Abstract
M-30 and 4HNE adducts are two markers of active liver disease. M-30 is a serologic marker and 4HNE adducts are histologic markers. M-30 is a marker for apoptosis because it is a fragment of cytokeratin-18 left over from proteolysis by caspase 3. 4HNE is a marker of oxidative stress because it results from lipid peroxidation. Both markers are commonly found in nonalcoholic steatohepatitis and in alcoholic hepatitis. Liver biopsies from patients with steatohepatitis, 11 alcoholic and 11 non-alcoholics were stained for 4HNE and M-30. Almost all of the biopsies in both groups showed 4HNE and M-30 positive aggresomes in hepatocytes. Mallory Denk bodies (MDB) stained variably positive for M-30, whereas 4HNE was present in aggresomes independent of MDBs. However, they were sometimes located in hepatocytes which also contained MDBs as shown by confocal microscopy of double stained biopsies. The results indicate that the formation of M-30 and 4HNE aggresomes occurs through different pathways of liver cell injury in both types of steatohepatitis.
Keywords: Caspase 3, oxidative stress, steatohepatitis, Mallory Denk bodies, aggresomes
INTRODUCTION
Two markers of activity, M-30 and 4-hydroxynonenal (4 HNE) adducts, have been observed in nonalcoholic and alcoholic steatohepatitis (NASH) and (ASH) in prior studies. 4HNE adducts were visualized in liver biopsies by immunohistochemical stains in ASH (1) and NASH (2). 4HNE is a reliable marker of lipid peroxidation (3) and both malondialdehyde and 4HNE are increased in the liver of rats fed ethanol chronically intragastrically (4–7). In human biopsies 4HNE adducts were found in 78.3% of fatty liver and 100% of NASH patients (2). 4HNE adducts were located in the cytoplasm of hepatocytes in NASH as well as in sinusoidal cells, mainly in Zone 3. In ASH, 92.5% of biopsies had 4 HNE adducts, when 4HNE adducts were located in the hepatocytic cytoplasm and sinusoids. 4HNE adducts were associated significantly with MDBs (1). The question here is are 4HNE adducts found in Mallory bodies (MDBs) and were they found in macrophages?
Elevated levels of M-30 have been found in the serum of patients with NASH and acute hepatic injury (8–10) but have not been reported in biopsies of ASH. M30 has not been reported in the liver biopsies of either NASH or ASH. M-30 is a degradation product of cytokeratin 18 in liver cells (11–14) and it is derived from cleavage of CK18 by caspase 3 and 7 during the early stages of apoptosis (15). Antibodies to M-30 label early apoptotic cells (16). The presence of M30 in serum was first reported in hepatitis C (17). It is not clear whether M-30 antigen aggregate occurs in MDBs in NASH and ASH livers. In the present study, ASH and ASH liver biopsies were stained for the presence of 4HNE and MDBs. The results showed that M-30 localized in MDBs. 4HNE adducts were localized in aggresomes and phagasomes, but not in MDBs.
Method
Archived liver biopsies from patients with active ASH and NASH from Harbor-UCLA Medical Center and the Department of Medicine were collected over a 15 year period. The liver biopsies from UC Davis were collected upon enrollment of alcoholic patients with clinical features of active ASH in a prospective study. They were fixed in zinc formalin buffered at pH 7.4. Four-micron thick sections were cut from zinc formalin-fixed, paraffin-embedded liver biopsies and mounted on poly-L-lysine-coated slides. The sections were deparaffinized in xylenes and hydrated gradually through graded alcohols. They were washed in 100%, 95% and 70% ethanol for 5 minutes each, and finally washed in PBS pH 7.4. Antigen retrieval was done treating the slides with CITRA (Biogenex, San Ramon, CA), and microwaving them. They were blocked with 5% normal second antibody host serum.
