Abstract
The small GTPase Rho is believed to regulate the actin cytoskeleton and cell adhesion through its specific targets. We previously identified the Rho targets: protein kinase N, Rho-associated kinase (Rho- kinase), and the myosin-binding subunit (MBS) of myosin phosphatase. We found that in MDCK epithelial cells, MBS accumulated at the tetradecanoylphorbol-13-acetate (TPA)-induced membrane ruffling area, where moesin, a member of the ERM (ezrin, radixin, and moesin) family, was localized. Neither membrane ruffling nor an accumulation of moesin and MBS at the free-end plasma membrane was induced when MDCK cells were stimulated with TPA after the microinjection of C3, which ADP-ribosylates and inactivates Rho. MBS was colocalized with moesin at the cell–cell contact sites in MDCK cells. We also found that moesin was coimmunoprecipitated with MBS from MDCK cells. Recombinant MBS interacted with the amino-terminal domains of moesin and ezrin. Myosin phosphatase composed of the catalytic subunit and MBS showed phosphatase activity toward moesin, which was phosphorylated by Rho-kinase. The phosphatase activity was inhibited when MBS was phosphorylated by Rho-kinase. These results suggest that MBS is recruited with moesin to the plasma membrane and that myosin phosphatase and Rho-kinase regulate the phosphorylation state of moesin downstream of Rho.
The small GTPase Rho has GDP-bound inactive (GDP·Rho) and GTP-bound active (GTP·Rho) forms (Nobes and Hall, 1994; Takai et al., 1995). When cells are stimulated with certain extracellular signals such as lysophosphatidic acid, GDP·Rho is thought to be converted to GTP·Rho, which binds to specific targets and then exerts its biological functions. Rho participates in signaling pathways that regulate actin cytoskeletons, such as stress fibers, and cell substratum adhesions, such as focal adhesions in fibroblasts (Ridley and Hall, 1992, 1994). Rho is also involved in the regulation of cell morphology (Paterson et al., 1990), cell aggregation (Tominaga et al., 1993), cadherin-mediated cell–cell adhesion (Braga et al., 1997), cell motility (Takaishi et al., 1994), cytokinesis (Kishi et al., 1993; Mabuchi et al., 1993), membrane ruffling (Nishiyama et al., 1994), smooth muscle contraction (Hirata et al., 1992; Gong et al., 1996), c-fos gene expression (Hill et al., 1995), and the synthesis of phosphatidylinositol 4,5-diphosphate (4,5-PIP2)1 via phosphatidylinositol 5-kinase (PI5-kinase) (Chong et al., 1994). In budding yeast, RHO1 (a homologue of RhoA) is implicated in the regulation of cell morphology and budding (Bussey, 1996). We identified three Rho targets: protein kinase N (Amano et al., 1996b ; Watanabe et al., 1996); Rho-associated kinase (Rho-kinase) (Matsui et al., 1996), which was also identified as ROKα (Leung et al., 1995); and the myosin-binding subunit (MBS) of myosin phosphatase (Kimura et al., 1996). p160 ROCK is an isoform of Rho-kinase (Ishizaki et al., 1996). We showed that Rho-kinase phosphorylates MBS and consequently inactivates myosin phosphatase (Kimura et al., 1996). Rho-kinase also phosphorylates myosin light chain (MLC) and thereby activates myosin ATPase in vitro (Amano et al., 1996a ) and in vivo (Chihara et al., 1997). Another group of investigators has identified different Rho targets: Rhophilin, Rhotekin, citron, and mDia (Madaule et al., 1995; Reid et al., 1996; Watanabe, G., et al., 1996; Watanabe, N., et al., 1997). PI5-kinase is shown to be activated by GTP·Rho (Chong et al., 1994). Among these Rho targets, Rho-kinase appears to be involved in the formation of stress fibers and focal adhesions (Leung et al., 1996; Amano et al., 1997; Ishizaki et al., 1997), smooth muscle contraction (Kureishi et al., 1997), and neurite retraction (Amano et al., 1998) downstream of Rho.
The ERM family, which consists of the three closely related proteins ezrin, radixin, and moesin, is thought to function as cross-linking protein between plasma membranes and actin filaments (Bretscher, 1983; Pakkanen et al., 1987; Tsukita et al., 1989; Algrain et al., 1993; Arpin et al., 1994). The ERM family is expressed and localized at the specific regions where actin filaments are associated with plasma membranes such as microvilli, ruffling membranes, cell–cell adhesion sites, and cell–substratum adhesion sites (Lankes et al., 1988; Tsukita et al., 1989, 1992; Sato et al., 1991, 1992; Berryman et al., 1993; Amieva et al., 1994; Takeuchi et al., 1994). The ERM family appears to be essential for the interaction between plasma membranes and actin filaments because the suppression of the ERM family expression by antisense oligonucleotide disrupts microvilli, cell–cell adhesion sites, and cell–substratum adhesion sites (Takeuchi et al., 1994). The NH2-terminal and COOH-terminal domains of the ERM family are thought to directly bind to some integral membrane proteins and actin filaments, respectively (Tsukita et al., 1992; Turunen et al., 1994; Pestonjamasp et al., 1995). Recently, it has been reported that the NH2-terminal domain of ezrin is also involved in F-actin binding (Roy et al., 1997). The ERM family proteins are immunoprecipitated with CD44 from cultured cells (Tsukita et al., 1994). CD44 is a polymorphic cell surface glycoprotein that may serve as an adhesion molecule (Haynes et al., 1989, 1991; Lesley et al., 1993). CD44 is precisely colocalized with the ERM family, and the cytoplasmic domain of CD44 interacts with the ERM family in vitro, indicating that CD44 is one of the membrane partners of the ERM family (Tsukita et al., 1994; Hirao et al., 1996). Since the association of plasma membranes with actin filaments is dynamically regulated, the complex formation between CD44 and the ERM family appears to be regulated dynamically. Consistently, recent evidence suggests that Rho regulates the complex formation between CD44 and the ERM family, presumably through 4,5-PIP2 synthesis (Takaishi et al., 1995; Hirao et al., 1996).
In light of these observations, we examined the relationship between moesin, a member of the ERM family, and Rho targets. We found that MBS is colocalized with moesin at the membrane ruffling area and cell–cell contact sites and interacts with moesin both in vivo and in vitro, and that myosin phosphatase and Rho-kinase regulate the phosphorylation state of moesin.
Materials and Methods
Materials and Chemicals
MDCK cells were kindly provided by Dr. S. Tsukita (Kyoto University, Kyoto, Japan) and maintained in DME containing 10% calf serum, streptomycin, and penicillin. The expression plasmid of C3 transferase (pGEX-C3) was kindly provided by Dr. A. Hall (University College, London, UK). The cDNA encoding mouse moesin (1–577 amino acids [aa]) was kindly provided by Dr. S. Tsukita (Hirao et al., 1996). Recombinant COOH-terminal domain (307–577 aa) of mouse moesin (C-moesin), rat anti–moesin mAb (M22), and mouse anti–moesin mAb (CR22) were kindly provided by Dr. S. Tsukita (Takeuchi et al., 1994; Tsukita et al., 1994; Hirao et al., 1996). Rabbit anti–rat MBS polyAb and chicken myosin phosphatase holoenzyme were kindly provided by Dr. M. Ito (Mie University, Japan) (Shimizu et al., 1994; Kimura et al., 1996). Anti–37-kD catalytic subunit of myosin phosphatase polyAb and anti–Rho GDI polyAb were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antihemagglutinin (HA) mAb (12CA5) was purchased from Boehringer Mannheim (Indianapolis, IN). [γ-32P]ATP and [35S]methionine were purchased from Amersham Corp. (Buckinghamshire, UK). All materials used in the nucleic acid study were purchased from Takara Shuzo Corp. (Kyoto, Japan). Other materials and chemicals were obtained from commercial sources.
Preparation of Recombinant Proteins
C3, human ezrin (1–323 aa), human moesin (1–323 aa), and Rho-GDI were expressed as glutathione-S-transferase (GST) fusion protein in Escherichia coli and purified as described previously (Kikuchi et al., 1992; Takeshima et al., 1994). For some experiments, human moesin (1–323 aa) was expressed as GST-HA fusion protein in Escherichia coli. Purified GST-C3, GST-Rho GDI, and GST-HA-moesin (1–323 aa) was then cleaved with thrombin to remove the GST according to the manufacturer's instructions. GST-catalytic domain (6–553 aa) of Rho-kinase (GST-CAT) was produced and purified from Sf9 cells as described previously (Matsuura et al., 1987; Amano et al., 1996a ).
