Abstract
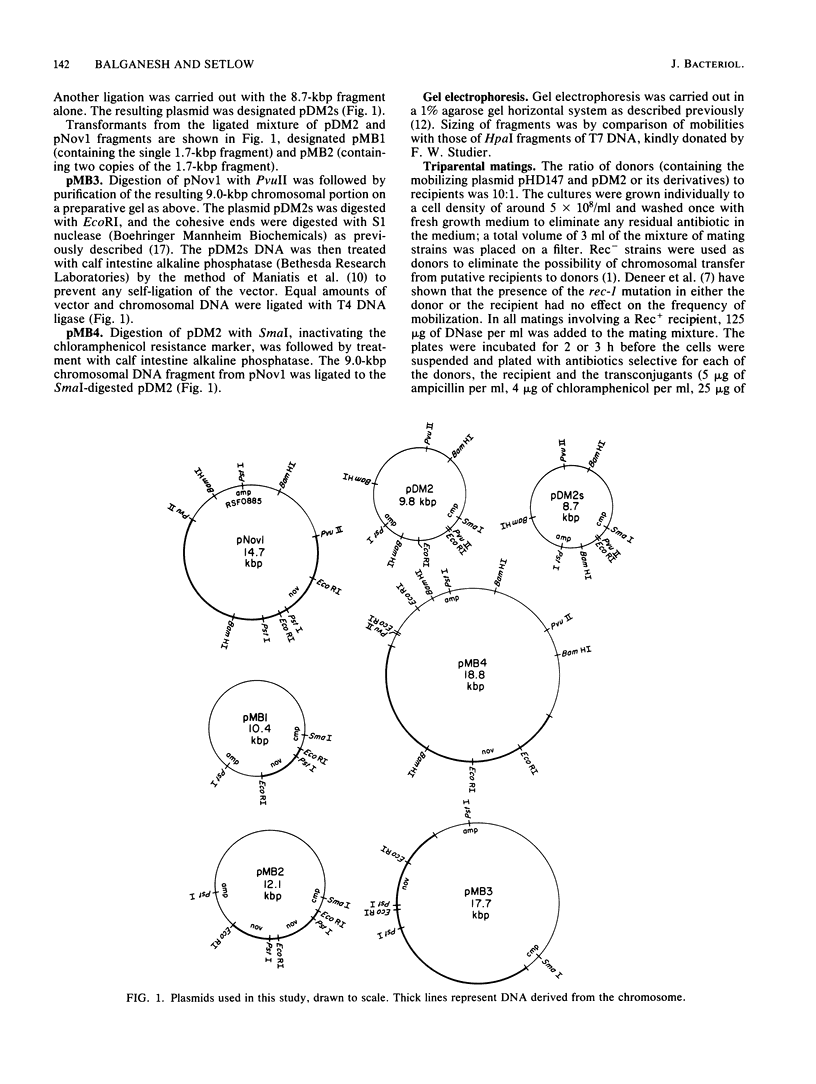
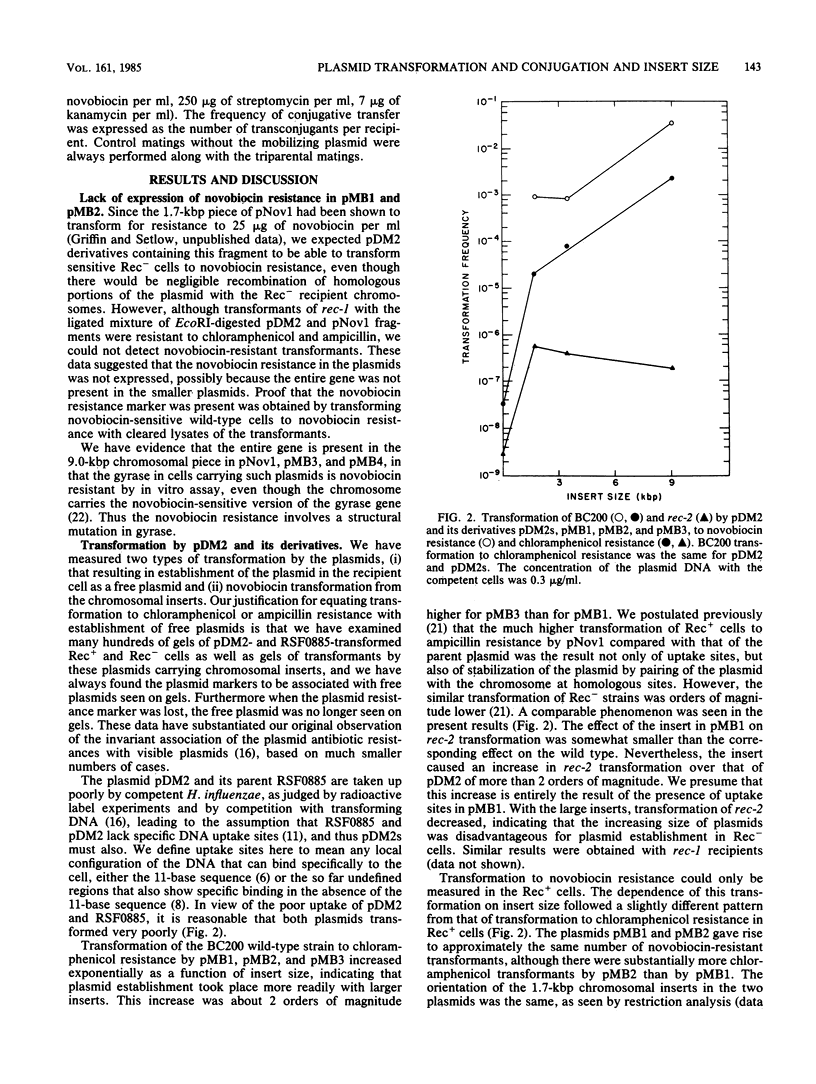
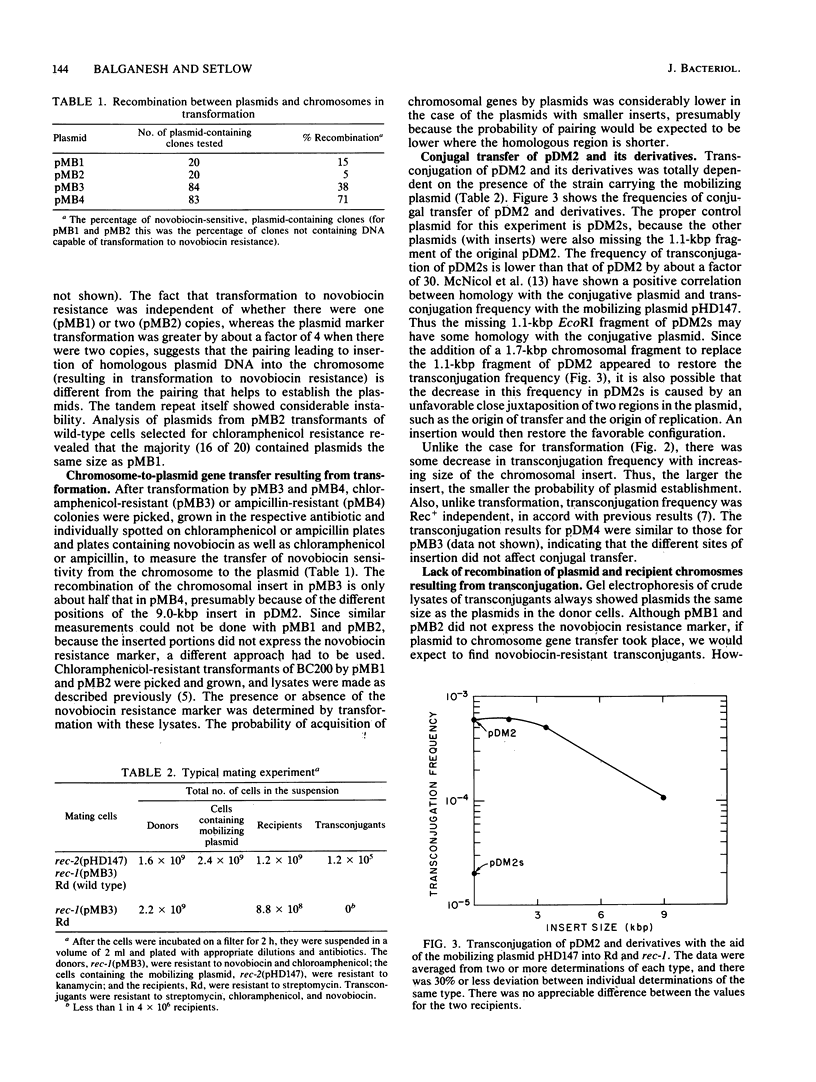
Plasmids with chromosomal insertions were constructed by removal of a 1.1-kilobase-pair piece from the 9.8-kilobase-pair vector plasmid pDM2 by EcoRI digestion and inserting in its place various lengths of chromosomal DNA (1.7, 3.4, and 9.0 kilobase pairs) coding for resistance to novobiocin. A fourth plasmid was constructed by insertion of the largest piece of chromosomal DNA into the SmaI site of pDM2. The plasmids without inserts were taken up poorly by competent cells and thus were considered not to contain specific DNA uptake sites. The presence of even the smallest insert of chromosomal DNA caused a large increase in transformation of Rec+ and Rec- strains. The frequency of plasmid establishment in Rec+ cells by transformation increased exponentially with increasing insert size, but in Rec- cells there was less transformation by the larger plasmids. Conjugal transfer of these plasmids was carried out with the 35-kilobase-pair mobilizing plasmid pHD147. The frequency of establishment of plasmids by this method not only was not markedly affected by the presence of the insertions, but also decreased somewhat with increase in insert size and was independent of rec-1 and rec-2 genes. Recombination between plasmid and chromosome was readily detected after transformation, but could not be detected after transconjugation even when the recipient cells were Rec+ and made competent. These data suggested that there is a special processing of plasmid DNA that enters the competent cells in transformation that makes possible recombination of homologous regions of the plasmid with the chromosome and pairing with the chromosome that aids plasmid establishment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton W. L., Setlow J. K., Slaney L. Transfer of Haemophilus influenzae chromosomal genes by cell-to-cell contact. J Bacteriol. 1982 Dec;152(3):1066–1070. doi: 10.1128/jb.152.3.1066-1070.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barany F., Kahn M. E., Smith H. O. Directional transport and integration of donor DNA in Haemophilus influenzae transformation. