Abstract
Marijuana-dependent young adults (N = 136), all referred by the criminal justice system, were randomized to 1 of 4 treatment conditions: a motivational/skills-building intervention (motivational enhancement therapy/cognitive–behavioral therapy; MET/CBT) plus incentives contingent on session attendance or submission of marijuana-free urine specimens (contingency management; CM), MET/CBT without CM, individual drug counseling (DC) plus CM, and DC without CM. There was a significant main effect of CM on treatment retention and marijuana-free urine specimens. Moreover, the combination of MET/CBT plus CM was significantly more effective than MET/CBT without CM or DC plus CM, which were in turn more effective than DC without CM for treatment attendance and percentage of marijuana-free urine specimens. Participants assigned to MET/CBT continued to reduce the frequency of their marijuana use through a 6-month follow-up.
Keywords: cognitive–behavioral therapy, contingency management, criminal justice populations, marijuana dependence, motivational enhancement therapy
Marijuana is the most commonly used illicit substance in the United States, with approximately 5.5 million regular weekly users (Anthony, Warner, & Kessler, 1994) and high prevalence among young adults (Kandel, Chen, Warner, Kessler, & Grant, 1997). In recent years, there has been a significant increase in marijuana use as well as in rates of marijuana use disorders among adults in the 18- to 29-year-old age range, most markedly among members of ethnic minority groups (Compton, Grant, Colliver, Glantz, & Stinson, 2004). Increased prevalence of marijuana use is significant because longitudinal epidemiological studies have consistently identified marijuana as a gateway drug for progression to use of other illicit substances among young adults (Kandel & Faust, 1975; Kandel, Yamaguchi, & Chen, 1992). Furthermore, frequent marijuana use during young adulthood significantly increases the risk of lifetime experiences and greater involvement with other illicit drugs, earlier onset of substance dependence, poorer educational and occupational outcomes, multiple health and psychiatric problems, and higher levels of involvement with the criminal justice system (Chen & Kandel, 1995; Ellickson, Martino, & Collins, 2004; Windle & Wiesner, 2004).
Because frequent marijuana use in early adulthood is the best predictor of persistent use, and initiation of marijuana use after age 29 is comparatively rare, providing effective interventions for marijuana use disorders to young adults is a strategy of great potential significance in preventing progression to other drug use disorders as well as harmful consequences of chronic marijuana use (Chen & Kandel, 1995). Although increasing numbers of individuals seek treatment specifically for a primary problem of marijuana dependence (National Institute on Drug Abuse, 2003), most do not do so until their mid-30s (Stephens, Roffman, & Simpson, 1994). Young adults tend to present for treatment only when compelled to do so by school officials, their parents, or the criminal justice system (Commission on Adolescent Substance and Alcohol Abuse, 2005; Deas & Thomas, 2001; Szapocznik et al., 1988). The point at which drug abusers confront legal consequences of their substance use may be a particularly important opportunity to intervene, given that more drug users are involved with the legal system than with the drug abuse treatment system (Weisner & Schmidt, 1995).
However, despite increased demand for effective interventions for marijuana dependence, only a few randomized clinical trials evaluating well-defined treatments for individuals with a primary problem of marijuana dependence have been conducted to date. In general, these have focused on motivational or skills-building approaches. For example, Stephens, Roffman, and Simpson (1994) compared a relapse prevention group with a social support interactional group for 212 marijuana-dependent adults (mean age = 31 years) and suggested that, although both treatments were associated with significant and sustained reductions in reported marijuana use, there were no significant effects by treatment condition. A subsequent trial (with adults with a mean age of 33 years) by this group suggested that a 2-session motivational intervention was not significantly different from an 18-session relapse prevention group, but both interventions were significantly more effective than a delayed-treatment control condition (Stephens, Roffman, & Curtin, 2000). The Marijuana Treatment Project (MTP Research Group, 2004; Stephens, Babor, Kadden, Miller, & MTP Research Group, 2002), a large multisite trial, randomized 450 adult marijuana-dependent individuals (mean age = 36 years) to a 9-session individual treatment that combined elements of motivational interviewing and cognitive–behavioral therapy (CBT), a 2-session motivational intervention, or a delayed-treatment control condition. Participants assigned to the 9-session intervention reduced their frequency of marijuana use and associated consequences significantly more than those assigned to the 2-session intervention. Moreover, both interventions were associated with significantly greater reductions in marijuana use compared with the delayed-treatment control condition (MTP Research Group, 2004).
In the only published report to date evaluating contingency management (CM) for marijuana dependence, Budney, Higgins, Radonovich, and Novy (2000) randomized 60 marijuana-dependent adults (mean age = 32 years) to four individual sessions of motivational enhancement therapy (MET), 14 sessions of a MET and CBT combination, or the MET/CBT combination plus voucher-based CM for marijuana-free urine specimens. Marijuana use outcomes were significantly better for the CM condition compared with either behavioral condition delivered without CM.
Thus, several recent randomized trials now have been conducted that suggest the efficacy and durability of behavioral approaches for adult marijuana users, particularly those treatments that have been found to be effective among other populations of drug users (Carroll & Onken, 2005). None of these trials, however, has focused on younger adults or those whose contact with the criminal justice system precipitated their involvement in treatment. As noted earlier, this is an important and growing population that has proven quite difficult to engage in treatment (Commission on Adolescent Substance and Alcohol Abuse, 2005; Deas & Thomas, 2001; Santisteban et al., 1996) and has markedly high rates of attrition from treatment (Sinha, Easton, & Kemp, 2003).