4HNE and ubiquitin double staining was performed using primary antibodies; mouse and rabbit. The slides were incubated with 4HNE mouse monoclonal antibody (1:4 dilution) (Jaica, Japan), overnight at 4ºC. The binding of this antibody to the antigen was detected using fluorescein (FITC) - conjugated donkey anti mouse (1:100 dilution) (Jackson immunoresearch, West Grove, PA, USA) for one hour at room temperature. The sections were then incubated with polyclonal rabbit anti-ubiquitin antibody (1:50 dilution) (DakoCytomation, Switzerland) (1:50 dilution) overnight at 4ºC. The signal was detected by Texas red donkey anti rabbit antibody (1:100 dilution) (Jackson immunoresearch, West Grove, PA, USA) for one hour at room temperature. DAPI was used to stain the nuclei. The slides were examined using a Nikon 400 fluorescent microscope with a triple color band cube to detect simultaneously FITC for 4HNE, Texas red for ubiquitin and DAPI for nuclei. A yellow fluorescence indicated co localization of the two different antigens. Selected cases were visualized using a Leica confocal microscope.
M30 was detected by the avidin-biotin-peroxidase complex (ABC) method. The slides were treated in the same way as the 4HNE and ubiquitin slides, except that 3% hydrogen peroxide in methanol was used in order to block endogenous peroxidase activity before incubating the slides with primary antibody. The slides were incubated with mouse monoclonal anti M30 (1:50 dilution) (Roche, Germany) overnight at 4ºC. They were then incubated with a biotin-conjugated donkey anti-mouse antibody (1:100 dilution) (Jackson Immunoresearch, West Grove, PA, USA) for one hour at room temperature.
Biotin-streptavidin peroxidase binding was performed by adding streptavidin/HRP (Dako cytomation, Denmark) (1:50 dilution) for 30 minutes at room temperature. The brownish signal was developed using a peroxidase substrate kit (Vector, Burlingame, CA). The slides were counterstained with hematoxylin, dehydrated and mounted. The stained slides were examined under light microscopy for immunoperoxidase staining.
RESULTS
M30 and 4HNE aggresomes had different locations in the liver cell cytoplasm. M-30 was located within MDBs as well as in smaller aggresomes (Fig. 1). MDBs were found in 100% of ASH biopsies and stained positive for the presence of M-30 in 100% (Table 1). Liver cytoplasmic aggresomes were found in 45% of ASH cases and 18% were in macrophage secondary lysosomes of the ASH cases (Table I).
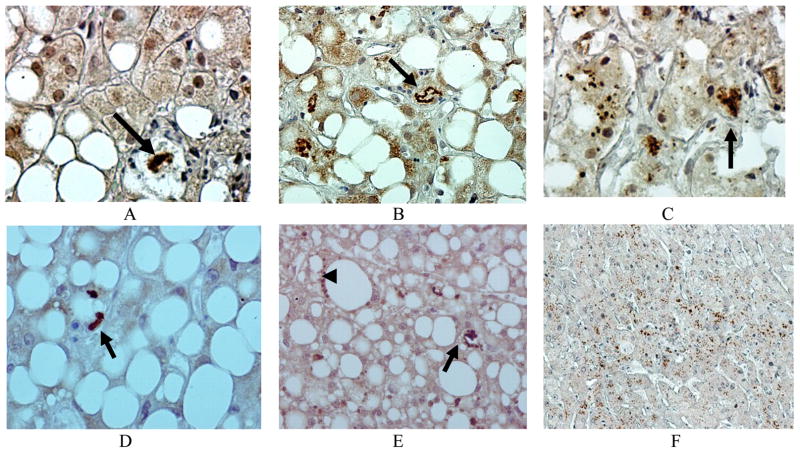
Fig 1.
M-30 was present in MBs (arrows) (A-E) and in smaller aggresomes (C) in ASH patients’ liver biopsies compared to the control (F). Immunoperoxidase stain. ×260 (F), ×520 (B,E), ×780 (A, C, D).
Table I.
