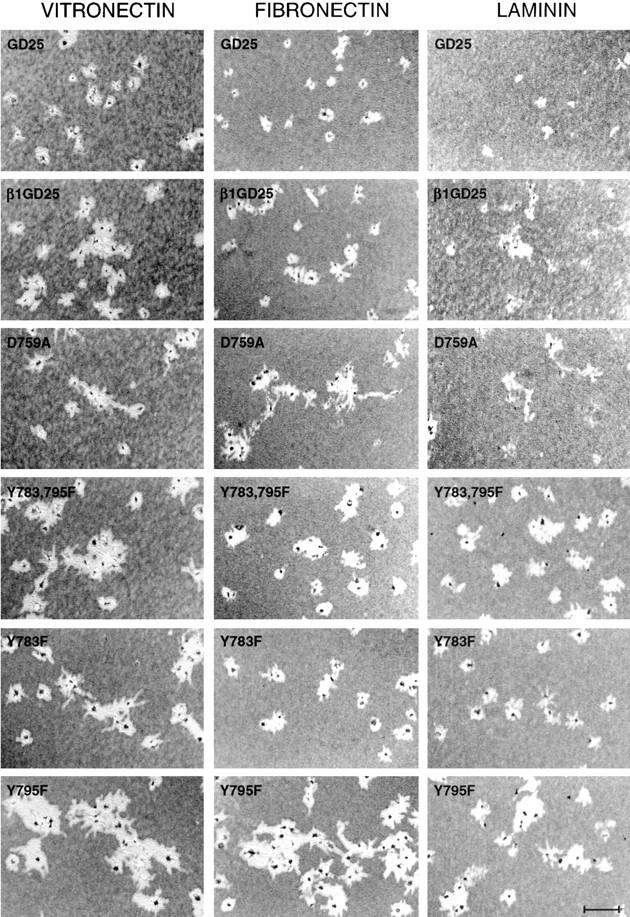
Cell motility as assessed by clearing of beads layered on vitronectin-, fibronectin-, or laminin-1–coated surfaces. Cells were added in the presence of PDGF, 10 ng/ ml. Wells were fixed 16 hours after seeding and photographed by phase microscopy. Cells, which were covered with beads, appear as dark specks in cleared-out areas. GD25, β1-deficient cells; β1GD25, GD25 cells expressing wild-type β1A; other cells are designated by mutation(s). Bar, 240 μm.
Transwell migration assays were performed in blind-well chambers fitted with filters coated with vitronectin, fibronectin, or laminin-1. In the absence of EGF or PDGF, 4- to 10-fold more cells moved across filters coated with fibronectin (Fig. 9) than filters coated with gelatin (<10 cells/0.16 mm2; not shown). This increase is presumed to represent a haptotactic response. The haptotactic responses to vitronectin were both less in magnitude and less variable than the haptotactic response to fibronectin, whereas the haptotactic responses to laminin-1 were equally variable (Fig. 9). GD25 and Y783,795F cells did not demonstrate haptotaxis through laminin-1–coated filters.
Figure 9.

Cell migration through vitronectin-, fibronectin-, or laminin-1–coated filters in response to EGF or PDGF. EGF (100 ng/ml) or PDGF (10 ng/ml), was in the lower chamber. Each bar represents the mean of cell number per 0.16-mm2 field. Error bars indicate ± SD of quadruplicate determinations. GD25, β1-deficient cells; β1GD25, GD25 cells expressing wild-type β1A; other cells are designated by mutation(s).
In response to EGF or PDGF, GD25 cells expressing wild-type β1A or the D759A mutant migrated two- to fourfold more than GD25 cells lacking β1A (Fig. 9). These results indicate that migration is enhanced by β1A integrins interacting with the adhesive substrates coating the filter, probably αvβ1A with vitronectin, α6β1A with laminin-1, and α5β1A with fibronectin. In response to EGF, D759A cells and cells expressing wild-type β1A migrated equivalently. In response to PDGF, D759A cells migrated better than cells expressing wild-type β1A. Y783,785F cells, in contrast, migrated much less than cells expressing wild-type β1A and no more than cells lacking β1A. Y783F and Y795F cells had migratory behavior that was intermediate between Y783,795F cells and cells expressing wild-type β1A. In all experiments, however, migration of cells expressing the Y783F mutation was less than migration of cells expressing the Y795F mutation. Differences in migration ability among cells expressing various mutant β1As were found over a 30-fold range of concentrations (3–100 ng/ml) of both of the chemotactic agents (not shown).
In the bead clearing assay, Y795F cells were most active, and the Y783,795F cells were as active as cells expressing wild-type β1A (Fig. 8). The discrepancy in behavior between the bead clearing assay and the chemotaxis assay shown in Fig. 9 was investigated by checkerboard analysis in which different concentrations of chemotactic agent are added to the upper and lower chamber of the apparatus (Table I). Such an analysis differentiates directed migration across the filter in response to a gradient of chemotactic agent (chemotaxis) from increased random motility because of the presence of the chemotactic agent per se (chemokinesis). Cells expressing wild-type β1A and the D759A mutant responded to a gradient of PDGF by chemotaxis but demonstrated only a low and variable chemokinetic response to increasing concentrations of PDGF in both chambers. The chemokinetic responses of wild-type and D759A cells were more than twofold less than the chemotactic responses. Migration of Y795F cells, in contrast, was greater when PDGF was present in both chambers than when there was a gradient of PDGF, indicating that the chemokinetic response was the main effect. Migration of Y783,795F and Y783F cells was low in magnitude and variable between fibronectin- and vitronectin-coated filters, making it difficult to classify the migration as chemotaxis or chemokinesis.