Another important gap in the substance abuse treatment literature is systematic data on combinations of different behavioral approaches to maximize outcome. That is, despite CM’s demonstrated efficacy and ability to target specific behaviors, an under-explored strategy is evaluating the extent to which CM can be used to enhance outcome for other types of behavioral therapy. Just as CM has been used to target medication compliance and hence improve outcomes for some pharmacotherapies (Carroll et al., 2001; Preston et al., 1999), it is possible that CM could improve response to other behavioral approaches, for example, by exposing more individuals to higher levels of effective interventions or the active ingredients of those interventions. There have been no studies evaluating the extent to which CM may be differentially effective when combined with different types of well-specified, manual-guided behavioral therapies. For example, acceleration of the onset of abstinence (and its possible beneficial relationship with improved motivation, cognition, or memory) via CM may be particularly helpful in promoting better response to some therapies. Similarly, particular therapies might improve individuals’ response to CM; for example, enhanced motivation to reduce substance use via MET or better coping skills developed via CBT could increase individuals’ exposure to the reinforcers and hence to the benefits of CM.
In this article, we present findings from a 2 × 2 factorial study that compared four treatment conditions among young marijuana-dependent individuals referred for treatment through the criminal justice system. Two different individual psychotherapy conditions were contrasted: a motivational/skills-building approach (MET/CBT; MTP Research Group, 2004; Steinberg et al., 2005) versus a manualized individual drug counseling (DC) approach. In addition, participants were randomized to receive either voucher-based CM (in which participants received vouchers contingent on session attendance or submission of marijuana-free urine specimens) compared with no CM. Thus, the four treatment conditions were MET/CBT plus CM, DC plus CM, MET/CBT without CM, and DC without CM. We hypothesized a main effect for the contrast of MET/CBT plus DC and a main effect for the contrast of CM with no CM for the primary outcome measures (retention in treatment and reduction in marijuana use). We also predicted best outcomes overall for the combination of MET/CBT plus CM: MET/CBT plus CM > MET/CBT without CM or DC plus CM > DC without CM, hypothesizing that emphasis on both external motivation (reinforcement of retention and abstinence through CM) and internal motivation (heightened recognition of personal goals and skill acquisition through MET/CBT) would maximize outcomes within treatment and possibly reduce rebound effects after termination of the incentive system at the end of treatment. A secondary aim was to confirm findings from the cocaine and emerging marijuana treatment literature regarding the prognostic significance of an initial urine specimen, in which submission of a drug-free urine specimen early in treatment tends to be associated with better outcome overall and in some cases with differential response to some treatments (Alterman et al., 1997; Ehrman, Robbins, & Cornish, 2001; Kampman et al., 2001; Moore & Budney, 2002; Sofuoglu, Gonzalez, Poling, & Kosten, 2003).
Method
Participants and Setting
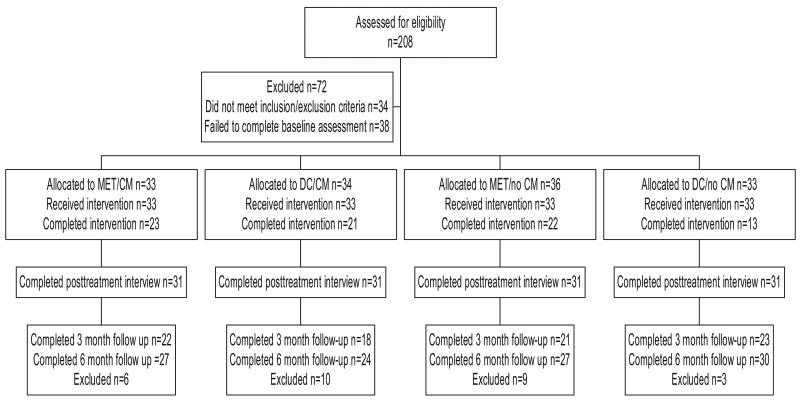
Participants were individuals between the ages of 18 and 25 years who were referred for treatment for marijuana dependence by the Office of Adult Probation to the Substance Abuse Treatment Unit in New Haven, Connecticut, and who met criteria for current marijuana dependence. Of 208 individuals screened, 174 met inclusion–exclusion criteria. Exclusion criteria and the number of individuals excluded for each reason were severe substance dependence that required inpatient treatment and detoxification (n = 7), failure to meet Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM–IV; American Psychiatric Association, 1994) criteria for current marijuana dependence or failure to submit a marijuana-positive urine specimen at baseline (n = 6), current physical dependence on alcohol or opioids (n = 4), current psychotic disorder (n = 4), involvement in other treatment for marijuana dependence within the past 60 days (n = 4), current homicidal risk (n = 3), inability to commit to the 1-year follow-up (n = 2), score of less than 25 on the Mini-Mental State examination (Folstein, Folstein, & McHugh, 1975; n = 2), no referral to treatment by the criminal justice system (n = 1), and severe medical problems (n = 1). Thirty-eight individuals dropped out during the screening process before eligibility could be determined. Thus, 136 individuals provided written informed consent and were randomized to one of the four treatment conditions. Of these, two were incarcerated and two more dropped out prior to their first session; hence, 132 individuals were exposed to the study treatments (as summarized in the participant flow diagram in Figure 1).
Figure 1.
Participant flow and data availability through the trial. Motivational enhancement therapy/cognitive–behavioral therapy (MET/CBT) = motivational/skills-building intervention; DC = individual drug counseling; CM = contingency management.