Summary of Results
| Number of Biopsies | MB present % | MB positive % | Liver Cell Aggresomes % | Macrophage 2º Lysosome % | |
|---|---|---|---|---|---|
| 4HNE-ASH | 9 | 88.8 | 0 | 55.5 | 55.5 |
| 4HNE-NASH | 11 | 63.6 | 0 | 72.7 | 54.5 |
| M30-ASH | 11 | 100 | 100 | 45 | 18 |
| M30-NASH | 10 | 40 | 100 | 40 | 30 |
MDBs were present in 40% of NASH biopsies and all the MDBs stained for M30 (Fig 2, Table 1). M30 was also found in the liver cell cytoplasm in small aggresomes in 40% of the biopsies. It was also in macrophages in scars and in the Kupffer cells in 30% of biopsies (Fig 2, Table 1).
Fig 2.
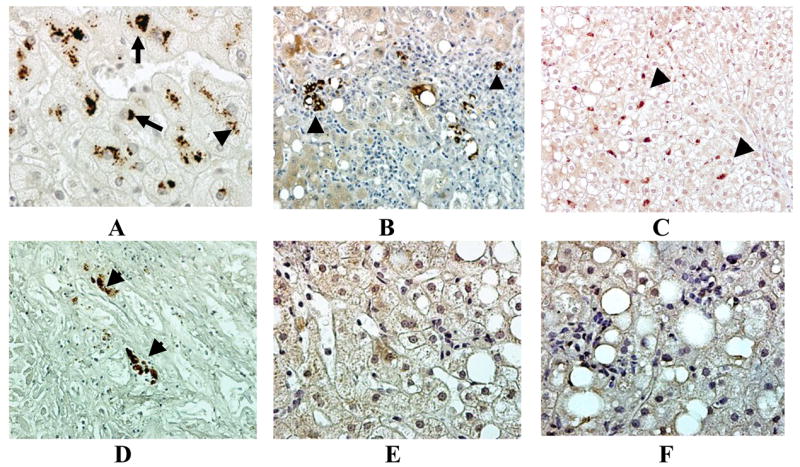
M-30 was present in MDBs (arrows) in NASH patients’ liver biopsies (A). M-30 stained aggresomes were present in hepatocytes and macrophages (arrow heads) (A-D). Some livers were negative for any positive staining for M30 in the presence of steatosis (E) or steatohepatitis (F). Immunoperoxidase ×260 (B, C, D), ×520 (A, E, F).
4HNE was found in aggresomes in the cytoplasm of 55.1% hepatocytes and in secondary lysosomes in 55.5% of ASH patient biopsies (Fig 3, Table 1). MDBs were unstained by the antibody to 4HNE in ASH biopsies as determined by Confocal microscopy (Fig. 3, Table 1).
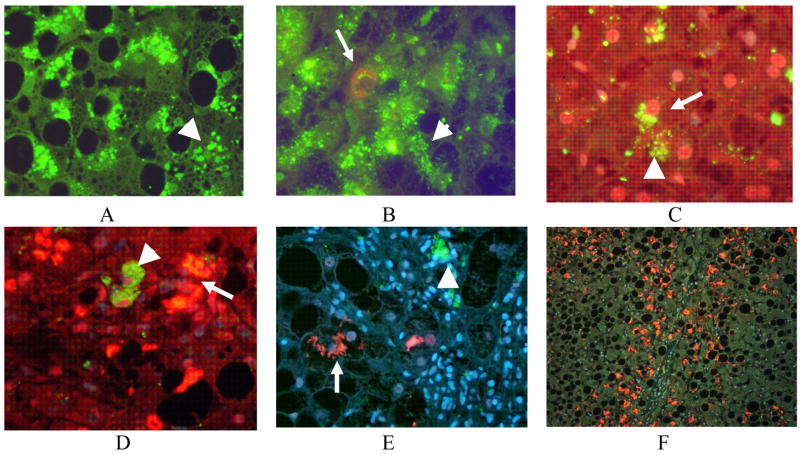
Fig 3.