Table I.
Checkerboard Analysis of PDGF-induced Cell Migration of β1-deficient GD25, and GD25 Wild-Type or Mutant β1A
| Vitronectin | Fibronectin | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration of PDGF | Upper compartment | Concentration of PDGF | Upper compartment | |||||||||||
| 0 | 10 | 30 | 0 | 10 | 30 | |||||||||
| GD25 | GD25 | |||||||||||||
| Lower compartment | ng/ml | Lower compartment | ng/ml | |||||||||||
| 0 | 34 ± 4 | 37 ± 4 | 35 ± 2 | 0 | 27 ± 2 | 31 ± 2 | 34 ± 4 | |||||||
| 10 | 87 ± 10 | 88 ± 8 | 90 ± 5 | 10 | 90 ± 2 | 60 ± 3 | 56 ± 3 | |||||||
| 30 | 105 ± 6 | 104 ± 5 | 114 ± 3 | 30 | 72 ± 6 | 67 ± 4 | 75 ± 5 | |||||||
| β1GD25 | β1GD25 | |||||||||||||
| Lower compartment | Lower compartment | |||||||||||||
| 0 | 37 ± 6 | 48 ± 3 | 55 ± 7 | 0 | 84 ± 4 | 50 ± 6 | 32 ± 2 | |||||||
| 10 | 155 ± 12 | 76 ± 12 | 56 ± 3 | 10 | 129 ± 7 | 57 ± 3 | 36 ± 6 | |||||||
| 30 | 198 ± 10 | 91 ± 9 | 63 ± 6 | 30 | 105 ± 3 | 78 ± 4 | 58 ± 5 | |||||||
| D759A | D759A | |||||||||||||
| Lower compartment | Lower compartment | |||||||||||||
| 0 | 36 ± 2 | 43 ± 3 | 52 ± 3 | 0 | 73 ± 4 | 53 ± 3 | 37 ± 5 | |||||||
| 10 | 206 ± 4 | 127 ± 13 | 96 ± 5 | 10 | 172 ± 17 | 109 ± 12 | 83 ± 8 | |||||||
| 30 | 221 ± 13 | 144 ± 7 | 104 ± 4 | 30 | 242 ± 14 | 165 ± 8 | 103 ± 6 | |||||||
| Y783, 795F | Y783, 795F | |||||||||||||
| Lower compartment | Lower compartment | |||||||||||||
| 0 | 25 ± 2 | 28 ± 4 | 18 ± 2 | 0 | 31 ± 2 | 26 ± 2 | 31 ± 2 | |||||||
| 10 | 51 ± 2 | 32 ± 2 | 25 ± 2 | 10 | 59 ± 3 | 48 ± 2 | 46 ± 5 | |||||||
| 30 | 44 ± 3 | 34 ± 4 | 37 ± 3 | 30 | 56 ± 3 | 50 ± 3 | 57 ± 3 | |||||||
| Y783F | Y783F | |||||||||||||
| Lower compartment | Lower compartment | |||||||||||||
| 0 | 25 ± 2 | 29 ± 3 | 31 ± 5 | 0 | 44 ± 3 | 41 ± 2 | 38 ± 4 | |||||||
| 10 | 87 ± 6 | 43 ± 4 | 35 ± 3 | 10 | 70 ± 2 | 60 ± 3 | 52 ± 5 | |||||||
| 30 | 61 ± 4 | 42 ± 5 | 32 ± 3 | 30 | 62 ± 6 | 61 ± 3 | 69 ± 3 | |||||||
| Y795F | Y795F | |||||||||||||
| Lower compartment | Lower compartment | |||||||||||||
| 0 | 30 ± 3 | 54 ± 4 | 62 ± 4 | 0 | 58 ± 8 | 84 ± 8 | 92 ± 4 | |||||||
| 10 | 117 ± 8 | 123 ± 5 | 114 ± 4 | 10 | 102 ± 3 | 122 ± 7 | 130 ± 9 | |||||||
| 30 | 120 ± 3 | 125 ± 8 | 129 ± 16 | 30 | 112 ± 7 | 125 ± 5 | 144 ± 12 | |||||||
Cell migration assays through fibronectin- or vitronectin-coated filters were performed as described in Materials and Methods except that variable concentrations of PDGF were added to the upper as well as the lower chamber. Results are expressed as the mean ± SD (n = 4) of cells that had migrated to the lower surface of the filter. GD25, β1-deficient cells; β1GD25, GD25 cells expressing wild-type β1A; other cells are designated by mutation(s).
Focal Contact Formation and Actin Cytoskeleton Organization in Cells Expressing Wild-Type β1A or Mutant β1As
Cells expressing wild-type β1A and the D759A and Y783,795F mutants were analyzed for focal contacts with antipaxillin (Fig. 10 A) and antivinculin (Fig. 10 B) and for F-actin–containing cytoskeleton with rhodamine phalloidin (Fig. 10 B). As assessed by paxillin or vinculin staining, focal contacts formed by D759A cells on fibronectin were larger, coarser, and more numerous than focal contacts formed by cells expressing wild-type β1A. More numerous focal contacts were also formed by Y783,795F cells on fibronectin. Compared with the focal contacts formed by D759A cells, focal contacts of Y783,795F cells were finer and more uniform in size. The differences in the patterns of paxillin and vinculin staining among the three cell types on fibronectin were similar to the difference in staining patterns of β1A (Fig. 7; more obvious in studies not shown in which only single fluorescence for β1 was done). The difference between vinculin-containing focal contacts of D759A and Y783,795F cells was also found when the cells were cultured on vitronectin-coated surfaces (Fig. 10 B) even though β1A could not be demonstrated in focal contacts (see Fig. 7). On the vitronectin-coated substratum, vinculin-staining focal contacts of cells expressing wild-type β1A were coarser and more heterogenous than wild-type cells on the other substrates. On a laminin-1–coated substratum, more vinculin-staining focal contacts were present in cells expressing wild-type β1A or the D759A mutant than in Y783,795F cells.