To obtain the recombinant full-length MBS, the cDNA encoding the full-length rat MBS (Kimura et al., 1996) was inserted into the BamHI site of pAcYMI. Also, the cDNA encoding mouse moesin (1–577 aa) was subcloned into the KpnI site of pAcYMI-HA to yield HA-full-length moesin. The full-length rat MBS and HA-full-length moesin were produced in High Five and Sf9 cells, respectively, with the use of a baculovirus system (Matsuura et al., 1987). The cells expressing the full-length rat MBS or HA-full-length moesin were suspended in homogenizing buffer (20 mM Tris/HCl, pH 8.0, 500 mM NaCl, 1 mM EDTA, 1 mM DTT, 10 μM [p-amidinophenyl]-methanesulfonyl fluoride [A-PMSF], 10 μg/ml leupeptin). The suspensions were sonicated and centrifuged at 100,000 g for 1 h at 4°C. The supernatants were diluted with buffer A (20 mM Tris/HCl, pH 7.5, 1 mM EDTA, 1 mM DTT) and applied onto Mono S or Mono Q column, respectively (Pharmacia LKB Biotechnology AB, Uppsala, Sweden), which had been equilibrated with buffer A. After the columns were washed, the proteins were eluted with a linear concentration gradient of NaCl (0–600 mM) in buffer A. Either MBS or HA-moesin was eluted with about 200 mM NaCl.
To obtain the in vitro–translated NH2- and COOH-terminal domains of MBS, the cDNA fragments encoding the NH2-terminal domain (1–707 aa) and COOH-terminal domain (699–976 aa) of rat MBS were cloned into the BamHI site of pCRII and pGEM-HA, respectively. The NH2-terminal and COOH-terminal domains of rat MBS were translated in vitro using the TNT T7-coupled reticulocyte lysate system (Promega Corp., Madison, WI) under the conditions described in the manufacturer's instruction manual.
Immunofluorescence Analysis
MDCK cells were fixed with 3.7% formaldehyde in PBS for 10 min and treated with PBS containing 0.2% Triton X-100 for 10 min. After being washed with PBS three times, the cells were incubated with anti-MBS Ab or antimoesin Ab (M22) for 1 h at room temperature. The cells were washed with PBS three times, followed by incubation with FITC-conjugated anti–rabbit Ig Ab or FITC-conjugated anti–rat Ig Ab for 1 h at room temperature. For double immunostaining, MDCK cells were incubated with antimoesin Ab (M22) together with anti-MBS Ab for 1 h at room temperature. Then MDCK cells were incubated with Texas red–conjugated anti–rat Ig Ab and FITC-conjugated anti–rabbit Ig Ab. After being washed with PBS three times, the cells were examined using a laser scanning confocal microscope (Carl Zeiss, Inc., Thornwood, NY).
Microinjection
MDCK cells were seeded at the density of 2.5 × 103 cells per 13-mm cover glass in 60-mm tissue culture dishes and incubated for 8 h. Then, the cells were deprived of serum for 24 h. For microinjection, C3 was concentrated, and during the concentration the buffer was replaced by microinjection buffer (20 mM Tris/HCl, pH 7.4, 20 mM NaCl, 1 mM MgCl2, 0.1 mM EDTA, 5 mM 2-mercaptoethanol). 0.2 mg/ml C3 was microinjected along with a marker protein (rabbit or mouse IgG at 1 mg/ml) into the cytoplasm of cells. 30 min after the microinjection, the cells were incubated in DME containing 200 nM tetradecanoylphorbol-13-acetate (TPA) for 10 min at 37°C. MBS and moesin were visualized as described above.
Immunoprecipitation Assay
MDCK cells were grown in 100-mm tissue culture dishes. After being washed with PBS, the cells were lysed with 1 ml of extraction buffer (20 mM Tris/HCl, pH 8.0, 150 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.1% NP-40, 10 μM A-PMSF, 10 μg/ml leupeptin). The lysate was removed from the dishes with a rubber policeman, incubated in a 1.5-ml tube for 15 min, and then clarified by centrifugation at 12,000 g for 15 min. The soluble supernatant was incubated with 10 μl of anti-MBS Ab or preimmuneserum. The immunocomplex was then precipitated with protein A–Sepharose CL 4B (Pharmacia LKB Biotechnology AB). The immunocomplex was washed five times with the extraction buffer containing 0.5% NP-40, eluted by boiling in sample buffer for SDS-PAGE, and subjected to silver staining and immunoblot analysis using antimoesin Ab (CR22) as described (Harlow and Lane, 1988).
Interaction of Recombinant Full-Length MBS with Ezrin and Moesin in a Cell-free System
GST-ezrin (1–323 aa), GST-moesin (1–323 aa), and GST (150 pmol) were separately immobilized onto 35 μl of glutathione–Sepharose 4B beads (Pharmacia LKB Biotechnology AB). The immobilized beads were incubated with the recombinant MBS (1–976 aa) (0.3 μM) in buffer A containing 1 mg/ml BSA for 1 h at 4°C. The beads were washed six times with 116 μl (3.3 vol) of buffer A, and the bound proteins were eluted three times with GST-ezrin and GST-moesin by the addition of 116 μl (3.3 vol) of buffer A containing 10 mM glutathione. The first eluates were subjected to SDS-PAGE, and the proteins were detected by silver staining. For some experiments, HA-moesin (1–323 aa) and GST-HA-moesin (1–323 aa) (50 pmol each) were separately immobilized onto protein A–Sepharose CL 4B (20 μl) with anti-HA mAb and incubated with MBS (0.3 μM). HA-full-length moesin was also immobilized the same as HA-moesin (1–323 aa). Instead of MBS, Rho GDI (2.0 μM) was incubated with HA-full-length moesin or GST-moesin (1–323 aa) (50 pmol each) immobilized beads and the bound Rho GDI were detected by immunoblotting. For K d estimation, 50 pmol of GST-moesin (1–323 aa) or HA-full-length moesin–immobilized beads (20 μl) were incubated with the various concentration of MBS in 100 μl of buffer A containing 50 mM NaCl and 1 mg/ml BSA. The amount of bound MBS to each moesin was detected by Coomassie brilliant blue staining and estimated with a densitograph (ATTO, Tokyo, Japan).
In Vitro Binding Assay between NH2- and COOH-terminal Domains of MBS and Ezrin
The in vitro–translated NH2- and COOH-terminal domains of MBS were separately incubated with GST-ezrin (1–323 aa) or GST (1 nmol each) immobilized beads, and the bound proteins were eluted as described above. The eluates were subjected to SDS-PAGE and vacuum-dried, followed by autoradiography.
Interaction of Myosin Phosphatase with Ezrin and Moesin
Bovine brain membrane extract was obtained as described previously (Amano et al., 1996b ). The extract was loaded onto 0.25 ml glutathione-Sepharose columns containing 6.5 nmol of GST-ezrin and GST-moesin. After the columns were washed with 0.85 ml (3.3 vol) of buffer A and then 0.85 ml (3.3 vol) of buffer A containing 50 mM NaCl, three times with each, the bound proteins were eluted three times by the addition of 0.85 ml (3.3 vol) of buffer A containing 10 mM glutathione. The first eluates were subjected to silver staining or immunoblot analysis using antibodies against the MBS and the 37-kD catalytic subunit of myosin phosphatase.
Protein Phosphatase Assay
C-moesin (300 ng of protein) was phosphorylated with GST-CAT (120 ng of protein) in 20 μl of kinase buffer (50 mM Tris/HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT) containing 100 μM [γ-32P]ATP for 1 h at 30°C, and the reaction was stopped by the addition of 200 nM staurosporine. Native myosin phosphatase (5–75 ng) was preincubated in 30 μl of reaction mixture containing 0.3 mM CoCl2, with or without 100 μM adenosine 5′-O-(3-thiotriphosphate) (ATPγS) in the presence of GST-CAT (80 ng of protein) for 15 min at 30°C, and the reaction was stopped by the addition of 200 nM staurosporine. The phosphatase reaction was then performed in 50 μl of the reaction mixture (30 mM Tris/HCl, pH 7.5, 3 mM MgCl2, 0.4 mM EDTA, 0.55 mM EGTA, 0.1 mg/ml BSA, 5–75 ng of myosin phosphatase preincubated as described above, 300 ng of 32P-labeled C-moesin) for 15 min at 30°C. The reaction mixture was then boiled in sample buffer for SDS-PAGE and resolved by SDS-PAGE. The 32P-labeled band corresponding to C-moesin was visualized and estimated with an image analyzer (model BAS-2000; Fuji, Tokyo, Japan).
Other Procedures
SDS-PAGE was performed as described (Laemmli, 1970). Protein concentrations were determined with BSA as the reference protein as described (Bradford, 1976).