Demographic, substance use, and psychosocial functioning variables at baseline for the 136 randomized individuals by treatment condition are presented in Table 1. The mean age of the sample was 21 years (SD = 2.1), and 90% of the participants were male. Sixty percent identified themselves as African American, 13% as Latin American, and 23% as European American. Nearly half (48%) had not completed high school; 35% were high school graduates; and 18% had completed some college-level work. Most (96%) had never been married, relatively few held a full-time job (21%), and 51% were unemployed. The sample reported an average of five previous arrests (with a mean age of 16 at their first arrest); they also reported that they had been incarcerated an average total of 9 months during their lifetimes.
Table 1.
Baseline Characteristics, by Treatment
| Treatment
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MET/CBT + CM (n = 33)
|
DC + CM (n = 34)
|
MET/CBT (n = 35)
|
DC (n = 33)
|
|||||||||
| Variable | % | M | SD | % | M | SD | % | M | SD | % | M | SD |
| Men | 88 | 94 | 94 | 82 | ||||||||
| Women | 12 | 6 | 6 | 18 | ||||||||
| Ethnicity | ||||||||||||
| African American | 52 | 77 | 53 | 58 | ||||||||
| European American | 27 | 9 | 28 | 23 | ||||||||
| Latin American | 18 | 15 | 17 | 3 | ||||||||
| Education | ||||||||||||
| Some college | 15 | 27 | 11 | 18 | ||||||||
| High school | 36 | 26 | 44 | 30 | ||||||||
| <High school | 49 | 47 | 44 | 52 | ||||||||
| Single or divorced | 91 | 100 | 97 | 97 | ||||||||
| Employment | ||||||||||||
| Full time | 24 | 29 | 8 | 21 | ||||||||
| Part time | 30 | 24 | 25 | 33 | ||||||||
| Unemployed | 46 | 47 | 67 | 46 | ||||||||
| DSM-IV disorders | ||||||||||||
| Lifetime alcohol use disorder | 12 | 21 | 36 | 24 | ||||||||
| Lifetime anxiety disorder | 24 | 15 | 23 | 27 | ||||||||
| Lifetime depressive disorder | 18 | 18 | 3 | 6 | ||||||||
| Antisocial personality disorder | 39 | 53 | 49 | 36 | ||||||||
| Age (years) | 21.0 | 2.2 | 21.5 | 2.4 | 21.1 | 1.8 | 21.2 | 2.2 | ||||
| Arrests, lifetime (n) | 4.9 | 3.8 | 5.9 | 5.7 | 5.0 | 5.2 | 5.2 | 5.0 | ||||
| Time incarcerated (months) | 11.8 | 16.6 | 10.6 | 15.7 | 7.1 | 12.2 | 5.2 | 10.9 | ||||
| Age at first alcohol use (years) | 14.0 | 2.8 | 14.3 | 3.5 | 17.5 | 14.2 | 14.9 | 2.1 | ||||
| Age at first marijuana use (years) | 14.4 | 1.8 | 14.4 | 2.0 | 14.9 | 2.4 | 14.7 | 1.9 | ||||
| Marijuana use in past 28 days (days) | 13.8 | 10.3 | 13.7 | 11.2 | 12.4 | 9.8 | 12.5 | 10.3 | ||||
| Alcohol use in past 28 days (days) | 1.9 | 2.9 | 1.7 | 3.4 | 4.1 | 4.9 | 3.3 | 5.3 | ||||
Note. Motivational enhancement therapy/cognitive–behavioral therapy (MET/CBT) = motivational enhancement plus skills-building therapy; DC = individual drug counseling; CM = contingency management.
Regarding substance use and comorbid psychopathology, the participants reported they first used alcohol at age 15 and first used marijuana at age 14. They reported using marijuana a mean of 13.0 days (SD = 10.3) and using alcohol a mean of 2.8 days (SD = 4.3) during the previous 28 days. Five percent met criteria for a current DSM–IV alcohol use disorder (24.4% lifetime), 11% met criteria for a lifetime diagnosis of a depressive disorder, 22% met criteria for lifetime anxiety disorder, and 43% met criteria for antisocial personality disorder. There were no statistically significant differences by treatment condition on any of the variables in Table 1.
Treatments
All treatments were manualized and delivered as individual weekly sessions over an 8-week period. Therapists were 14 clinicians who had a mean age of 33 years and an average of 7 years of postdegree experience. Three doctoral- and 4 master’s-level clinicians delivered MET/CBT; 5 master’s-level clinicians, 1 doctoral-level clinician, and 1 high school-level clinician delivered DC. All clinicians completed a 2-day didactic training seminar and at least one closely supervised training case. In addition, clinicians were required to demonstrate competence in the protocol treatments by meeting prespecified criteria for competence in either MET/CBT or DC on the basis of ratings of their training cases using a validated treatment process rating system (Carroll, Connors, et al., 1998; Carroll et al., 2000), before being certified to treat trial participants.
Motivational enhancement/skills training (MET/CBT)
This condition emphasized the development of motivation for change and the implementation of skills to reduce marijuana use, using the manualized approach developed for the Marijuana Treatment Project (MTP Research Group, 2004; Steinberg et al., 2005). In this condition, clinicians were encouraged to make use of an empathic therapeutic style associated with motivational interviewing (Miller & Rollnick, 2002) that was intended to resolve ambivalence, heighten discrepancies about personal goals and marijuana use, and elicit motivation to change. Exposure to CBT techniques and skills training (e.g., understanding the patterns of substance use, strategies for recognizing and coping with craving, problem solving, managing thoughts about marijuana, improving decision-making skills to avoid risky decisions) was initiated once ambivalence about reducing marijuana use had been addressed. Throughout treatment, skills training was delivered using a therapeutic style compatible with motivational interviewing.