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7274–7278. doi: 10.1073/pnas.80.23.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart B. J., Cox S. H. Radiation-sensitive and radiation-resistant mutants of Haemophilus influenzae. J Bacteriol. 1968 Jul;96(1):280–282. doi: 10.1128/jb.96.1.280-282.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie K. L., Setlow J. K. Repair of ultraviolet-irradiated transforming deoxyribonucleic acid in Haemophilus influenzae. J Bacteriol. 1970 Mar;101(3):808–812. doi: 10.1128/jb.101.3.808-812.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner D. B., Deich R. A., Sisco K. L., Smith H. O. An eleven-base-pair sequence determines the specificity of DNA uptake in Haemophilus transformation. Gene. 1980 Nov;11(3-4):311–318. doi: 10.1016/0378-1119(80)90071-2. [DOI] [PubMed] [Google Scholar]
- Deneer H. G., Slaney L., Maclean I. W., Albritton W. L. Mobilization of nonconjugative antibiotic resistance plasmids in Haemophilus ducreyi. J Bacteriol. 1982 Feb;149(2):726–732. doi: 10.1128/jb.149.2.726-732.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodgal S. H. DNA uptake in Haemophilus transformation. Annu Rev Genet. 1982;16:169–192. doi: 10.1146/annurev.ge.16.120182.001125. [DOI] [PubMed] [Google Scholar]
- McCarthy D., Clayton N. L., Setlow J. K. A plasmid cloning vehicle for Haemophilus influenzae and Escherichia coli. J Bacteriol. 1982 Sep;151(3):1605–1607. doi: 10.1128/jb.151.3.1605-1607.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- McNicol P. J., Albritton W. L., Ronald A. R. Characterization of ampicillin resistance plasmids of Haemophilus ducreyi and Neisseria gonorrhoeae with regard to location of origin of transfer and mobilization by a conjugative plasmid of Haemophilus ducreyi. J Bacteriol. 1983 Oct;156(1):437–440. doi: 10.1128/jb.156.1.437-440.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K., Joshi V. R., Allison D. P. Molecular basis for the transformation defects in mutants of Haemophilus influenzae. J Bacteriol. 1972 Jun;110(3):1171–1180. doi: 10.1128/jb.110.3.1171-1180.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K., McCarthy D., Clayton N. L. Transformation of Haemophilus influenzae by plasmid RSF0885. J Bacteriol. 