Figure 10.
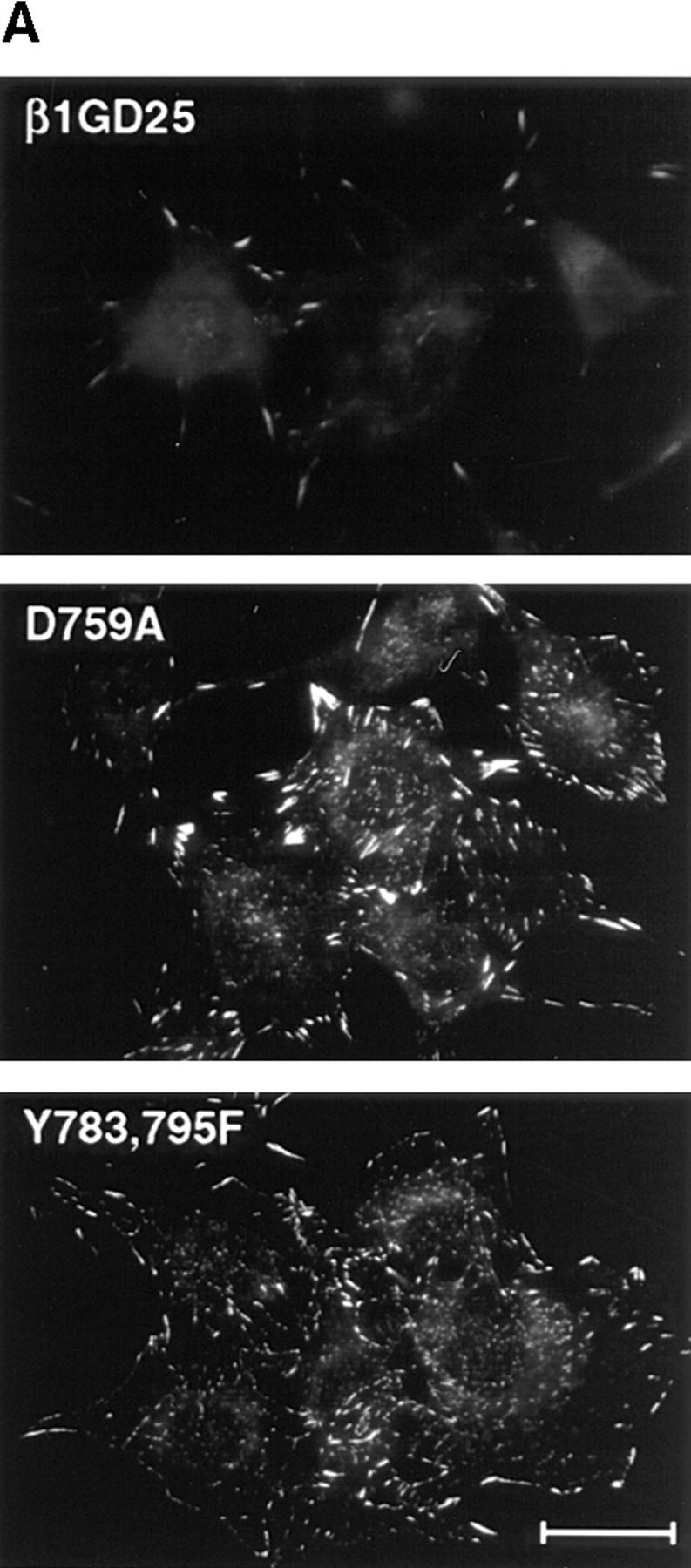
Visualization of focal contacts and associated structures in β1-deficient GD25, wild-type or mutant β1A cells. (A) Immunofluorescent detection of paxillin in focal contacts of cells expressing wild-type β1A, or D759A, or Y783,795F mutations. Cells were cultured for 4 h on a fibronectin-coated substratum. (B) Double fluorescence of vinculin (green) and rhodamine–phallondin (red). Cells were incubated for 4 h on a fibronectin-, laminin-1–, or vitronectin-coated substratum. GD25, β1-deficient cells; β1GD25, GD25 cells expressing wild-type β1A; other cells are designated by mutation(s). Bar, 25 μm.
Cells expressing wild-type β1A or the D759A mutant, when stained with rhodamine phalloidin, contained thick cables that ran through most of the length of the cells (Fig. 10 B). These cables terminated in the coarse vinculin-staining focal contacts. The F-actin network of Y783,795F cells was more peripheral than in cells expressing wild-type β1A or the D759A mutant and terminated in the fine vinculin-staining focal contacts. The differences in F-actin distribution were noted on all three substrata. For each of the transfected cell types, however, the F-actin network was less when cells were on vitronectin. Each of the transfected cell types had a better developed F-actin network than nontransfected GD25 cells on vitronectin or fibronectin (Fig. 10 B).