Results
Colocalization of MBS and Moesin at the TPA-induced Membrane Ruffling Area in MDCK Cells
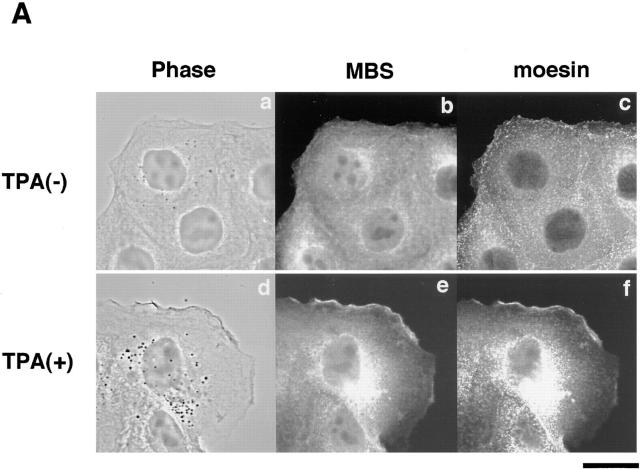
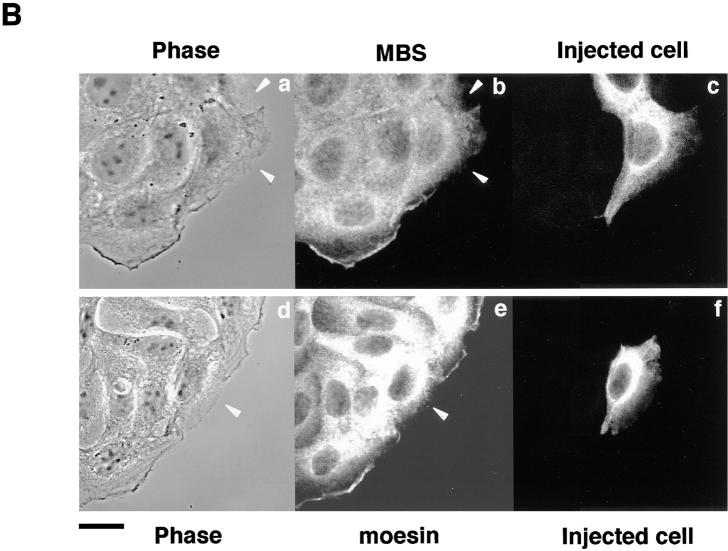
Rho is believed to be involved in the hepatocyte growth factor– and phorbol ester–induced membrane ruffling in MDCK and KB epithelial cells (Nishiyama et al., 1994; Takaishi et al., 1995). Rho and the ERM family are localized at the TPA-induced membrane ruffling area (Takaishi et al., 1995). We examined whether MBS is localized at the TPA-induced membrane ruffling area. When serum-starved MDCK cells were stimulated with TPA, membrane ruffling was induced within 10 min, and moesin (one of the ERM family) accumulated at the membrane ruffling area as previously shown (Takaishi et al., 1995) (Fig. 1 A). MBS also accumulated at the membrane ruffling area and was colocalized with moesin (Fig. 1 A). The TPA-induced membrane ruffling is inhibited by a prior microinjection of C3, which ADP-ribosylates and inactivates Rho (Takaishi et al., 1995). When serum-starved MDCK cells were stimulated with TPA 30 min after a microinjection of C3, neither membrane ruffling nor the accumulation of moesin and MBS at the free-end plasma membrane was induced (Fig. 1 B).
Figure 1.
Colocalization of MBS and moesin at membrane ruffling area in MDCK cells. (A) Colocalization of MBS and moesin at the TPA-induced ruffling area. Serum-starved MDCK cells were stimulated with 200 nM TPA and doubly stained with anti-MBS Ab and antimoesin Ab (M22), followed by FITC-conjugated anti–rabbit Ig Ab for MBS and Texas red– conjugated anti–rat Ig Ab for moesin and analyzed by a confocal microscopy. (a–c) Nonstimulated serum-starved MDCK cells. (d–f) Serum-starved MDCK cells stimulated with TPA. (a and d) Phase-contrast images (b and e), MBS staining, and (c and f) moesin staining. (B) Inhibition of the TPA-induced translocation of MBS and moesin by C3. Microinjection with 0.2 mg/ml C3 and TPA stimulation were performed as described in Materials and Methods. After TPA stimulation, cells were stained with anti-MBS Ab (b) or antimoesin Ab (e), followed by FITC-conjugated anti–rabbit Ig Ab for MBS and FITC-conjugated anti–rat Ig Ab for moesin. The mouse IgG or rabbit IgG microinjected as a marker with C3 were stained by Texas red–conjugated anti–mouse (c) or rabbit (f) Ig Ab, and microinjected cells were indicated. The arrowheads indicate the microinjected cells. (a and d) Phase-contrast images. The results shown are representative of three independent experiments. Bars, 10 μm.
We have recently shown that MBS accumulates at cell– cell contact sites apart from myosin fibers in polarized MDCK epithelial cells, whereas MBS is colocalized with myosin fibers in REF52 fibroblasts (Inagaki et al., 1997). The ERM family and Rho are localized at the cell–cell contact sites of MDCK epithelial cells, and the activity of the ERM family is thought to be regulated by Rho (Takaishi et al., 1995; Hirao et al., 1996; Kotani et al., 1997). We then compared the localization of MBS with that of moesin in confluent MDCK cells. A high level of MBS immunoreactivity was observed at the basolateral membrane of the cell–cell contact sites but not at a free end and showed a granular dotlike pattern as found earlier (Inagaki et al., 1997). Double immunofluorescence analysis revealed that MBS showed a distribution similar to that of moesin at the basolateral membrane of cell–cell contact sites (data not shown). On the other hand, MBS was not localized at the microvilli of the apical membrane, where moesin was concentrated. The localization of MBS was different from that of ZO-1 as a molecular marker of tight junctions, which showed a thin beltlike pattern and the localization at the apical side of cell–cell contact sites as previously described (Stevenson et al., 1986) (data not shown). These results indicate that MBS is partly colocalized with moesin in MDCK cells.
Complex Formation between MBS and Moesin
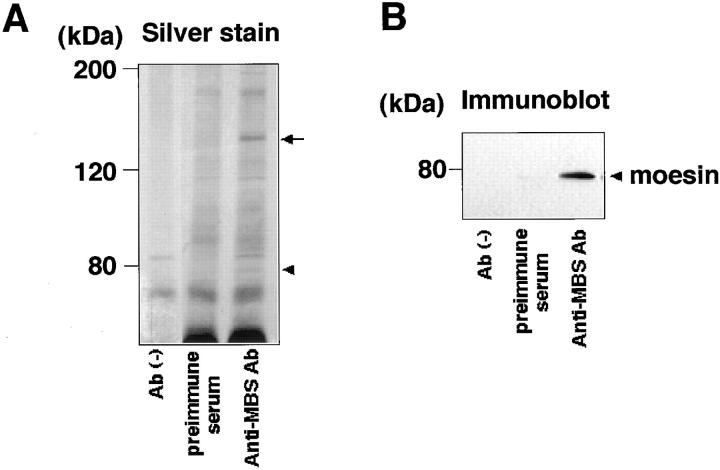
Because the localization and behavior of MBS are partly similar to those of moesin in MDCK cells, we examined whether MBS forms a complex with the ERM family in vivo. From the lysate of MDCK cells, MBS was immunoprecipitated with anti-MBS antibody, and the immunoprecipitates were subjected to immunoblot analysis to detect the ERM family. The immunoreactive band was detected in the immunoprecipitate with anti-MBS antibody (Fig. 2 B). Because the mAb CR22 predominantly reacts to moesin of the ERM family members in immunoblotting (Tsukita et al., 1994), the immunoreactive band was likely to correspond to moesin under these conditions. About 10% of the total MBS was immunoprecipitated, and about 0.5% of the total moesin was recovered with the immunoprecipitated MBS under the conditions. Moesin appeared to associate with MBS with a stoichiometry of about one moesin per three MBS in the immunoprecipitates. Little moesin was immunoprecipitated with control preimmuneserum or without antibody. Similar results were obtained when MBS was immunoprecipitated from KB cells instead of MDCK cells (data not shown). Using the other antibodies that can specifically react with each member of the ERM family, we examined whether ezrin and radixin other than moesin were coimmunoprecipitated with MBS from MDCK cells. Under the conditions, neither ezrin nor radixin was detected in the immunoprecipitate with anti-MBS antibody (data not shown). Radixin appeared to be undetectable in the immunoprecipitate with anti-MBS antibody because only ezrin and moesin but not radixin were detected in the start lysate of the MDCK cells. MDCK cells may express radixin in a small amount. We do not know the exact reason why ezrin was not coimmunoprecipitated with MBS.
Figure 2.
Coimmunoprecipitation of moesin with MBS. MDCK cells were lysed and solubilized with extraction buffer. This sample was incubated without antibody, with preimmuneserum, or with anti-MBS Ab. Then, the immunocomplexes were precipitated with protein A–Sepharose 4B. The immunocomplexes were subjected to silver staining (A) and immunoblot analysis using antimoesin Ab (CR22) (B). The arrow and arrowhead denote the positions of MBS and moesin, respectively. The results shown are representative of three independent experiments.