Individual DC
This condition was intended as a standardized version of the counseling that is typically offered in community-based clinics, as well as to provide a treatment condition that was sharply discriminable in technique and theoretical basis from MET/CBT. The treatment manual (Baker, 1998; Mercer & Woody, 1999) places strong emphasis on achieving abstinence from marijuana and other drugs through use of self-help groups and concepts compatible with a 12-step approach. Clinicians were instructed to use a clear, authoritative, and directive style throughout treatment. Session topics that could be selected to meet the needs of the individual participant included (a) education regarding marijuana use; (b) people, places, and things associated with drug use; (c) structuring one’s time; (d) craving; (e) coping with dangerous situations; (f) coping with shame and guilt; (g) social pressures to use; (h) postacute withdrawal symptoms; (i) use of other drugs; and (j) 12-step participation.
CM
Participants assigned to this condition received vouchers redeemable for goods or services purchased by study staff using the system described by Higgins and colleagues (Budney & Higgins, 1998; Higgins et al., 1991). Because marijuana remains detectable in urine for up to 3 weeks after the initiation of abstinence (Hawks & Chiang, 1986), and CM’s benefits are generally strongest among individuals who have exposure to the reinforcers (Petry, 2000), the CM system was designed to provide high levels of exposure to reinforcement (and protocol treatments) by reinforcing attendance at treatment sessions. It was also intended to provide comparatively high levels of reinforcement for a more challenging target behavior, that is, submission of marijuana-free urine specimens. This was done through a two-track incentive system found to be effective in previous trials (Carroll et al., 2001; Carroll, Sinha, Nich, Babuscio, & Rounsaville, 2002). Thus, participants received a voucher worth $25 for the first session attended, and the value of the vouchers increased in $5 increments for each consecutive session attended to a maximum of $340 in vouchers if the individual attended all eight sessions. In addition, participants received $50 in vouchers for the first marijuana-free urine specimen submitted, with increments of $5 for each consecutive marijuana-free urine specimen thereafter, to a maximum of $540 if all urine specimens submitted were negative for marijuana. Thus, if the participant attended all eight sessions and submitted eight consecutive negative urine specimens, he or she would earn a total of $880 worth of vouchers. As in the Higgins system, the value of the points earned in either track was reset to its original level if an individual missed a session, submitted a urine specimen that tested positive for drugs, or failed to submit a specimen. The two tracks were independent in that if a participant submitted a marijuana-positive urine specimen but attended the scheduled treatment session, only the urine track was reset. The voucher system was implemented by the research staff; however, therapists in both conditions were encouraged to discuss the incentive system during sessions, praise participants for earning vouchers, and discuss how earnings from vouchers might be used to reach the individual’s goals.
Assessment of Treatment Fidelity
All treatment sessions were videotaped for supervision and assessment of fidelity to manual guidelines. To evaluate treatment fidelity, therapists’ level of skill in implementing the interventions, and discriminability of the study treatments, 357 session videotapes were rated by evaluators who were unaware of the participants’ treatment assignment (54% of all sessions; these included randomly selected early [Sessions 1, 2, and 3] and late [Session 6 or 7] sessions for all participants who initiated treatment). The Yale Adherence and Competence Scale (YACS; Carroll et al., 2000), which includes several scales evaluating interventions characterizing specific therapies (e.g., MET, DC, CBT, CM) as well as several scales tapping interventions common to many therapies (e.g., assessment, general support), was used for process ratings. The YACS has been demonstrated to have excellent reliability, concurrent and factorial validity, and ability to discriminate therapies in several previous studies (Carroll, Connors, et al., 1998; Carroll et al., 2000).
Estimates of interrater reliability were done on the basis of a sample of 10 tapes rated by all seven raters (e.g., a complete block design). The mean intraclass correlation coefficient estimates from the random effects model (Shrout & Fleiss, 1979) were .76 for the treatment adherence ratings (CBT, DC, MET, CM) and .71 for the skill ratings, averaged across those four scales. Simple analyses of variance (ANOVAs) suggested that the study treatments were highly discriminable in the expected directions across the evaluated sessions. For example, on the basis of all ratings from Session 2 (103 tapes), ratings on the MET adherence scale were higher for clinicians assigned to the MET/CBT compared with the DC condition, F(1, 99) = 6.9, p = .01, and ratings on the DC adherence scale were higher for participants assigned to DC than MET/CBT, F(1, 99) = 83.9, p = .00. Clinicians assigned to CM had significantly higher ratings on the CM adherence scale than those not assigned to CM, F(1, 99) = 13.5, p < .01. There were no significant differences in MET/CBT or DC adherence ratings by CM condition. As expected, there were very few significant differences by treatment condition on the scales assessing common factors and interventions, such as provision of support and assessment of substance use and functioning. Similarly, although clinicians implementing MET/CBT tended to have more formal training, there were no significant differences by treatment condition on skill ratings for any of the scales (CBT, MET, DC, CM, or the nonspecific scales).
Assessments
Participants were assessed at baseline, weekly during treatment, at the 8-week treatment termination point, and at follow-up through 6 months. Weekly assessments included urinalysis (Varian OnTrak Testcup 5 with adulterant checks), as well as self-reports of substance use (marijuana, cocaine, alcohol, methamphetamines, opioids, benzodiazepenes, and other illicit drugs), which were collected via the Timeline Followback Method (TLFB), a reliable and valid method for assessing substance use on a day-by-day basis (Babor, Steinberg, Anton, & Del Boca, 2000; Fals-Stewart, O’Farrell, Freitas, McFarlin, & Rutigliano, 2000; Miller & Del Boca, 1994; Sobell & Sobell, 1992). Current and lifetime psychiatric diagnoses were evaluated at baseline using the Structured Clinical Interview for DSM–IV (First, Spitzer, Gibbon, & Williams, 1995). Psychosocial functioning was assessed using the Addiction Severity Index (ASI; McLellan et al., 1992) at baseline, 4 weeks after randomization, at the 8-week posttreatment interview, and at follow-up.