1981 Dec;148(3):812–816. doi: 10.1128/jb.148.3.812-816.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani N., Goodgal S. H. On the nature of recombinants formed during transformation in Hemophilus influenzae. J Gen Physiol. 1966 Jul;49(6):197–209. doi: 10.1085/jgp.49.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick B., Setlow J. K. Single-stranded regions in transforming deoxyribonucleic acid after uptake by competent Haemophilus influenzae. J Bacteriol. 1976 Feb;125(2):588–594. doi: 10.1128/jb.125.2.588-594.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Beattie K. L., Kimball R. F. A complex of recombination and repair genes in Haemophilus influenzae. J Mol Biol. 1972 Jul 21;68(2):361–378. doi: 10.1016/0022-2836(72)90218-5. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Notani N. K., McCarthy D., Clayton N. L. Transformation of Haemophilus influenzae by plasmid RSF0885 containing a cloned segment of chromosomal deoxyribonucleic acid. J Bacteriol. 1981 Dec;148(3):804–811. doi: 10.1128/jb.148.3.804-811.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Spikes D., Ledbetter M. Loss of plasmids containing cloned inserts coding for novobiocin resistance or novobiocin sensitivity in Haemophilus influenzae. J Bacteriol. 1984 Jun;158(3):872–877. doi: 10.1128/jb.158.3.872-877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Danner D. B., Deich R. A. Genetic transformation. Annu Rev Biochem. 1981;50:41–68. doi: 10.1146/annurev.bi.50.070181.000353. [DOI] [PubMed] [Google Scholar]
- Steinhart W. L., Herriott R. M. Fate of recipient deoxyribonucleic acid during transformation in Haemophilus influenzae. J Bacteriol. 1968 Nov;96(5):1718–1724. doi: 10.1128/jb.96.5.1718-1724.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H. Plasmid transfer in Haemophilus influenzae. J Bacteriol. 1979 Aug;139(2):520–529. doi: 10.1128/jb.139.2.520-529.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne G. M., Farrar W. E., Jr Transfer of ampicillin resistance between strains of Haemophilus influenzae type B. J Infect Dis. 1975 Sep;132(3):276–281. doi: 10.1093/infdis/132.3.276. [DOI] [PubMed] [Google Scholar]