Discussion
We studied eight cytoplasmic mutant of β1A—four active (D759A, Y783F, Y795F, and Y783,795F) and four inactive (P781A, T788P, P793A, and P781,793A) as judged by reactivity with the 9EG7 anti-β1 antibody, adhesion to laminin-1, and ability to support fibronectin assembly. (Recently, it was reported that human foreskin fibroblasts do not assemble fibronectin when cultured on vitronectin [18]. In our hands, human foreskin fibroblasts do assemble fibronectin when cultured on vitronectin, and the inability to assemble fibronectin when cultured on vitronectin is a specific property of cells deficient in active β1 integrin [Zhang, Q., T. Sakai, R. Fässler, J. Nowlen, and D.F. Mosher, manuscript in preparation]). The active β1As were associated with distinctive cellular phenotypes. D759A cells had thick F-actin–containing microfilaments that terminated in coarse vinculin-containing focal contacts. Y783,795F cells had thinner and more peripheral F-actin–containing microfilaments that terminated in fine focal contacts. The cytoskeletal phenotypes were found when cells were on vitronectin-coated substrates, in which β1A was associated with assembling fibronectin, as well as on fibronectin- or laminin-1–coated substrates, in which β1A was associated both with focal contacts and assembling fibronectin. Motility phenotypes were associated with the cytoskeletal phenotypes. D759A cells were as active as cells expressing wild-type β1A in chemotaxis assays whereas Y783,795F cells were no more active than cells lacking β1A and Y795F demonstrated a strong chemokinetic response.
Cell migration is a complex process that involves lamellipodial extension, generation of intracellular force, integrin clustering in focal contacts, integrin avidity, and integrin signaling (34, 39, 51, 61). Focal contacts are enriched in a large number of kinases, at least one phosphatase, and various adapter and connector proteins (11, 22, 27, 38, 44, 70). Phe lacks the hydroxyl group that is the acceptor site for tyrosine phosphorylation. The results suggest, therefore, that conversion of tyrosines between nonphosphorylated and phosphorylated states is critical for movement on adhesive substrates (Fig. 11). Phosphorylated integrins are hypothesized to initiate a pathway leading to changes in F-actin–containing cytoskeleton and generation of the polarity required for directional movement. NPXY motifs are also hypothesized to regulate cellular localization of β1A integrins. Cells lacking focal adhesion tyrosine kinase (FAK) exhibit reduced motility and increased numbers of focal contacts whereas overexpression of FAK causes increased motility (14, 36). Enhancement of tyrosine phosphorylation causes loss of focal contacts (20, 47). Phosphorylated β1 integrins of src-transformed fibroblasts do not localize to focal contacts (37). Solubilized phosphorylated integrins from src-transformed fibroblasts, in contrast to integrins from normal fibroblasts, fail to bind to an extracellular ligand, fibronectin, or cytoplasmic protein, talin (63). These observations all suggest that upon phosphorylation of the NPXY motifs by tyrosine kinase(s), the integrin loses its affinity for both extracellular ligand and cytoplasmic components of the focal contacts and exits the focal contact. Dephosphorylation of the motifs by unknown tyrosine phosphatase(s) would allow the integrin to participate in a new round of ligation and focal contact formation. The two hypotheses shown in Fig. 11 could be linked in the sense that the cytoskeleton may polarize the cycling of integrins.
Figure 11.
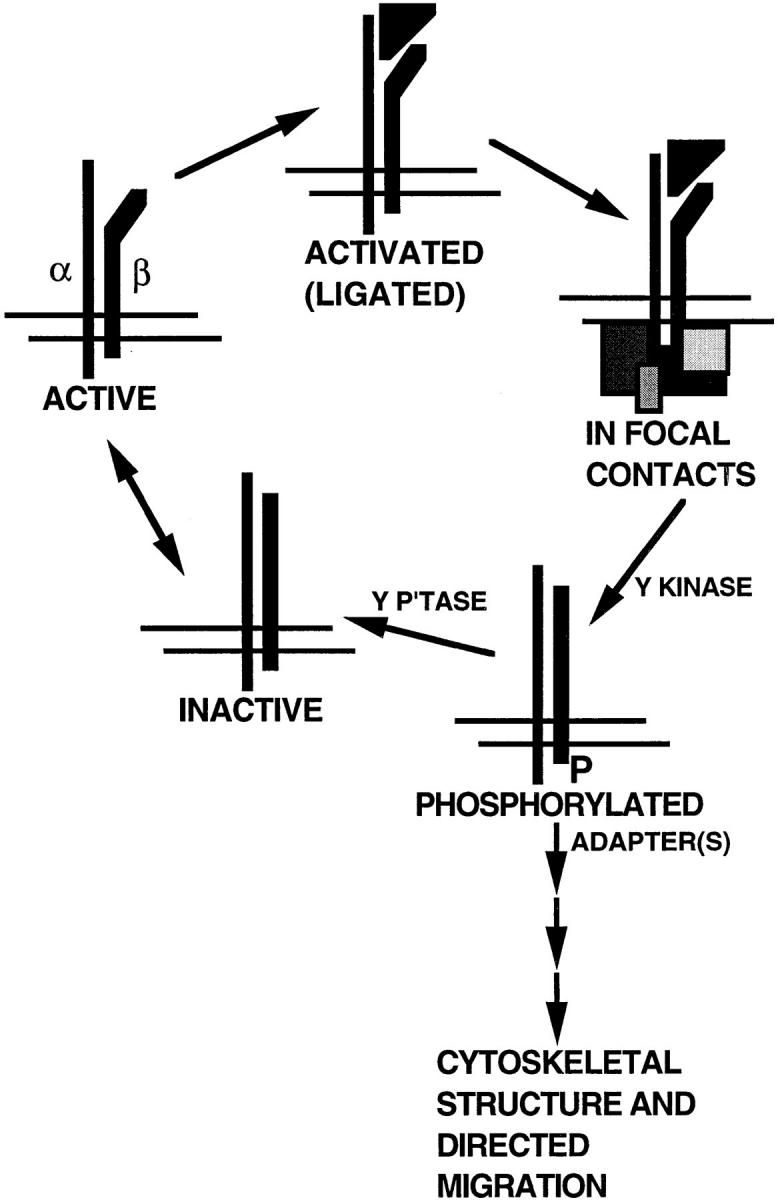
Models whereby directed cell migration may be accomplished by phosphorylation/dephosphorylation of tyrosines in the cytoplasmic domain of β1A. The integrin heterodimer is depicted as being in an equilibrium between inactive and active forms. Upon binding of extracellular ligand (triangle), the integrin complexes with cytoplasmic components of focal contacts (various boxes). Phosphorylation (P) of β1A by a tyrosine kinase (Y Kinase) causes the integrin to dissociate from both extracellular ligand and focal contact components. Dephosphorylation by a tyrosine phosphatase (Y P'tase) regenerates the integrin in an inactive form. Alternatively or additionally, binding of adapter molecule(s) to phosphorylated NPXY motifs may initiate a pathway leading to cytoskeletal rearrangement, polarization, and directional integrin cycling.