In cases in which a randomized participant did not initiate treatment or dropped out of treatment, he or she was followed and interviewed at the 8-week posttreatment interview point and at all follow-ups. The TLFB was used to collect data on each participant’s daily substance use from the time of dropout to the 8-week assessment point. Of the 4 participants who did not begin treatment and the 53 who did not complete treatment, 93% (53 of 57) were successfully tracked and interviewed at the 8-week point. Thus, complete self-report data for the 8-week treatment period were available for 131 of 136 (96%) of the randomized sample (see Figure 1).
Participant self-reports of marijuana use were verified through urine toxicology screens that were obtained weekly during treatment and at each follow-up interview. Of 587 urine specimens collected during treatment, 436 were collected from participants who had contributed at least 7 days of self-report data from which to calculate agreement between self-reports and results of urine toxicology screens. Of these, 83% were consistent with participant self-report; 13% were positive for marijuana when the participant denied use in the past 7 days; and 4% were negative, although the participant had reported that he or she had used marijuana within 7 days of the assessment. Using a 14-day, rather than a 7-day, cutoff to calculate agreement, 85% of the samples were consistent with self-report, and 10% of the urine specimens were positive for marijuana when the participant had denied use in the past 14 days.
In addition, one third of all specimens collected via the rapid detection system were also sent to a commercial laboratory for independent confirmation. In only 13 of 286 samples verified was there disagreement between the laboratory and on-site test results (κ = .89). Urine specimens were also routinely screened for adulterants, and only 11 of 752 samples tested had evidence of adulteration; 6 of these 11 samples also tested positive for marijuana.
Participants were contacted for follow-up interviews that were scheduled 3 months and 6 months after the 8-week treatment period. The follow-up interviews included collection of urine and breath samples and administration of the ASI and the TLFB. For the latter, participants who missed the 3-month follow-up interview were asked to provide information about their marijuana use for the full period since the last interview completed. Of the 136 individuals randomized to treatment, 64% were reached at the 3-month follow-up, and 85% were interviewed at the 6-month follow-up. There were no significant differences by treatment condition in rates of follow-up.
Furthermore, given the comparatively high vulnerability of the study population because of their age and legal status, all prospective participants were informed that a federal Certificate of Confidentiality had been obtained from the National Institutes of Health to protect the confidentiality of their research records. They were also assured that information regarding their drug use and other data would not be shared with representatives of the State of Connecticut Department of Corrections or Adult Probation. Permission was also received from the Yale School of Medicine Human Investigations Committee and the Department of Corrections to interview participants who became incarcerated during follow-up. Of 26 participants who were incarcerated during the follow-up period, 23 were interviewed (12 while incarcerated, 11 after their release).
Data Analyses
Random-effect regression models (Gibbons et al., 1993) were used to evaluate change across time for primary marijuana use outcome variables (e.g., likelihood of submitting a marijuana-positive urine specimen across time) with the following three contrasts: MET/CBT versus DC (MET/CBT plus CM and MET/CBT without CM vs. DC plus CM and DC without CM), CM versus no CM (MET/CBT plus CM and DC plus CM vs. MET/CBT without CM and DC without CM), and the interaction of MET/CBT and CM (MET/CBT plus CM < MET/CBT without CM = DC plus CM < DC without CM). ANOVAs, with the same contrasts, were used to analyze variables that summarized outcomes across treatment (e.g., longest period of continuous abstinence during treatment, percentage of marijuana-free urine specimens).
In addition to the principal analyses that were conducted on the 136 participants randomized to treatment (intention-to-treat sample), supplemental analyses also evaluated treatment effects for the 132 participants who initiated treatment (treatment-exposed sample) and the 79 participants who completed treatment. Unless otherwise noted, results were consistent across analysis samples and only results from the intention to treat sample are presented.
Results
Retention
There were significant differences across conditions in numbers of sessions completed and rates of completing treatment (defined as completing at least one session during the final week of treatment). Overall, those who initiated treatment completed a mean of 5.1 of the 8 sessions offered (SD = 2.5), with significantly higher rates for the combination of CM and MET/CBT (Cohen’s d = .45, 95% confidence interval [CI] = .05, .84) and for CM compared with no CM (d = .47, 95% CI = .12, .81). Sixty percent of the participants completed treatment, with significantly higher rates for those who were assigned to MET/CBT (MET/CBT plus CM = 69.7%, MET/CBT without CM = 66.7%, DC plus CM = 63.68%, DC without CM = 39.4%), MET/CBT versus DC, χ2(1, N = 136) = 3.8, p = .05.