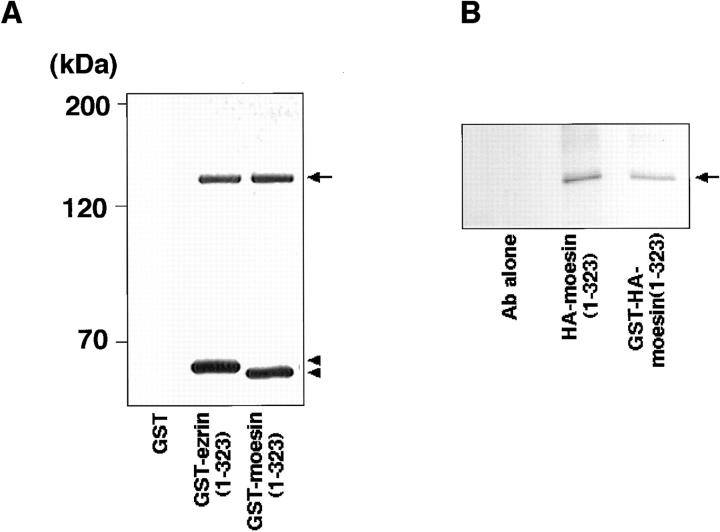
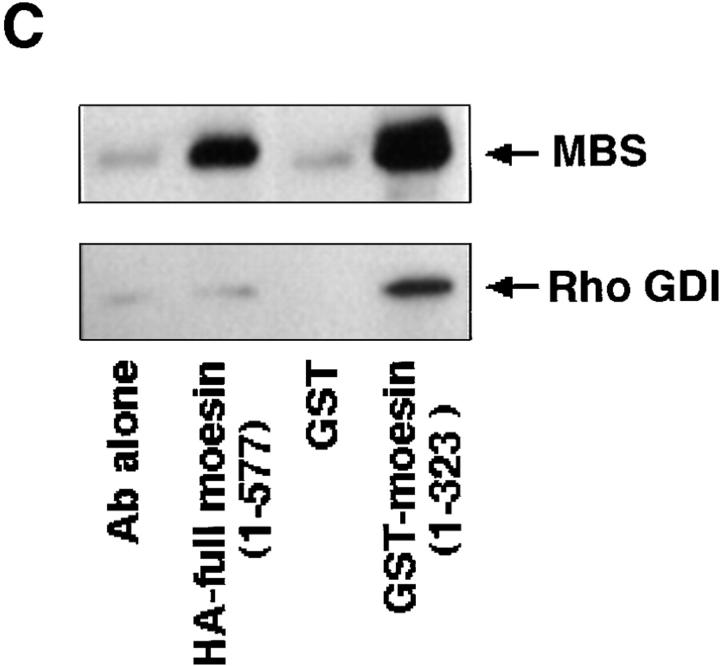
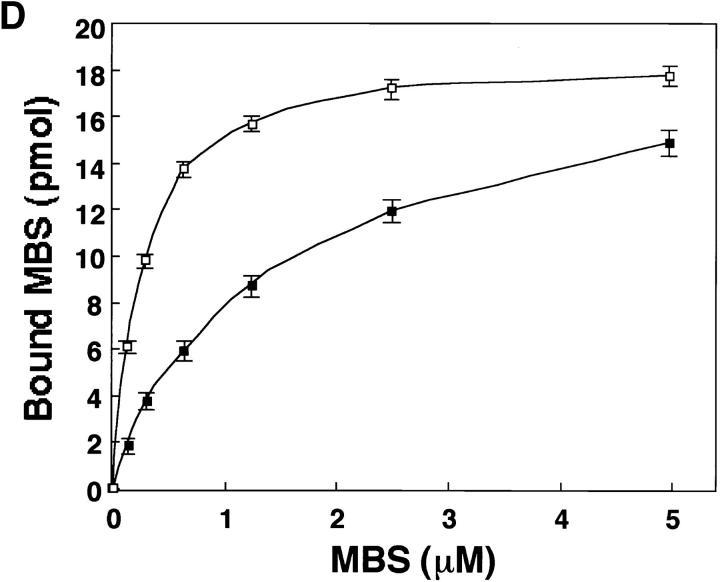
Since in the ERM family members the amino acid sequence of their NH2-terminal halves (∼300 aa) is highly conserved (∼85% identity for any pair) (Sato et al., 1992), we tested whether MBS binds directly to the NH2-terminal domain of ezrin and moesin in a cell-free system. The purified recombinant MBS (1–976 aa) was incubated with GST-ezrin (1–323 aa), GST-moesin (1–323 aa), or GST-immobilized beads. After washing the beads, the GST fusion proteins were eluted by the addition of glutathione. Recombinant MBS was coeluted with GST-ezrin (1–323 aa) and GST-moesin (1–323 aa), but not with GST (Fig. 3 A). To confirm that the NH2-terminal domain of moesin without GST shows the same behavior as GST-NH2-terminal domain of moesin (1–323 aa), we produced HA-NH2-terminal domain of moesin (1–323 aa) by cleaving GST of GST-HA-moesin (1–323 aa). Fig. 3 B shows that MBS bound not only to GST-HA-moesin (1–323 aa) but also to HA-moesin (1–323 aa). We confirmed that using a gel filtration chromatography, MBS bound to the untagged NH2-terminal domain of moesin (1–323 aa) stoichiometrically (data not shown). Because moesin was coimmunoprecipitated with MBS from the cultured cells, we next tested whether recombinant MBS also binds to the full-length moesin. Recently, Rho GDI has been reported to bind to the NH2-terminal domain of the ERM family but not to the full-length ERM family (Takahashi et al., 1997). HA-tagged full-length moesin (1–577 aa) was produced by use of a baculovirus system. As shown in Fig. 3 C, MBS bound to full-length moesin, whereas Rho GDI did not bind to full-length moesin as previously described (Takahashi et al., 1997). We further performed the kinetic study on the binding of MBS to moesin. As shown in Fig. 3 D, purified MBS bound to GST-moesin (1–323 aa) and HA-full-length moesin (1–577 aa) in a dose-dependent manner, and the interactions were almost saturable under the conditions. The K d values for the binding of MBS to GST-moesin (1–323 aa) and HA-full-length moesin (1–577 aa) were 0.26 ± 0.03 and 1.23 ± 0.09 μM, respectively. The stoichiometry of the binding of MBS to GST-moesin (1– 323 aa) was about 0.4. Although we do not know the exact reason why the stoichiometry did not reach to about one, either recombinant MBS or moesin may not be completely renatured. We also carried out this assay at a physiological ionic strength (at 150 mM NaCl). The K d value for the binding of MBS to GST-moesin (1–323 aa) at 150 mM NaCl was about the same as that under the above conditions (at 50 mM NaCl) (data not shown).
Figure 3.
Direct interaction between MBS and ezrin or moesin in a cell-free system. (A) Interaction of recombinant MBS and GST-ezrin (1– 323 aa) or GST-moesin (1– 323 aa). Purified MBS (1–976 aa) was incubated with GST-ezrin (1–323 aa), GST-moesin (1–323 aa), or GST-immobilized beads. After being washed by buffer A, the bound proteins were eluted with GST fusion proteins by the addition of glutathione. The eluates were subjected to SDS-PAGE and detected by silver staining. The arrow denotes the position of the recombinant MBS (1–976 aa). The arrowheads denote the positions of GST-ezrin or GST-moesin. (B) Interaction of recombinant MBS and HA-moesin (1–323 aa) without GST. Purified MBS (1–976 aa) was incubated with the protein A–Sepharose on which HA-moesin (1–323 aa) or GST-HA-moesin (1–323 aa) were immobilized with anti-HA mAb. As the control beads, anti-HA mAb alone was separately immobilized. After being washed with buffer A, the bound proteins were eluted by buffer A containing 500 mM NaCl and detected by silver staining. The arrow denotes the position of the recombinant MBS (1–976 aa). (C) Interaction of recombinant MBS and HA-full-length moesin (1–577 aa). Purified MBS (1–976 aa) and Rho GDI were separately incubated with the protein A–Sepharose which HA-full-length moesin was immobilized with anti-HA mAb. As the control beads, anti-HA mAb alone was separately immobilized. Also, purified MBS and Rho GDI were separately incubated with the glutathione Sepharose 4B on which GST or GST-moesin (1– 323 aa) was immobilized. After being washed with buffer A, the bound MBS and Rho GDI were eluted as described above and detected by immunoblot analysis. The results shown are representative of three independent experiments. (D) The kinetic study of the MBS binding to the NH2-terminal domain of moesin and the full-length moesin. The various doses of MBS (1–976 aa) were incubated with 50 pmol of GST-moesin (1–323 aa) (white boxes) or HA-full-length moesin (1–577 aa) (black boxes) immobilized beads in 100 μl of buffer A containing 50 mM NaCl. The amount of bound MBS was plotted after subtraction of the background binding to control beads. The values shown are means ± SE of triplicates.
To identify the region responsible for MBS binding in the NH2-terminal domain of moesin, we produced further truncated constructs of the NH2-terminal domain of moesin as GST fusion proteins. MBS could not bind to the NH2-terminal moesin that lacked at least the region between amino acids 234 and 323 (data not shown). This result indicates that the region between amino acids 234 and 323 is important for MBS binding to moesin. Recently, the regulatory subunit of protein kinase A type II (RII) has been reported to bind to ezrin through an amphipathic helix located between residues 417–430 and also to bind to moesin and radixin (Dransfield et al., 1997). It is likely that MBS and RII bind to the distinct regions of moesin.