For phosphorylated integrins to initiate a pathway leading to changes in F-actin–containing cytoskeleton, one or more adaptor molecules specific for the phosphorylated NPXY motifs would be required. Tyrosine-phosphorylated intracellular domains interact with Grb2 and Shc in the case of β3 (40) and with the P85 of phosphoinositide-3 kinase (PI-3) kinase in the case of β1A (37). However, the double Phe for Tyr substitution did not impair the ability of human β1A transfected into mouse 3T3 cells to signal via Shc when cross-linked by mouse anti–human β1 antibody (66). D759A and Y783,795F cells each had increased numbers of focal contacts and upregulation of fibronectin assembly. Increased focal contacts also correlate with increased assembly when cells are treated with LPA (2, 45, 55, 72, 73). The patterns of focal contacts in D759A and Y783,795F cells, however, were strikingly different. The different patterns and the divergent behavior of the two cells in the migration assay suggest that the mutations influence focal contacts by impinging at different points in the pathway shown in Fig. 11. The D759A mutation likely increases the avidity of β1A integrins for extracellular ligands and the probability that integrins will bind ligand and enter focal contacts (32). Increased avidity of integrins for ligands does not slow migration but rather lowers the density of ligands required for maximum migration (12, 34, 39, 51). The Y783,795F mutations, as above, are hypothesized to cause persistence of ligated β1A integrins in focal contacts.
β3 in which the tyrosine homologous to Y783 in β1A was mutated to phenylalanine failed to induce adhesion and clot retraction by cells in which β3 integrins are constitutively inactive (9). Our tyrosine mutants of β1A were constitutively active in GD25 cells. We do not know whether expression of the mutant β1As in cells in which the integrins are constitutively inactive would reveal additional functions for the phosphorylation. Indeed, the argument that phosphorylation rather than the simple absence of the hydroxyl group explains the observed phenotype of transfected GD25 cells is circumstantial. The argument is based on the fact that β1A can, in principle, be phosphorylated as evidenced by studies of transformed cells and presumes that only a small, undetectable fraction of β1A is phosphorylated at any one time in untransformed cells.
Several of the substitutions generated in the present investigation are found naturally in other β subunits (Fig. 1). In β1D, the conserved Thr in the sequence between the NPXY motifs is an Asn, and a Pro is present in the sequence. β1D is a muscle-specific integrin found in junctions where there is high tensile strength (5). β1D-transfected GD25 display retarded spreading, reduced cell migration, enhanced contractility, and robust ability to assemble fibronectin matrix when compared with β1A-expressing counterparts (6). Transfected β1D is targeted to focal contacts and is also more strongly associated with detergent-insoluble cytoskeleton than β1A. These results suggest that alternative splicing of β1 is a means to strengthen the cytoskeleton–matrix link where an extremely stable association is required for contraction (6). In β2, Tyr of both NPXY motifs are replaced by Phe. β2 integrins interact with counter receptors on other cells, thus localizing lymphocytes and leukocytes in inflammatory and immunological reactions and facilitating interactions among these cells (13, 26, 30, 35, 56). These and other (10, 24, 30, 35, 52, 53, 64, 68) natural variations in NPXY sequences in β subunits may determine the kinetics with which integrins enter and exit focal contacts and the relative abilities of integrins to mediate cell migration, stable adhesions, endocytosis, and assembly of extracellular matrix.
Acknowledgments
We gratefully acknowledge J. Nowlen, K. Schell, and C. Dizack for valuable technical help, W. Busse for his interest and encouragement, S. Miyamoto for suggestions regarding cDNA constructs, and M. Salmon for preparation of the manuscript.
This work was supported by National Institutes of Health grants HL21644 and HL54462, fellowship funds from the Cell Science Research Foundation and Marion Merrill Dow (to T. Sakai), and a grant from the Swedish Medical Research Foundation.
Abbreviations used in this paper
- FAK
focal adhesion kinase
- LPA
lysophosphatidic acid
- LRSC
lissamine rhodamine B sulfonyl chloride
Footnotes
Address all correspondence to Deane F. Mosher, Departments of Medicine and Biomolecular Chemistry, University of Wisconsin-Madison, 1300 University Avenue, Madison, WI 53706. Tel.: (608) 262-1576. Fax: (608) 263-4969.