Effects of Study Treatments on Marijuana Use
Primary marijuana use outcomes within treatment are presented in Table 2. Simple ANOVAs evaluating the effects of MET/CBT versus DC, CM versus no CM, and the interaction suggested that participants assigned to the CM condition had significantly longer durations of continuous abstinence than those not assigned to CM, t(124) = 2.1, p = .04, d = .45. These analyses did not indicate any statistically significant differences for MET/CBT versus DC or the interaction. Regarding outcomes based on urine specimens collected during treatment, participants assigned to CM submitted significantly more consecutive marijuana-free urine samples, t(127) = 2.7. p = .01, d = .29, 95% CI for effect size = −.06, .63, and significantly more total negative urine samples, t(127) = 2.8, p = .01, d = .29, 95% CI = −.06, .64, than those not assigned to CM. No significant effects of MET/CBT versus DC were seen. The interaction was also significant, suggesting that participants treated with the combination of CM and MET/CBT had significantly more consecutive marijuana-free urine specimens than those treated with MET/CBT without CM or DC without CM, t(127) = 1.99, p < .05, d = .25, 95% CI = −.17, .62, as well as a lower percentage of marijuana-positive urine specimens during treatment, t(127) = 2.24, p < .05, d = .28, 95% CI = −.12, .67.
Table 2.
Retention and Marijuana Use Outcomes by Treatment Condition
| Treatment
|
Contrast
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MET/CBT + CM
|
DC + CM
|
MET/CBT
|
DC
|
Interaction (t)
|
|||||||||
| Variable | M | SE | M | SE | M | SE | M | SE | MET/CBT vs. DC (t) | CM/vs. no CM (t) | dfs | n | |
| Total no. of sessions | 6.0 | 0.44 | 5.4 | 0.4 | 4.9 | 0.41 | 4.2 | 0.43 | 1.47 | 2.72* | 2.19* | 1, 131 | 136 |
| Days of marijuana use (%) | 0.64 | 0.06 | 0.75 | 0.1 | 0.73 | 0.05 | 0.71 | 0.06 | 1.03 | 0.6 | 0.63 | 1, 126 | 131a |
| Longest duration of continuous abstinence, days | 27.3 | 3.6 | 26.4 | 3.6 | 21.5 | 3.58 | 17.3 | 4.83 | 1.21 | 2.06* | 1.17 | 1, 124 | 129b |
| No. of marijuana-negative urine specimens submitted | 2.3 | 0.38 | 2.0 | 0.4 | 1.3 | 0.37 | 0.9 | 0.37 | 0.87 | 2.79* | 1.82 | 1, 127 | 132c |
| No. of consecutive marijuana-negative urine specimens | 2.2 | 0.36 | 1.8 | 0.4 | 1.3 | 0.35 | 0.8 | 0.35 | 1.21 | 2.73* | 1.99* | 1, 127 | 132c |
| Marijuana-positive urine specimens (%) | 0.5 | 0.07 | 0.7 | 0.1 | 0.7 | 0.07 | 0.7 | 0.07 | 1.27 | 1.39 | 2.24* | 1, 127 | 132c |
Note. Motivational enhancement therapy/cognitive–behavioral therapy (MET/CBT) = motivational enhancement plus skills therapy; DC = individual drug counseling; CM = contingency management. Interaction = contrast of MET/CBT + CM > DC/CM = MET/CBT without CM > DC/CM.
N = 131, ns = 33, 32, 34, and 32 for the MET/CBT + CM, DC + CM, MET/CBT without CM, and DC without CM groups, respectively.
N = 129, ns = 33, 32, 32, and 32 for the MET/CBT + CM, DC + CM, MET/CBT without CM, and DC without CM groups, respectively.
N = 132, ns = 33, 33, 33, and 33 for the MET/CBT + CM, DC + CM, MET/CBT without CM, and DC without CM groups, respectively.
p < .05.
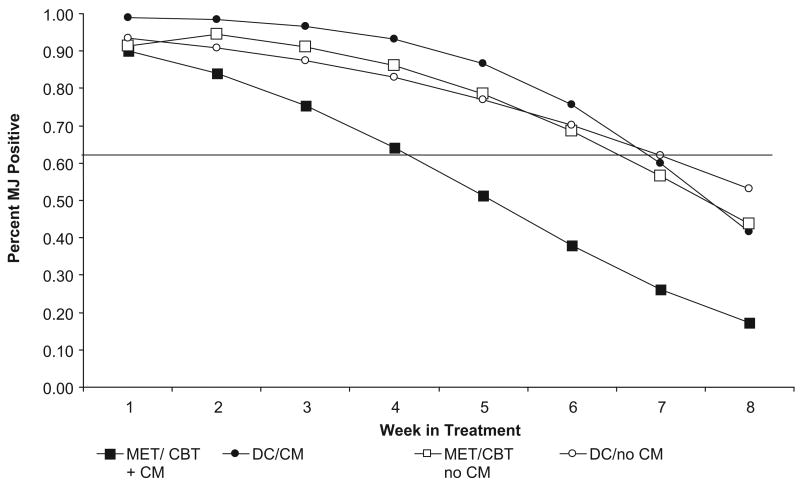
Although the ANOVAs addressed marijuana use outcomes summarized over the course of treatment, they did not address rates of change over time. Thus, random-effect regression models (Gibbons et al., 1993; Hedeker & Gibbons, 1996), using MIXOR software, were used to evaluate the likelihood of submitting a marijuana-positive urine specimen over time. For these analyses, there was an overall effect for time, indicating that the likelihood of submitting marijuana-positive urine samples decreased over time for the sample as a whole (z = −6.23, p < .05). Moreover, as shown in Figure 2, there was a significant interaction by time effect (z = −1.99, p < .05) in which participants who were assigned to the MET/CBT plus CM combination were less likely to submit marijuana-positive urine specimens during the course of treatment compared with participants assigned to MET/CBT without CM, DC plus CM, or DC without CM.
Figure 2.
Likelihood of submitting a marijuana-positive urine specimen, by week and treatment condition (estimates based on random regression analyses). MJ = marijuana; motivational enhancement therapy/cognitive–behavioral therapy (MET/CBT) = motivational/skills-building intervention; DC = individual drug counseling; CM = contingency management.