4HNE positive aggresomes were found in hepatocytic cytoplasm of ASH biopsies (arrow) (A-C). MDBs stained negative with the 4HNE antibody where they were formed in ASH (A). This was confirmed by confocal microscopy (E-F). 4HNE aggresomes were also found in macrophages in secondary lysosomes (arrow head) A, (arrows) (C). ×218(B), ×436 (C-D), ×654 (A), ×1090 (E-G).
4HNE was found in liver cell cytoplasmic aggresomes in 72.7% of NASH patients (Fig 4, Table 1). 4HNE aggresomes were numerous, small and closely packed within the liver cell cytoplasm. MDBs did not stain for 4HNE (Fig 4). Some liver biopsies showed numerous MDBs and 4HNE aggresomes (Fig 4). Macrophages contained secondary lysosomes that stained positive for 4HNE in 54.5% of NASH patient biopsies (Fig. 4, Table I).
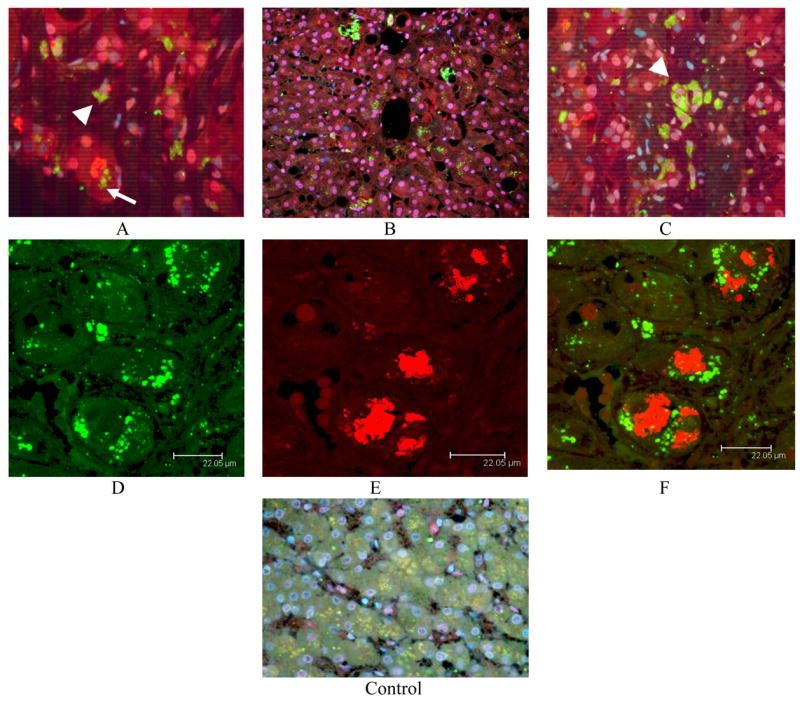
Fig 4.
4HNE positive aggresomes were found in hepatocytic cytoplasm in the NASH patient biopsies (arrow heads) A and B and (arrow) C. MDBs failed to stain positive for 4HNE (arrow) B, D-F. Macrophages contained 4HNE positive secondary lysosomes in scarred areas (arrow head) C-D. Numerous MBs were found in the absence of 4HNE aggresomes in some cases (F). Immunocytochemistry stain (x130 F, ×520 A, B, E, ×780, C, D).
DISCUSSION
There were no obvious differences between NASH and ASH pathology using the two parameters measured. M-30 was found in MDBs, liver cell aggresomes and secondary lysosomes in macrophages in both diseases. 4HNE was found in liver cell aggresomes and secondary lysosomes in macrophages in both diseases. 4HNE was absent in MDBs in both. The implications are that oxidative stress and apoptosis are present in both ASH and NASH and that 4HNE adducts are not always associated with MDB formation.
M30 antigen, a product of early apoptosis, was found in both aggresomes and MDBs. M30 was found in secondary lysosomes in macrophages in the majority of the liver biopsies from ASH and NASH patients. Apoptosis was frequently found in both ASH (18–20) and NASH (21) biopsies. An increase in M30 antigen in the serum is used as a biomarker of liver disease severity in NASH and fulminating hepatitis (9–10).