References
- 1.Albelda SM, Buck CA. Integrins and other cell adhesion molecules. FASEB (Fed Am Soc Exp Biol) J. 1990;4:2868–2880. [PubMed] [Google Scholar]
- 2.Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 3.Bansal A, Gierasch LM. The NPXY internalization signal of the LDL receptor adopts a reverse turn conformation. Cell. 1991;67:1195–1201. doi: 10.1016/0092-8674(91)90295-a. [DOI] [PubMed] [Google Scholar]
- 4.Bazzoni G, Shib DT, Buck CA, Hemler ME. Monoclonal antibody 9EG7 defines a novel β1integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem. 1995;270:25570–25577. doi: 10.1074/jbc.270.43.25570. [DOI] [PubMed] [Google Scholar]
- 5.Belkin AM, Zhidkova NI, Balzac F, Altruda F, Tomatis D, Maier A, Tarone G, Koteliansky VE, Burridge K. β1D integrin displaces the β1A isoform in striated muscles: localization at junctional structures and signaling potential in nonmuscle cells. J Cell Biol. 1996;132:211–226. doi: 10.1083/jcb.132.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belkin AM, Retta SF, Pletjushkina OY, Balzac F, Silengo L, Fassler R, Koteliansky VE, Burridge K, Tarone G. Muscle β1D integrin reinforces the cytoskeleton–matrix link: Modulation of integrin adhesive function by alternative splicing. J Cell Biol. 1997;139:1583–1595. doi: 10.1083/jcb.139.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bittorf SV, Williams EC, Mosher DF. Alteration of vitronectin: Characterization of changes induced by treatment with urea. J Biol Chem. 1993;268:24838–24846. [PubMed] [Google Scholar]
- 8.Blystone SD, Lindberg FP, Williams MP, McHugh KP, Brown EJ. Inducible tyrosine phosphorylation of the β3 integrin requires the αvintegrin cytoplasmic tail. J Biol Chem. 1996;271:31458–31462. doi: 10.1074/jbc.271.49.31458. [DOI] [PubMed] [Google Scholar]
- 9.Blystone SD, Williams MP, Slater SE, Brown EJ. Requirement of integrin α3 tyrosine 747 for β3 tyrosine phosphorylation and regulation αvβ3avidity. J Biol Chem. 1997;272:28757–28761. doi: 10.1074/jbc.272.45.28757. [DOI] [PubMed] [Google Scholar]
- 10.Boylan AM, Sanan DA, Sheppard D, Broaddus VC. Vitronectin enhances internalization of crocidolite asbestos by rabbit pleural mesothelial cells via the integrin alpha v beta 5. J Clin Invest. 1995;96:1987–2001. doi: 10.1172/JCI118246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Mol Biol Cell. 1996;8:1415–1425. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 12.Burridge K, Fath K, Kelly T, Nuckolls G, Turner CE. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- 13.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 14.Cary LA, Chang JF, Guan JL. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J Cell Sci. 1996;109:1787–1794. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Sottile J, O'Rourke KM, Dixit VM, Mosher DF. Properties of recombinant mouse thrombospondin 2 expressed in Spodopteracells. J Biol Chem. 1994;269:32226–32232. [PubMed] [Google Scholar]
- 16.Chen YP, Djaffar I, Pidard D, Steiner B, Cieutat AM, Caen JP, Rosa JP. Ser 752 → Pro mutation in the cytoplasmic domain of integrin β3 subunit and defective activation of platelet integrin αIIbβ3(glycoprotein IIb IIIa) in a variant of Glanzmann thrombasthenia. Proc Natl Acad Sci USA. 1992;89:10169–10173. doi: 10.1073/pnas.89.21.10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YP, O'Toole TE, Ylänne J, Rosa JP, Ginsberg MH. A point mutation in the integrin β3 cytoplasmic domain (S752 → P) impairs bidirectional signaling through αIIbβ3(platelet glycoprotein IIbIIIa) Blood. 1994;84:1857–1865. [PubMed] [Google Scholar]
- 18.Christopher RA, Kowalczyk AP, McKeown-Longo PJ. Localization of fibronectin matrix assembly sites on fibroblasts and endothelial cells. J Cell Sci. 1997;110:569–581. doi: 10.1242/jcs.110.5.569. [DOI] [PubMed] [Google Scholar]
- 19.Cone RI, Weinacker A, Chen A, Sheppard D. Effects of β subunit cytoplasmic domain deletions on the recruitment of the integrin αvβ6to focal contacts. Cell Adhesion Commun. 1994;2:101–113. doi: 10.3109/15419069409004430. [DOI] [PubMed] [Google Scholar]
- 20.Crowley E, Horwitz AF. Tyrosine phosphorylation and cytoskeletal tension regulate the release of fibroblast adhesions. J Cell Biol. 1995;131:525–537. doi: 10.1083/jcb.131.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Damsky CH, Werb Z. Signal transduction by integrin receptors for extracellular matrix: cooperative processing of extracellular information. Curr Opin Cell Biol. 1992;4:772–781. doi: 10.1016/0955-0674(92)90100-q. [DOI] [PubMed] [Google Scholar]
- 22.Dedhar S, Hannigan GE. Integrin cytoplasmic interactions and bidirectional transmembrane signalling. Curr Opin Cell Biol. 1996;8:657–669. doi: 10.1016/s0955-0674(96)80107-4. [DOI] [PubMed] [Google Scholar]
- 23.Fässler R, Pfaff M, Murphy J, Noegel AA, Johansson S, Timpl R, Albrecht R. Lack of β1 integrin gene in embryonic stem cells affects morphology, adhesion, and migration but not integration into the inner cell mass of blastocysts. J Cell Biol. 1995;128:979–988. doi: 10.1083/jcb.128.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fässler R, Georges-Labouesse E, Hirsch E. Genetic analyses of integrin function in mice. Curr Opin Cell Biol. 1996;8:641–646. doi: 10.1016/s0955-0674(96)80105-0. [DOI] [PubMed] [Google Scholar]