Participants assigned to CM earned an average of $363.11 in vouchers ($399.39 for MET/CBT plus CM, $326.82 for DC plus CM, ns). Of the participants who initiated treatment, all earned at least one voucher for attendance, and 37 (58% of those in MET/CBT plus CM, 51% of those in DC plus CM) earned at least one voucher for abstinence.
In terms of clinical significance, evaluation of meaningful change is complex in studies of substance-dependent populations in that concepts such as falling within the range of functioning of the functional or normal population (Jacobson & Truax, 1991) are not easily translatable to substance use treatment research (Cisler, Kowalchulk, Saunders, Zweben, & Trinh, 2005; Maisto, Kaczynski, & Ammerman, 1996), in part because illegal drug use involves nonnormative behaviors (especially illegal behaviors such as drug use; Kazdin, 1999). Valid norms on marijuana use in the general population are not easily obtained because the modal frequency of marijuana use in the general population would be complete abstinence. Problems with absolute but insensitive criteria such as percentage of participants completely abstinent are well known in the substance use literature (Babor et al., 1994).
Thus, for the purposes of this brief 8-week abstinence initiation trial, clinically significant improvement was defined as (a) completing treatment (given the robust nature of the relationship between retention and outcome in substance abuse treatment; Simpson, Joe, & Broome, 2002; Simpson, Joe, & Brown, 1997) and (b) submission of at least one marijuana-free urine specimen during treatment (indicative of attaining at least 14 days of abstinence), given that attainment of a stable period of abstinence has been associated with improved long-term functioning in several studies with substance-using populations (Higgins, Wong, Badger, Haug-Ogden, & Dantona, 2000). Percentages of participants meeting this definition were 46% for MET/CBT plus CM, 44% for DC plus CM, 31% for MET/CBT without CM, and 21% DC without CM (contrast for CM vs. no CM, z = 2.03, p < .05).
Prognostic Significance of Initial Urine Specimen
As in previous studies among cocaine-dependent (Kampman et al., 2001; Sofuoglu et al., 2003) and marijuana-dependent populations (Moore & Budney, 2002), whether or not the participant submitted a marijuana-positive urine specimen early in treatment was a strong predictor of treatment outcome. Ninety-three participants submitted a marijuana-positive urine specimen during the 1st week of treatment (79% of the 117 who submitted a valid urine specimen between Days 1 through 10 of treatment; all participants were required to submit a marijuana-positive urine during the screening process). These individuals were significantly less likely to attain a meaningful period of abstinence during treatment than those whose first-submitted urine specimen was negative. That is, 31.2% of participants whose first urine specimen was positive reached 21 days of continuous abstinence during treatment versus 87.5% of those whose first urine was negative for marijuana, χ2(1, N = 117) = 24.72, p < .01. Similarly, rates of those who reached 7 days of continuous abstinence were 56% versus 100%, respectively, χ2(1, N = 117) = 12.9, p < .01. Those whose first urine specimen was positive for marijuana were also somewhat less likely to complete treatment, although this difference did not reach statistical significance (58% vs. 74%), χ2(1, N = 117) = 2.3, p = .13. Within the group that submitted a marijuana-positive urine specimen early in treatment, there were significant effects of CM on the percentage of urine specimens that were negative for marijuana—CM versus no CM, F(1, 84) = 5.99, p = .02—but no evidence of an effect for the MET/CBT versus DC contrast, nor any other evidence that the study treatments were differentially effective by whether or not the first urine submitted was positive for marijuana.
Effects of Study Treatments on Secondary Outcomes
Regarding the ASI composite scores, there was a significant time effect in the marijuana, medical, legal, family, and psychological composite scores, with no significant overall reductions on the ASI employment, alcohol, or drug use composite scores. There was a significant treatment condition (MET/CBT vs. DC) by time effect on the legal composite (z = 3.01, p = .05), with participants assigned to DC reporting greater reductions in the legal composite score than those assigned to MET/CBT. For the more complete sample, there was a significant CM by time effect (z = −2.23, p = .03), suggesting a greater reduction in marijuana composite scores for participants assigned to CM than those who did not receive CM.
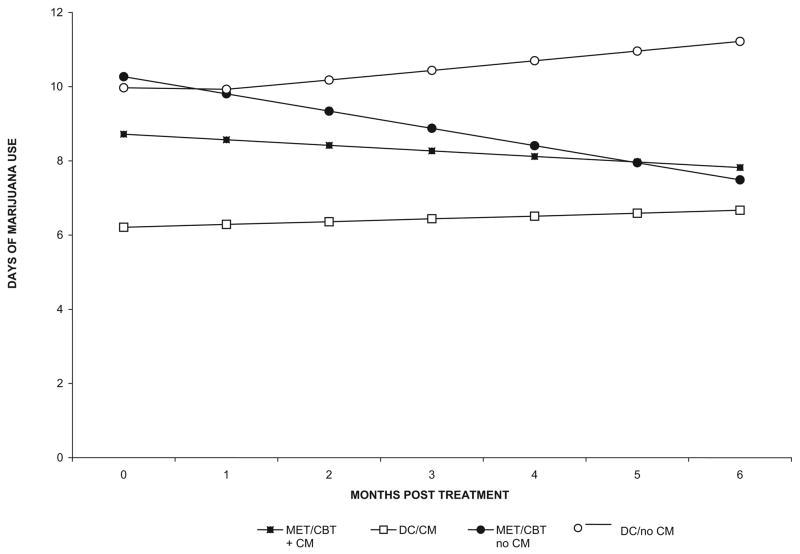
Marijuana Use Outcomes During Follow-Up
Marijuana use outcomes were assessed 3 and 6 months after the treatment period using the TLFB to evaluate frequency of marijuana use by month. As shown in Figure 3, random-effect regression analyses (using the same contrasts as for the active treatment period) indicated, first, that the effect for time was not significant, suggesting that participants as a group did not change their frequency of marijuana use between the end of the 8-week treatment period and the 6-month point (z = −.61, p = .54). Second, there was a significant MET/CBT versus DC by time effect (z = −2.3, p = .02), suggesting that participants who had been assigned to MET/CBT decreased their frequency of marijuana use over time compared with those assigned to DC. The effects for CM condition by time and the interaction by time were not significant, nor were main effects for these contrasts, suggesting no significant change or differential improvement for these conditions during the follow-up period.