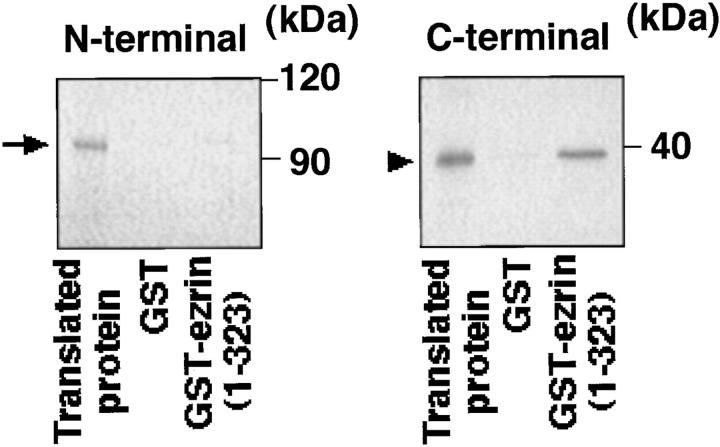
We further examined which domain of MBS interacts with the NH2-terminal domain of the ERM family using the in vitro–translated NH2- and COOH-terminal domains of MBS. Immobilized GST-ezrin (1–323 aa) was mixed with the in vitro–translated NH2- or COOH-terminal domain of MBS, and interacting proteins were eluted with GST-ezrin by the addition of glutathione. As shown in Fig. 4, the COOH-terminal domain of MBS bound to the NH2-terminal domain of ezrin, whereas the NH2-terminal domain of MBS did not bind. Essentially identical results were obtained when GST-moesin (1–323 aa) was used instead of GST-ezrin (1–323 aa) (data not shown), indicating that the COOH-terminal domain of MBS is responsible for the binding of MBS to the NH2-terminal domain of the ERM family. Taken together, these findings indicate that MBS binds to ezrin and moesin of the ERM family in vitro and at least to moesin in vivo, and the interaction is specific and physiological.
Figure 4.
Binding of the COOH-terminal domain of MBS to the NH2-terminal domain of ezrin. The in vitro–translated NH2- or COOH-terminal domain of MBS was incubated with GST-ezrin (1–323 aa) or GST-immobilized beads. After the beads were washed, the bound proteins were eluted with GST-ezrin or GST by the addition of glutathione. The eluates were subjected to SDS-PAGE and vacuum-dried, followed by autoradiography. The in vitro–translated NH2-terminal (arrow) and COOH-terminal (arrowhead) domains of MBS were shown. The results shown are representative of three independent experiments.
Phosphatase Activity of Myosin Phosphatase toward Moesin Phosphorylated by Rho-Kinase
It has been reported that in thrombin-activated platelets, moesin is phosphorylated at Thr558 (Nakamura et al., 1995). Thr558 is located in the sequence K555YKTL559, which is highly conserved among the ERM family and is involved in the actin binding of the ERM family (Turunen et al., 1994; Pestonjamasp et al., 1995). By further analysis using phosphorylation site–specific antibody, it is speculated that the phosphorylation at Thr558 of moesin is required for the stable interaction with actin of moesin at filopodia (Nakamura et al., 1996). In the preceding paper (Matsui et al., 1998), we found that Rho-kinase phosphorylates the ERM family. The phosphorylation site of moesin by Rho-kinase is identified as Thr558.
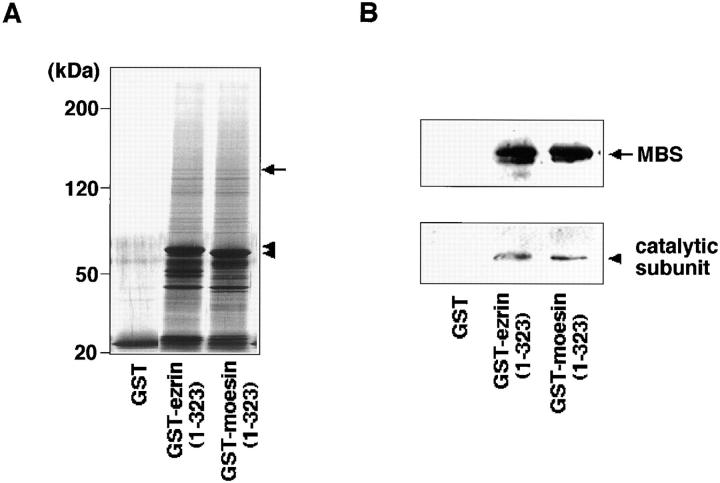
We speculated that myosin phosphatase might also regulate the phosphorylation states of moesin downstream of Rho, as described for MLC (Kimura et al., 1996). We tested whether only the MBS or also the 37-kD catalytic subunit of myosin phosphatase interacts with ezrin and moesin, using GST-ezrin (1–323 aa) and GST-moesin (1– 323 aa) affinity columns (Fig. 5). Bovine brain membrane extract was loaded onto a glutathione-Sepharose affinity column on which GST, GST-ezrin, or GST-moesin was immobilized. The proteins bound to the affinity columns were then coeluted with GST fusion proteins by the addition of glutathione. Fig. 5 A shows some molecules interacted with the NH2-terminal domain of ezrin and moesin (1–323 aa). Immunoreactive bands corresponding to the catalytic subunit as well as MBS were specifically detected in the eluates from the GST-ezrin and GST-moesin affinity columns (Fig. 5 B). We estimated that MBS and the catalytic subunit were about 20-fold enriched on GST-ezrin and GST-moesin affinity columns from bovine brain membrane extract. This result indicates that myosin phosphatase composed of MBS and the catalytic subunit interacts with ezrin and moesin, presumably through MBS.
Figure 5.
Specific interaction of myosin phosphatase with ezrin and moesin. Bovine brain membrane extract was loaded onto 0.25 ml of glutathione-Sepharose columns containing 6.5 nmol of GST-ezrin (1–323 aa), GST-moesin (1–323 aa), or GST. After the columns were washed, the proteins were eluted by the addition of glutathione. The eluates were subjected to silver staining (A) and immunoblot analysis (B) using antibodies against MBS (B, top) and the 37-kD catalytic subunit of myosin phosphatase (B, bottom). (A) The arrow denotes the position of MBS. The arrowheads denote the positions of GST-ezrin (1–323 aa) or GST-moesin (1–323 aa). (B) The arrow denotes the position of MBS. The arrowhead denotes the position of the 37-kD catalytic subunit of myosin phosphatase. The results shown are representative of three independent experiments.
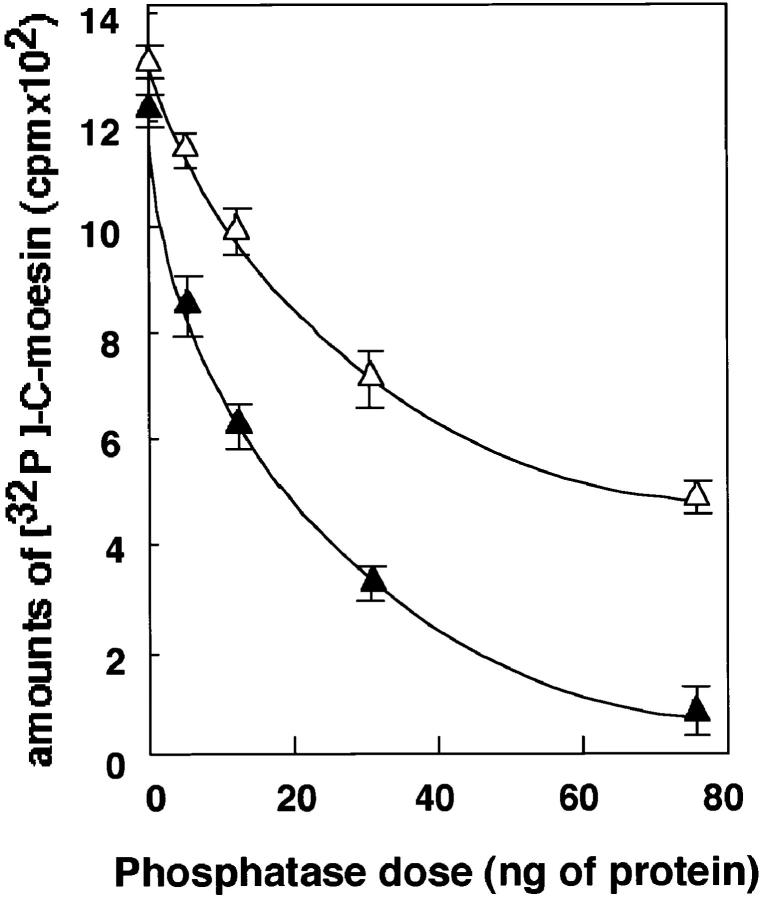
We next examined whether myosin phosphatase dephosphorylates moesin that is phosphorylated by Rho- kinase. The myosin phosphatase showed phosphatase activity toward the C-moesin (307–577 aa), which was phosphorylated by Rho-kinase (Fig. 6). The myosin phosphatase also showed phosphatase activity toward full-length moesin (data not shown). We previously observed that the MBS of the native myosin phosphatase is thiophosphorylated with Rho-kinase in the presence of ATPγS and that this thiophosphorylation of MBS is associated with a decrease of phosphatase activity toward MLC (Kimura et al., 1996). Here, we examined whether Rho- kinase also modulates the phosphatase activity of myosin phosphatase toward moesin through the thiophosphorylation of MBS. We found that the thiophosphorylation of MBS by Rho-kinase was associated with a decrease of phosphatase activity toward C-moesin, although the inhibition of phosphatase activity was not strong (Fig. 6).