- 25.Filardo EJ, Brooks PC, Deming SL, Damsky C, Cheresh DA. Requirement of the NPXY motif in the integrin β3 subunit cytoplasmic tail for melanoma cell migration in vitro and in vivo. J Cell Biol. 1995;130:441–450. doi: 10.1083/jcb.130.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gahmberg CG. Leukocyte adhesion: CD11/CD18 integrins and intercellular adhesion molecules. Curr Opin Cell Biol. 1997;9:643–650. doi: 10.1016/s0955-0674(97)80117-2. [DOI] [PubMed] [Google Scholar]
- 27.Giancotti FG. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol. 1997;9:691–700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- 28.Grinblat Y, Zusman S, Yee G, Hynes RO, Kafatos FC. Functions of the cytoplasmic domain of the βPS integrin subunit during Drosophiladevelopment. Development (Camb) 1994;120:91–102. doi: 10.1242/dev.120.1.91. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi Y, Haimovich B, Reszka A, Boettinger D, Horwitz A. Expression and function of chicken integrin β1 subunit and its cytoplasmic domain mutants in mouse NIH 3T3 cells. J Cell Biol. 1990;110:175–184. doi: 10.1083/jcb.110.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemler ME. VLA proteins in the integrin family: Structure, functions and their roles on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- 31.Hirst R, Horwitz A, Buck C, Rohrschneider L. Phosphorylation of the fibronectin receptor complex in cells transformed by oncogenes that encode tyrosine kinases. Proc Natl Acad Sci USA. 1986;83:6470–6474. doi: 10.1073/pnas.83.17.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes PE, Diaz-Gonzalez F, Leong L, Wu C, McDonald JA, Shattil SJ, Ginsberg MH. Breaking the integrin hinge. A defined structural constraint regulates integrin signaling. J Biol Chem. 1996;271:6571–6574. doi: 10.1074/jbc.271.12.6571. [DOI] [PubMed] [Google Scholar]
- 33.Hughes PE, Renshaw MW, Pfaff M, Forsyth J, Keivens VM, Schwartz MA, Ginsberg MH. Suppression of integrin activation: a novel function of a Ras/Raf–initiated MAP kinase pathway. Cell. 1997;88:521–530. doi: 10.1016/s0092-8674(00)81892-9. [DOI] [PubMed] [Google Scholar]
- 34.Huttenlocher A, Sandborg RR, Horwitz AF. Adhesion in cell migration. Curr Opin Cell Biol. 1995;7:697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 35.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 36.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T, Aizawa S. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 37.Johansson MW, Larsson E, Lüning B, Pasquale EB, Ruoslahti E. Altered localization and cytoplasmic domain-binding properties of tyrosine-phosphorylated β1 integrin. J Cell Biol. 1994;126:1299–1309. doi: 10.1083/jcb.126.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LaFlamme SE, Auer KL. Integrin signaling. Semin Cancer Biol. 1996;7:111–118. doi: 10.1006/scbi.1996.0016. [DOI] [PubMed] [Google Scholar]
- 39.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 40.Law DA, Nannizzi-Alaimo L, Phillips DR. Outside-in integrin signal transduction. αIIbβ3(GP IIbIIIa) tyrosine phosphorylation induced by platelet aggregation. J Biol Chem. 1996;18:10811–10815. doi: 10.1074/jbc.271.18.10811. [DOI] [PubMed] [Google Scholar]
- 41.Leahy DJ, Aukhil I, Erickson HP. 2.0 Å crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell. 1996;84:155–164. doi: 10.1016/s0092-8674(00)81002-8. [DOI] [PubMed] [Google Scholar]
- 42.Lenter M, Uhlig H, Hamann A, Jenö P, Imhof B, Vestweber D. A monoclonal antibody against an activation epitope on mouse integrin chain β1 blocks adhesion of lymphocytes to the endothelial integrin α6β1 . Proc Natl Acad Sci USA. 1993;90:9051–9055. doi: 10.1073/pnas.90.19.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKeown-Longo PJ, Mosher DF. Interaction of the 70,000-mol-wt amino-terminal fragment of fibronectin with the matrix-assembly receptor of fibroblasts. J Cell Biol. 1985;100:364–374. doi: 10.1083/jcb.100.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyamoto S, Akiyma SK, Yamada KM. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science. 1995;267:883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- 45.Moolenaar WH, Kranenburg O, Postma FR, Zondag GCM. Lysophosphatidic acid: G protein signalling and cellular responses. Curr Opin Cell Biol. 1997;9:168–173. doi: 10.1016/s0955-0674(97)80059-2. [DOI] [PubMed] [Google Scholar]
- 46.Mosher DF. Assembly of fibronectin into extracellular matrix. Curr Opin Struct Biol. 1993;3:214–222. [Google Scholar]
- 47.Nakamura TY, Yamamoto I, Nishitani H, Matozaki T, Suzuki T, Wakabayashi S, Shigekaw M, Goshima K. Detachment of cultured cells from the substratum induced by the neutrophil-derived oxidant NH2Cl: synergistic role of phosphotyrosine and intracellular Ca2+concentration. J Cell Biol. 1995;131:509–524. doi: 10.1083/jcb.131.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nhieu GT V, Krukonis ES, Reszka AA, Horwitz AF, Isberg RR. Mutations in the cytoplasmic domain of the integrin β1 chain indicate a role for endocytosis factors in bacterial internalization. J Biol Chem. 1996;271:7665–7672. doi: 10.1074/jbc.271.13.7665. [DOI] [PubMed] [Google Scholar]