The present study determined that M30 was localized in both MDBs and separate aggresomes of liver cells. The implication of this observation is that M30 a degraded protein, is incorporated into aggresomes. M30 it is a peptide that results from proteolysis. It persists in aggresomes as an insoluble protein fragment which has survived because the liver cells failed to complete the apoptotic process.
The question regarding the location of 4HNE adducts in liver cells and macrophages, was answered by the finding that 4HNE was in both liver aggresomes and in secondary lysosomes in macrophages when 4HNE forms adducts, which interfere with the function of the adducted proteins (22, 23).
Adducts form with proteasome subunits and inhibit proteolysis by the proteasome in experimental alcoholic liver disease (22). The finding that 4HNE adducts are sequestered in aggresomes and secondary lysosomes implies that 4HNE adducts are insoluble and resist proteolysis by the proteasome in ASH and NASH.
Acknowledgments
The authors appreciate Adriana Flores who typed the manuscript. This study was supported by Grants from NIH/NIAAA P50-011999 Alcoholic Center Grant on Liver and Pancreas, Morphology Core, 8116 and NIH/NIAAA R01-014562 to CHH at UC Davis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Seki S, Kitada T, Sakaguchi H, Nakatani K, Wakasa K. Pathological significance of oxidative cellular damage in human alcoholic liver disease. Histopathology. 2003;42:365–371. doi: 10.1046/j.1365-2559.2003.01599.x. [DOI] [PubMed] [Google Scholar]
- 2.Seki S, Kitada T, Yamada J, Sakaguchi H, Nakatini K, Wakasa K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J Hepatol. 2002;37:56–62. doi: 10.1016/s0168-8278(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 3.Esterbauer H, Schaur RJ, Zoliner H. Chemistry and Biochemistry of 4-hydroxynonenal, malondialdehyde and related aldehydes. Free Radical Biol Med. 1992;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 4.French SW, Wong K, Jui L, Albino E, Hagbjork AL, Ingelman-Sundberg M. Effect of ethanol on cytochrome P450 2E1 (CYP2E1), lipid peroxidation and serum protein adduct formation in relation to liver pathology pathogenesis. Exp Mol Pathol. 1993;58:61–75. doi: 10.1006/exmp.1993.1006. [DOI] [PubMed] [Google Scholar]
- 5.Li CJ, Nanji AA, Siakotos AN, Lin RC. Acetaldehyde-modified and 4-hydroxynonenal-modified proteins in the liver of rats with alcoholic liver disease. Hepatology. 1997;26:650–657. doi: 10.1002/hep.510260317. [DOI] [PubMed] [Google Scholar]
- 6.Mottaran E, Stewart SF, Rolla R, Vay D, Cipriani V, Moretti M, Vidali M, Sartori M, Rigamonti C, Day CP, Albino E. Lipid peroxidation contributors to immune reactions associated with alcoholic liver disease. Free Radical Biol Med. 2002;32:38–43. doi: 10.1016/s0891-5849(01)00757-2. [DOI] [PubMed] [Google Scholar]
- 7.Bardag-Gorce F, Li J, French BA, French SW. The effect of ethanol-induced CYP2E1 on proteasome activity: the role of 4-hydroxynonenal. Exp Mol Pathol. 2005;78:109–115. doi: 10.1016/j.yexmp.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Yimaz Y, Dolan E, Ulukaya E, Akgoz S, Keskin M, Kiyici M, Aker S, Yilmaztepe A, Gorel S, Gulten M, Nak SG. Soluble forms of extracellular cytokeratin 18 may differentiate simple steatosis from nonalcoholic steatohepatitis. World J Gastroenterol. 2007;14:837–844. doi: 10.3748/wjg.v13.i6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wieckowska A, Zern NW, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 10.Yasumi Y, Takikawa Y, Endo R, Suzuki K. Significance of a novel biomarker for hepatocellular apoptosis (Caspase 3-cleaved cytokeratin-18 product) in acute and fulminating hepatic injury. Gastroenterology. 2007;132:A87. [Google Scholar]