Figure 6.
Phosphatase activity of myosin phosphatase toward moesin. C-moesin was phosphorylated with GST-CAT as described in Materials and Methods. After the indicated amounts of myosin phosphatase were incubated with (white triangles) or without (black traingles) ATPγS in the presence of GST-CAT, the phosphatase activity toward the phosphorylated C-moesin was assayed for 10 min at 30°C. The reaction mixture was then boiled in sample buffer for SDS-PAGE and resolved by SDS-PAGE. The 32P-labeled bands corresponding to C-moesin were visualized, and the amounts of 32P-labeled C-moesin were estimated with an image analyzer (model BAS-2000; Fuji). The values shown are means ± SE of triplicates.
Discussion
Colocalization of MBS and Moesin at the TPA-induced Membrane Ruffling Area
The ERM family members, which belong to the band 4.1 family, are thought to act as cross-linkers between plasma membranes and actin filaments (Tsukita et al., 1989; Algrain et al., 1993; Arpin et al., 1994). The ERM family and Rho are localized at specialized plasma membrane areas, including cell–cell contact sites and membrane ruffling area of MDCK epithelial cells (Takaishi et al., 1995). The translocation of the ERM family to the plasma membranes is thought to be regulated by Rho (Takaishi et al., 1995; Hirao et al., 1996; Kotani et al., 1997). Here we showed that MBS is colocalized with moesin at the TPA-induced membrane ruffling area (Fig. 1) and cell–cell contact sites in MDCK epithelial cells and that myosin phosphatase interacts with ezrin and moesin (Fig. 5) and dephosphorylates moesin, which is phosphorylated by Rho-kinase (Fig. 6). Thus, it is likely that MBS regulates the state of phosphorylation of at least moesin of the ERM family at specialized areas of plasma membranes, such as ruffling area and cell–cell contact sites. On the other hand, the ERM family has been shown to be located at the microvilli of the apical membrane (Bretscher, 1983; Berryman et al., 1993), where MBS does not accumulate. Also, MBS has been shown to be localized with myosin in the stress fibers in REF52 fibroblasts (Inagaki et al., 1997), whereas the ERM family is not localized in the stress fibers. This observation is reasonable because MBS is isolated as the myosin-binding subunit of myosin phosphatase. We also assume that moesin is one of the substrates of myosin phosphatase, and it is not necessary for the enzyme to be completely colocalized with its substrates. These observations mean that MBS is colocalized with some part of moesin at the specialized area of plasma membranes such as ruffling area and cell–cell contact sites.
Complex Formation between MBS and Moesin
The ERM family interacts with some integral membrane proteins via their NH2-terminal domains, and with F-actin via their NH2- and COOH-terminal domains (Algrain et al., 1993; Roy et al., 1997). We showed that some population of moesin interacts with MBS in vivo and that MBS interacts with the NH2-terminal domains of ezrin and moesin in vitro (Figs. 2–5). The efficiency of coimmunoprecipitation of moesin with MBS is not so high. Nevertheless, we consider this interaction physiological because the enzyme– substrate interaction is usually labile, and MBS is colocalized with moesin at the membrane ruffling area and the cell–cell contact sites but not at the microvilli. We did not detect ezrin or radixin in the immunoprecipitates of MBS. As described in Results, the exact reason for this result is not known, but we cannot conclude that MBS binds only to moesin out of the ERM family because the NH2-terminal domain of the ERM family is highly conserved and MBS also binds to the NH2-terminal domain of ezrin in vitro. It is possible that the anti-MBS antibody preferentially immunoprecipitated the MBS/moesin complex.
The NH2- and COOH-terminal domains of the ERM family are thought to oppose each other, presumably through the head to tail association between the NH2- and COOH-terminal domains (Martin et al., 1995, 1997; Gary and Bretscher, 1995; Hirao et al., 1996). This interdomain association is thought to be released by acidic phospholipids such as 4,5-PIP2 (Hirao et al., 1996). Once the ERM family is activated, it appears to be translocated from the cytosol to the plasma membrane, where it serves as a cross-linker between actin filaments and its membrane partners, such as CD44 (Tsukita et al., 1994). Rho GDI has consistenly been shown to interact with the NH2-terminal domain of the ERM family but not with the full-length of the ERM family (Takahashi et al., 1997). We confirmed its observation (Fig. 3 C). On the other hand, Dransfield et al. (1997) has reported that RII binds to the full-length ezrin. Also, we found that MBS can stoichiometrically interact with the full-length moesin, although the affinity of MBS to the full-length moesin is lower than that to the NH2-terminal domain of moesin (Fig. 3 D). Thus, the simple head to tail interaction model cannot fully explain why the MBS/full-length moesin and RII/full-length ezrin interaction occur in vitro under the conditions where Rho GDI does not interact with the full-length ERM family. Although it is unknown whether MBS and RII bind to the ERM family in its inactive states in vivo and are required for the activation of the ERM family, this point may be important to clarify the activation process of the ERM family. Further studies are necessary to understand this point.
Is Moesin Activated by MBS?
Since the activity of the ERM family is thought to be regulated by Rho, and MBS is one of the Rho targets, we can speculate that MBS interacts with and activates moesin and is translocated with moesin to the plasma membrane. However, this may not be likely because the translocation of moesin is not induced when the myristoylated form of MBS is expressed in MDCK cells and is targeted to the plasma membrane (data not shown). This means that the MBS translocation is not sufficient for the translocation of moesin to the membrane. When MDCK cells are stimulated with TPA, the membrane ruffling is induced and the ERM family is translocated to the plasma membrane of ruffling area. This action of TPA may be mediated by Rho because the TPA-induced membrane ruffling is prevented by a prior microinjection of C3 transferase. The translocation of the ERM family to the plasma membrane requires the Rho function in vitro (Hirao et al., 1996). Taken together, these observations suggest that the activation of the ERM family is regulated by Rho. Further studies are necessary for a better understanding of the involvement of MBS in Rho-mediated ERM activation. Other Rho targets such as PI5-kinase may be responsible for the activation of the ERM family through the synthesis of 4,5-PIP2 (Hirao et al., 1996).
Dual Regulation of the Phosphorylation State of Moesin
Rho regulates MLC phosphorylation via two pathways through its targets, Rho-kinase and MBS, as follows. Activated Rho interacts with Rho-kinase and the MBS of myosin phosphates and activates Rho-kinase. The activated Rho-kinase subsequently phosphorylates MBS, thereby inactivating myosin phosphatase (Kimura et al., 1996). Rho-kinase by itself phosphorylates MLC at the same site that is phosphorylated by MLC kinase, and activates myosin ATPase (Amano et al., 1996a ). The phosphorylation of MLC results in the contraction of smooth muscle or the interaction of actin and myosin in nonmuscle cells, leading to stress fiber formation (Kamm and Stull, 1985; Hartshorne, 1987; Sellers and Adelstein, 1987; Giuliano and Taylor, 1995). The expression of the dominant active form of Rho-kinase in cells consistently induces the formation of stress fibers and focal adhesions (Leung et al., 1996; Amano et al., 1997; Ishizaki et al., 1997). We confirmed that the phosphorylation state of MLC is dually regulated through myosin phosphatase and Rho-kinase in permeabilized smooth muscle (Kureishi et al., 1997) and COS7 cells (Chihara et al., 1997). We also found that Rho-kinase phosphorylates the ERM family and that the phosphorylation of radixin by Rho-kinase inhibits the interdomain association between the NH2- and COOH-terminal domains of radixin (Matsui et al., 1998). This result suggests that the phosphorylation of the ERM family by Rho-kinase may release the interdomain suppression between the NH2- and COOH-terminal domains or may maintain the activity of the ERM family by interfering with the interdomain association. Here we showed that myosin phosphatase composed of MBS and the catalytic subunit specifically interacts with ezrin and moesin (Fig. 5). We also showed that myosin phosphatase dephosphorylates moesin, which is phosphorylated by Rho-kinase (Fig. 6). The phosphatase activity of myosin phosphatase toward moesin is inhibited by the thiophosphorylation of MBS with Rho- kinase (Fig. 6). These results mean not only that MBS is one of the new partners of moesin but also that the phosphorylation level of moesin is dually regulated through myosin phosphatase and Rho-kinase downstream of Rho in a manner similar to MLC. Taken together, it is likely that Rho can regulate the activity of moesin at least by regulating its phosphorylation state through MBS and Rho-kinase.