- 49.O'Toole TE, Ylanne J, Culley BM. Regulation of integrin affinity states through a NPXY motif in the β subunit cytoplasmic domain. J Biol Chem. 1995;270:8553–8558. doi: 10.1074/jbc.270.15.8553. [DOI] [PubMed] [Google Scholar]
- 50.Obeso J, Weber J, Auerbach R. A hemangioendothelioma- derived cell line: its use as a model for the study of endothelial cell biology. Lab Invest. 1990;63:259–269. [PubMed] [Google Scholar]
- 51.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 52.Panetti TS, McKeown-Longo PJ. The αvβ5 integrin receptor regulates receptor-mediated endocytosis of vitronectin. J Biol Chem. 1993;268:11492–11495. [PubMed] [Google Scholar]
- 53.Pasqualini R, Hemler ME. Contrasting roles for integrin β1 and β5cytoplasmic domains in subcellular localization, cell proliferation, and cell migration. J Cell Biol. 1994;125:447–460. doi: 10.1083/jcb.125.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reszka AA, Hayashi Y, Horwitz AF. Identification of amino acid sequences in the integrin β1 cytoplasmic domain implicated in cytoskeletal association. J Cell Biol. 1992;117:1321–1330. doi: 10.1083/jcb.117.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 56.Ruoslahti E. Integrins. J Clin Invest. 1991;87:1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sakai T, Ohta M, Furukawa Y, Saga Y, Aizawa S, Kawakatsu H, Saito M. TenascinC induction by the diffusible factor epidermal growth factor in stromal-epithelial interactions. J Cell Physiol. 1995;165:18–29. doi: 10.1002/jcp.1041650104. [DOI] [PubMed] [Google Scholar]
- 58.Sakai T, Furukawa Y, Chiquet-Ehrismann R, Nakamura M, Kitagawa S, Ikemura T, Matsumoto KI. TenascinX expression in tumor cells and fibroblasts: glucocorticoids as negative regulators in fibroblasts. J Cell Sci. 1996;109:2069–2077. doi: 10.1242/jcs.109.8.2069. [DOI] [PubMed] [Google Scholar]
- 59.Schwartz MA, Schaller MD, Ginsberg MH. Integrins: Emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 60.Shih DT, Horwitz AF, Grunwald GB, Buck CA. Structure/ function analysis of the integrin β1 subunit by epitope mapping. J Cell Biol. 1993;122:1361–1371. doi: 10.1083/jcb.122.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stossel TP. On the crawling of animal cells. Science. 1993;260:1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- 62.Tamkun JW, DeSimone DW, Fonda D, Patel RS, Buck C, Horwitz AF, Hynes RO. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell. 1986;46:271–282. doi: 10.1016/0092-8674(86)90744-0. [DOI] [PubMed] [Google Scholar]
- 63.Tapley P, Horwitz A, Buck C, Duggan K, Rohrschneider L. Integrins isolated from Rous sarcoma virus-transformed chicken embryo fibroblasts. Oncogene. 1989;4:325–333. [PubMed] [Google Scholar]
- 64.Varner JA, Cheresh DA. Integrins and cancer. Curr Opin Cell Biol. 1996;8:724–730. doi: 10.1016/s0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- 65.Vignoud L, Albigès-Rizo C, Frachet P, Block MR. NPXY motifs control the recruitment of the α5β1 integrin in focal adhesions independently of the association of talin with β1 chain. J Cell Sci. 1997;110:1421–1430. doi: 10.1242/jcs.110.12.1421. [DOI] [PubMed] [Google Scholar]
- 66.Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- 67.Wennerberg K, Lohikangas L, Gullberg D, Pfaff M, Johansson S, Fässler R. β1 integrin–dependent and –independent polymerization of fibronectin. J Cell Biol. 1996;132:227–238. doi: 10.1083/jcb.132.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wickham TJ, Filardo EJ, Cheresh DA, Nemerow GR. Integrin αvβ5 selectively promotes adenovirus mediated cell membrane permeabilization. J Cell Biol. 1994;127:257–264. doi: 10.1083/jcb.127.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu C, Keivens VM, O'Toole TE, McDonald JA, Ginsberg MH. Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell. 1995;83:715–724. doi: 10.1016/0092-8674(95)90184-1. [DOI] [PubMed] [Google Scholar]
- 70.Yamada KM, Miyamoto S. Integrin transmembrane signaling and cytoskeletal control. Curr Opin Cell Biol. 1995;7:681–689. doi: 10.1016/0955-0674(95)80110-3. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Q, Mosher DF. Crosslinking of the N-terminal region of fibronectin to molecules of large apparent molecular mass (LAMMs): characterization of fibronectin assembly sites induced by the treatment of fibroblasts with lysophosphatidic acid. J Biol Chem. 1996;271:33284–33292. doi: 10.1074/jbc.271.52.33284. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Q, Checovich WJ, Peters DM, Albrecht RM, Mosher DF. Modulation of cell surface fibronectin assembly sites by lysophosphatidic acid. J Cell Biol. 1994;127:1447–1459. doi: 10.1083/jcb.127.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Q, Magnusson MK, Mosher DF. Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Mol Biol Cell. 1997;8:1415–1425. doi: 10.1091/mbc.8.8.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]