- 11.Ueno T, Toi M, Linder S. Detection of epithelial cell death in the body by cytokeratin 18 measurement. Biomed Pharmacother. 2005;59(Suppl 2):S359–S362. doi: 10.1016/s0753-3322(05)80078-2. [DOI] [PubMed] [Google Scholar]
- 12.Caulin C, Salversen GS, Oshima RG. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol. 1997;138:1379–1394. doi: 10.1083/jcb.138.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ku NO, Liso J, Omary MB. Apoptosis generates stable fragments of human type 1 keratins. J Biol Chem. 1997;272:33197–33203. doi: 10.1074/jbc.272.52.33197. [DOI] [PubMed] [Google Scholar]
- 14.MacFarlane M, Merrison W, Dinsdale O, Cohen GM. Activate caspases and cleaved cytokeratins are sequestered into cytoplasmic inclusions in TRAIL-induced apoptosis. J Cell Biol. 2000;148:1239–1254. doi: 10.1083/jcb.148.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leers MP, Kolgen W, Bjorklund V, Bergman T, Tribbick G, Persson B, Bjorklund P, Ramaeters FC, Bjorklund B, Nap M, Jornvall H, Schutte B. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitopic exposed during early apoptosis. J Pathol. 1999;187:567–572. doi: 10.1002/(SICI)1096-9896(199904)187:5<567::AID-PATH288>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 16.Bantel H, Ruck P, Gregor M, Schulze-Oshoff K. Detection of elevated caspase activation and early apoptosis in liver disease. Eur J Cell Biol. 2001;80:230–239. doi: 10.1078/0171-9335-00154. [DOI] [PubMed] [Google Scholar]
- 17.Bantel H, Lugering A, Heidemann J, Volkmann X, Poremba C, Strassburg CP, Manns MP, Schultze-Osthoff K. Detection of apoptotic caspase activation in sera from patients with chronic HCV, infection is associated with fibrotic liver injury. Hepatology. 2004;40:1078–1082. doi: 10.1002/hep.20411. [DOI] [PubMed] [Google Scholar]
- 18.Chedid A, Mendenhall CL, Gartside P, French SW, Chu T, Rabain L. Alcoholic liver disease. Prognostic factors according to stage. Am J Gastroenterol. 1991;86:210–216. [PubMed] [Google Scholar]
- 19.French SW, Nash J, Shitabata P, Kachi K, Hara C, Chedid A, Mendenhall C. Pathology of alcoholic liver disease. Seminars Liver Dis. 1993;13:154–169. doi: 10.1055/s-2007-1007346. [DOI] [PubMed] [Google Scholar]
- 20.Ishii H, Adachi M, Fernandez-Checa JC, Cederbaum AI, Deaciuc IV, Nanji AA. Role of apoptosis in alcoholic liver injury. Alcohol Clin Exp Res. 2003;27:1207–1212. [PubMed] [Google Scholar]
- 21.Willner IR, Reuben AB. Alcohol and the liver. Current Opinion Gastroenterol. 2005;21:323–330. doi: 10.1097/01.mog.0000160044.87933.87. [DOI] [PubMed] [Google Scholar]
- 22.Bardag-Gorce F, Li J, French BA, French W. The effect of ethanol-induced CYP2E1 on proteasome activity: the role of 4-hydroxynonenal. Exp Mol Pathol. 2005;78:109–115. doi: 10.1016/j.yexmp.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Petersen DR, Doom JA. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radical Biol Med. 2004;37:937–945. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Bardag-Gorce F, French BA, Nan L, Song H, Nguyen SK, Yong H, Dedes J, French SW. CYP2E1 induced by ethanol causes oxidative stress and protease inhibition and cytokeratin aggresome (Mallory body-like) formation. Exp Mol Pathol. 2006;81:191–201. doi: 10.1016/j.yexmp.2006.07.007. [DOI] [PubMed] [Google Scholar]