Acknowledgments
We thank Dr. Alan Hall for providing pGEX-C3, Dr. Masaaki Ito for providing chicken gizzard myosin phosphatase and rabbit anti-MBS Ab, Dr. Sachiko Tsukita and Dr. Shoichiro Tsukita for providing MDCK cells, C-moesin, rat anti–moesin mAb (M22), mouse anti–moesin mAb (CR22), and cDNA of mouse moesin, and Dr. Masaki Inagaki for valuable discussions. We are also grateful to Akemi Takemura for secretarial assistance and Nagatoki Kinoshita for technical assistance.
Abbreviations used in this paper
- aa
amino acids
- A-PMSF
(p-amidinophenyl)-methanesulfonyl fluoride
- C-moesin
COOH-terminal domain of moesin
- ERM
ezrin, radixin, and moesin
- GDI
GDP dissociation inhibitor
- GST
glutathione-S-transferase
- HA
hemagglutinin
- MBS
myosin-binding subunit
- MLC
myosin light chain
- 4,5-PIP2
phosphatidylinositol 4,5-diphosphate
- PI5-kinase
phosphatidylinositol 5-kinase
- Rho-kinase
Rho-associated kinase
- TPA
tetradecanoylphorbol-13-acetate
Footnotes
This investigation was supported by grants-in-aid for Scientific Research and for Cancer Research from the Ministry of Education, Science, and Culture, Japan (1997) and by grants from the Mitsubishi Foundation and Kirin Brewery Company Limited.
Address all correspondence to Kozo Kaibuchi, M.D. and Ph.D., Division of Signal Transduction, Nara Institute of Science and Technology, 8916-5 Takayama, Ikoma 630-0101, Japan, Tel.: 81-743-72-5440. Fax: 81-743-72-5449. E-mail: kaibuchi@bs.aist-nara.ac.jp
References
- Algrain M, Turunen O, Vaheri A, Louvard D, Arpin M. Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J Cell Biol. 1993;120:129–139. doi: 10.1083/jcb.120.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996a;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Amano M, Mukai H, Ono Y, Chihara K, Matsui T, Hamajima Y, Okawa K, Iwamatsu A, Kaibuchi K. Identification of a putative target for Rho as a serine-threonine kinase, PKN. Science. 1996b;271:648–650. doi: 10.1126/science.271.5249.648. [DOI] [PubMed] [Google Scholar]
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- Amano, M., K. Chihara, N. Nakamura, Y. Fukata, T. Yano, M. Shibata, M. Ikebe, and K. Kaibuchi. 1998. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells. In press. [DOI] [PubMed]
- Amieva MR, Wilgenbus KK, Furthmayr H. Radixin is a component of hepatocyte microvilli in situ. Exp Cell Res. 1994;210:140–144. doi: 10.1006/excr.1994.1021. [DOI] [PubMed] [Google Scholar]
- Arpin M, Algrain M, Louvard D. Membrane-actin microfilament connections: an increasing diversity of players related to band 4.1. Curr Opin Cell Biol. 1994;6:136–141. doi: 10.1016/0955-0674(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Berryman M, Franck Z, Bretscher A. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells, whereas moesin is found primarily in endothelial cells. J Cell Sci. 1993;105:1025–1043. doi: 10.1242/jcs.105.4.1025. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell–cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. Purification of an 80,000-dalton protein that is a component of the isolated microvillus cytoskeleton, and its localization in nonmuscle cells. J Cell Biol. 1983;97:425–432. doi: 10.1083/jcb.97.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey H. Cell shape determination: a pivotal role for Rho. Science. 1996;272:224–225. doi: 10.1126/science.272.5259.224. [DOI] [PubMed] [Google Scholar]
- Chihara K, Amano M, Nakamura N, Yano T, Shibata M, Tokui T, Ichikawa H, Ikebe R, Ikebe M, Kaibuchi K. Cytoskeletal rearrangements and transcriptional activation of c-fosserum response element by Rho-kinase. J Biol Chem. 1997;272:25121–25127. doi: 10.1074/jbc.272.40.25121. [DOI] [PubMed] [Google Scholar]
- Chong LD, Traynor-Kaplan A, Bokoch GM, Schwartz MA. The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell. 1994;79:507–513. doi: 10.1016/0092-8674(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Dransfield DT, Bradford AJ, Smith J, Martin M, Roy C, Mangeat PH, Goldenring JR. Ezrin is a cyclic AMP-dependent protein kinase anchoring protein. EMBO (Eur Mol Biol Organ) J. 1997;16:35–43. doi: 10.1093/emboj/16.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary R, Bretscher A. Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site. Mol Biol Cell. 1995;6:1061–1075. doi: 10.1091/mbc.6.8.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano KA, Taylor DL. Measurement and manipulation of cytoskeletal dynamics in living cells. Curr Opin Cell Biol. 1995;7:4–12. doi: 10.1016/0955-0674(95)80038-7. [DOI] [PubMed] [Google Scholar]
- Gong MC, Iizuka K, Nixon G, Browne JP, Hall A, Eccleston JF, Sugai M, Kobayashi S, Somlyo AV, Somlyo AP. Role of guanine nucleotide-binding proteins—ras-family or trimeric proteins or both—in Ca2+sensitization of smooth muscle. Proc Natl Acad Sci USA. 1996;93:1340–1345. doi: 10.1073/pnas.93.3.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow, E., and D. Lane. 1988. Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 471–510.
- Hartshorne, D.J. 1987. Biochemistry of the contractile process in smooth muscle. In Physiology of the Gastrointestinal Tract. D.R. Johnson, editor. Raven Press, New York. 423–482.
- Haynes BF, Telen MJ, Hale LP, Denning SM. CD44—a molecule involved in leukocyte adherence and T-cell activation [published erratum appears 11(3):80] Immunol Today. 1989;10:423–428. doi: 10.1016/0167-5699(89)90040-6. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Liao HX, Patton KL. The transmembrane hyaluronate receptor (CD44): multiple functions, multiple forms. Cancer Cells. 1991;3:347–350. [PubMed] [Google Scholar]
- Hill CS, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- Hirao M, Sato N, Kondo T, Yonemura S, Monden M, Sasaki T, Takai Y, Tsukita S, Tsukita S. Regulation mechanism of ERM (Ezrin/Radixin/Moesin) protein/plasma membrane association: Possible involvement of phosphatidylinositol turnover and rho-dependent signaling pathway. J Cell Biol. 1996;135:37–51. doi: 10.1083/jcb.135.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata K, Kikuchi A, Sasaki T, Kuroda S, Kaibuchi K, Matsuura Y, Seki H, Saida K, Takai Y. Involvement of rho p21 in the GTP-enhanced calcium ion sensitivity of smooth muscle contraction. J Biol Chem. 1992;267:8719–8722. [PubMed] [Google Scholar]
- Inagaki N, Nishizawa M, Ito M, Fujioka M, Nakano T, Tsujino S, Matsuzawa K, Kimura K, Kaibuchi K, Inagaki M. Myosin binding subunit of smooth muscle myosin phosphatase at the cell-cell adhesion sites in MDCK cells. Biochem Biophys Res Commun. 1997;230:552–556. doi: 10.1006/bbrc.1996.5986. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160 kD Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO (Eur Mol Biol Organ) J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 1997;404:118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–603. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Kuroda S, Sasaki T, Kotani K, Hirata K, Katayama M, Takai Y. Functional interactions of stimulatory and inhibitory GDP/GTP exchange proteins and their common substrate small G protein. J Biol Chem. 1992;267:14611–14615. [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kishi K, Sasaki T, Kuroda S, Itoh T, Takai Y. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) J Cell Biol. 1993;120:1187–1195. doi: 10.1083/jcb.120.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani H, Takaishi K, Sasaki T, Takai Y. Rho regulates association of both the ERM family and vinculin with the plasma membrane in MDCK cells. Oncogene. 1997;14:1705–1713. doi: 10.1038/sj.onc.1200998. [DOI] [PubMed] [Google Scholar]
- Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, Kaibuchi K, Ito M. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1997;272:12257–12260. doi: 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lankes W, Griesmacher A, Grunwald J, Schwartz-Albiez R, Keller R. A heparin-binding protein involved in inhibition of smooth-muscle cell proliferation. Biochem J. 1988;251:831–842. doi: 10.1042/bj2510831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J, Hyman R, Kincade PW. CD44 and its interaction with extracellular matrix. Adv Immunol. 1993;54:271–335. doi: 10.1016/s0065-2776(08)60537-4. [DOI] [PubMed] [Google Scholar]
- Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem. 1995;270:29051–29054. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROKα is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi I, Hamaguchi Y, Fujimoto H, Morii N, Mishima M, Narumiya S. A rho-like protein is involved in the organization of the contractile ring in dividing sand dollar eggs. Zygote. 1993;1:325–331. doi: 10.1017/s0967199400001659. [DOI] [PubMed] [Google Scholar]
- Madaule P, Furuyashiki T, Reid T, Ishizaki T, Watanabe G, Morii N, Narumiya S. A novel partner for the GTP-bound forms of rho and rac. FEBS Lett. 1995;377:243–248. doi: 10.1016/0014-5793(95)01351-2. [DOI] [PubMed] [Google Scholar]
- Martin M, Andreoli C, Sahuquet A, Montcourrier P, Algrain M, Mangeat P. Ezrin NH2-terminal domain inhibits the cell extension activity of the COOH-terminal domain. J Cell Biol. 1995;128:1081–1093. doi: 10.1083/jcb.128.6.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, M., C. Roy, P. Montcourrier, A. Sahuquet, P. and Mangeat. 1997 Three determinants in ezrin are responsible for cell extension activity. Mol. Biol. Cell. 8:1543–1557. [DOI] [PMC free article] [PubMed]
- Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for the small GTP-binding protein Rho. EMBO (Eur Mol Biol Organ) J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S, Tsukita S. Rho-kinase phosphorylates carboxy-terminal threonines of ERM proteins and regulates their head-to-tail association. J Cell Biol. 1998;140:647–658. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura Y, Possee RD, Overton HA, Bishop DH. Baculovirus expression vectors: the requirements for high level expression of proteins, including glycoproteins. J Gen Virol. 1987;68:1233–1250. doi: 10.1099/0022-1317-68-5-1233. [DOI] [PubMed] [Google Scholar]
- Nakamura F, Amieva MR, Furthmayr H. Phosphorylation of threonine 558 in the carboxyl-terminal actin-binding domain of moesin by thrombin activation of human platelets. J Biol Chem. 1995;270:31377–31385. doi: 10.1074/jbc.270.52.31377. [DOI] [PubMed] [Google Scholar]
- Nakamura F, Amieva MR, Hirota C, Mizuno Y, Furthmayr H. Phosphorylation of 558T of moesin detected by site-specific antibodies in RAW264.7 macrophages. Biochem Biophys Res Commun. 1996;226:650–656. doi: 10.1006/bbrc.1996.1410. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Sasaki T, Takaishi K, Kato M, Yaku H, Araki K, Matsuura Y, Takai Y. rac p21 is involved in insulin-induced membrane ruffling and rho p21 is involved in hepatocyte growth factor- and 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced membrane ruffling in KB cells. Mol Cell Biol. 1994;14:2447–2456. doi: 10.1128/mcb.14.4.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes C, Hall A. Regulation and function of the Rho subfamily of small GTPases. Curr Opin Genet Dev. 1994;4:77–81. doi: 10.1016/0959-437x(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Pakkanen R, Hedman K, Turunen O, Wahlstrom T, Vaheri A. Microvillus-specific Mr 75,000 plasma membrane protein of human choriocarcinoma cells. J Histochem Cytochem. 1987;35:809–816. doi: 10.1177/35.8.3298422. [DOI] [PubMed] [Google Scholar]
- Paterson HF, Self AJ, Garrett MD, Just I, Aktories K, Hall A. Microinjection of recombinant p21rho induces rapid changes in cell morphology. J Cell Biol. 1990;111:1001–1007. doi: 10.1083/jcb.111.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestonjamasp K, Amieva MR, Strassel CP, Nauseef WM, Furthmayr H, Luna EJ. Moesin, ezrin, and p205 are actin-binding proteins associated with neutrophil plasma membranes. Mol Biol Cell. 1995;6:247–259. doi: 10.1091/mbc.6.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid T, Furuyashiki T, Ishizaki T, Watanabe G, Watanabe N, Fujisawa K, Morii N, Madaule P, Narumiya S. Rhotekin, a new putative target for Rho bearing homology to a serine/threonine kinase, PKN, and rhophilin in the rho-binding domain. J Biol Chem. 1996;271:13556–13560. doi: 10.1074/jbc.271.23.13556. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. Signal transduction pathways regulating Rho-mediated stress fibre formation: requirement for a tyrosine kinase. EMBO (Eur Mol Biol Organ) J. 1994;13:2600–2610. doi: 10.1002/j.1460-2075.1994.tb06550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy C, Martin M, Mangeat P. A dual involvement of the amino-terminal domain of ezrin in F- and G-actin binding. J Biol Chem. 1997;272:20088–20095. doi: 10.1074/jbc.272.32.20088. [DOI] [PubMed] [Google Scholar]
- Sato N, Yonemura S, Obinata T, Tsukita S, Tsukita S. Radixin, a barbed end-capping actin-modulating protein, is concentrated at the cleavage furrow during cytokinesis [published erratum appears 114:1101–1103] J Cell Biol. 1991;113:321–330. doi: 10.1083/jcb.113.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Funayama N, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites. J Cell Sci. 1992;103:131–143. doi: 10.1242/jcs.103.1.131. [DOI] [PubMed] [Google Scholar]
- Sellers, J.R., and R.S. Adelstein. 1987. Regulation of contractile activity. In The Enzymes. P. Boyer and E.G. Erevs, editors. Academic Press, San Diego, CA. 381–418.
- Shimizu H, Ito M, Miyahara M, Ichikawa K, Okubo S, Konishi T, Naka M, Tanaka T, Hirano K, Hartshorne DJ, Nakano T. Characterization of the myosin-binding subunit of smooth muscle myosin phosphatase. J Biol Chem. 1994;269:30407–30411. [PubMed] [Google Scholar]
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Sasaki T, Mammoto A, Takaishi K, Kameyama T, Tsukita S, Tsukita S, Takai Y. Direct interaction of the Rho GDP dissociation inhibitor with Ezrin/Radixin/Moesin initiates the activation of the Rho small G protein. J Biol Chem. 1997;272:23371–23375. doi: 10.1074/jbc.272.37.23371. [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Tanaka K, Nakanishi H. Rho as a regulator of the cytoskeleton. Trends Biochem Sci. 1995;20:227–231. doi: 10.1016/s0968-0004(00)89022-2. [DOI] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kato M, Yamochi W, Kuroda S, Nakamura T, Takeichi M, Takai Y. Involvement of Rho p21 small GTP-binding protein and its regulator in the HGF-induced cell motility. Oncogene. 1994;9:273–279. [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kameyama T, Tsukita S, Tsukita S, Takai Y. Translocation of activated Rho from the cytoplasm to membrane ruffling area, cell-cell adhesion sites and cleavage furrows. Oncogene. 1995;11:39–48. [PubMed] [Google Scholar]
- Takeshima H, Izawa I, Lee PS, Safdar N, Levin VA, Saya H. Detection of cellular proteins that interact with the NF2 tumor suppressor gene product. Oncogene. 1994;9:2135–2144. [PubMed] [Google Scholar]
- Takeuchi K, Sato N, Kasahara H, Funayama N, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Perturbation of cell adhesion and microvilli formation by antisense oligonucleotides to ERM family members. J Cell Biol. 1994;125:1371–1384. doi: 10.1083/jcb.125.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga T, Sugie K, Hirata M, Morii N, Fukata J, Uchida A, Imura H, Narumiya S. Inhibition of PMA-induced, LFA-1-dependent lymphocyte aggregation by ADP ribosylation of the small molecular weight GTP binding protein, rho. J Cell Biol. 1993;120:1529–1537. doi: 10.1083/jcb.120.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Hieda Y, Tsukita S. A new 82-kD barbed end-capping protein (radixin) localized in the cell- to-cell adherens junction: purification and characterization. J Cell Biol. 1989;108:2369–2382. doi: 10.1083/jcb.108.6.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Tsukita S, Nagafuchi A, Yonemura S. Molecular linkage between cadherins and actin filaments in cell-cell adherens junctions. Curr Opin Cell Biol. 1992;4:834–839. doi: 10.1016/0955-0674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Oishi K, Sato N, Sagara J, Kawai A, Tsukita S. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol. 1994;126:391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen O, Wahlstrom T, Vaheri A. Ezrin has a COOH-terminal actin-binding site that is conserved in the ezrin protein family. J Cell Biol. 1994;126:1445–1453. doi: 10.1083/jcb.126.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe G, Saito Y, Madaule P, Ishizaki T, Fujisawa K, Morii N, Mukai H, Ono Y, Kakizuka A, Narumiya S. Protein kinase N (PKN) and PKN-related protein rhophilin as targets of small GTPase Rho. Science. 1996;271:645–648. doi: 10.1126/science.271.5249.645. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Madaule P, Reid T, Ishizaki T, Watanabe G, Kakizuka A, Saito Y, Nakao K, Jockusch BM, Narumiya S. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO (Eur Mol Biol Organ) J. 1997;16:3044–3056. doi: 10.1093/emboj/16.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]