Figure 3.
Marijuana use during 6-month follow-up, by treatment condition and month (estimates based on random regression analyses). Motivational enhancement therapy/cognitive–behavioral therapy (MET/CBT) = motivational/skills-building intervention; DC = individual drug counseling; CM = contingency management.
Proportions of participants who reported no marijuana use in the 28 days prior to the 3-month follow-up (point prevalence rates) were .42 for MET/CBT plus CM, .33 for DC plus CM, .31 for MET/CBT without CM, and .19 for DC without CM (ns). Proportions of participants who reported no marijuana use in the 28 days prior to the 6-month follow-up were .33 for MET/CBT plus CM, .32 for DC plus CM, .54 for MET/CBT without CM, and .33 for DC without CM (ns). These proportions parallel rates of individuals who provided marijuana-free urine specimens at the 3-month follow-up (.45 for MET/CBT plus CM, .32 for DC plus CM, .29 for MET/CBT without CM, and .24 for DC without CM) and the 6-month follow-up (.46 for MET/CBT plus CM, .57 for DC plus CM, .50 for MET/CBT without CM, and .41 for DC without CM).
Discussion
In this randomized trial of voucher-based CM and MET/CBT for young marijuana-dependent individuals referred to treatment by the criminal justice system, there were consistent effects favoring CM for treatment retention and marijuana use outcomes, both of which were specifically targeted by the CM system used here. There were few significant main effects for MET/CBT over DC for the full sample during the active phase of treatment. However, there were several significant interaction effects suggesting that MET/CBT combined with CM was associated with better outcomes than MET/CBT without CM, DC plus CM, and that those three treatments were significantly more effective than DC without CM. Finally, there was evidence of continuing improvement during the 6-month follow-up for those assigned to the MET/CBT condition.
This study extends the literature supporting the efficacy of CM interventions in a range of substance-using populations in several ways. First, the study underscores the feasibility of CM procedures with marijuana-dependent populations. Given that the effectiveness of CM has been associated with the exposure of individuals to reinforcers (Petry, 2000) as well as rapid and accurate detection of target behaviors (in this case, the initiation of abstinence; Budney & Higgins, 1998; Higgins et al., 1991), the comparatively extended half-life of marijuana could potentially complicate use of CM procedures with this population, given that marijuana may be detectible in urine up to 3 weeks after the initiation of abstinence (Hawks & Chiang, 1986; Schwartz, 1988). During the 8-week active phase of treatment, 46% of participants submitted at least one marijuana-free urine sample, and more than half of the participants assigned to the CM condition (37/67) earned at least one voucher for urine-verified abstinence during the trial. Most participants were able to produce a marijuana-free urine specimen approximately 10 days after their last reported use of marijuana; moreover, the level of agreement between participants’ self-reports of marijuana use and urinalysis results was consistent with those reported among previous clinical trials of other types of drug users (Zanis, McLellan, & Randall, 1994). This is notable given that the participants, all of whom were referred by the criminal justice system, faced both significant negative consequences (i.e., the threat of incarceration for continued illegal behavior and drug use) and positive consequences for abstinence (i.e., earned goods and services if assigned to CM).
Second, the strategy of reinforcing both session attendance (so that most participants could have some exposure to the reinforcers simply by attending sessions) and abstinence (with a comparatively large incentive for the first marijuana-free urine specimen) appeared to be effective for this population, particularly when delivered in combination with MET/CBT. It should be noted that significant effects favoring CM were found primarily on those outcomes that were reinforced (session attendance and marijuana-free urine specimens) and did not appear to generalize to other indicators of outcome (e.g., ASI composite scores addressing other psychosocial outcomes). On the other hand, the 8-week trial was of comparatively short duration, and more time may have been necessary for treatment, or the initiation of abstinence, to have meaningful effects on other problem areas (e.g., legal and employment functioning).
Finally, although several studies have evaluated combinations of CM and CBT to enhance outcome (Budney & Higgins, 1998; Epstein, Hawkins, Covi, Umbricht, & Preston, 2003; Rawson et al., 2002), this is the first study to our knowledge to evaluate systematically whether outcomes could be enhanced through a combination of CM with different types of well-defined behavioral therapies, in this case MET/CBT versus a manualized DC condition that had strong empirical support (Carroll, Nich, Ball, McCance-Katz, & Rounsaville, 1998; Crits-Christoph et al., 1999). The combination of CM and MET/CBT was significantly more effective than any of the other conditions (MET/CBT without CM, DC plus CM, and DC without CM) in terms of several important outcome measures, including retention in treatment, number of consecutive marijuana-free urine specimens submitted, and the likelihood of submitting a marijuana-free urine specimen across the treatment period. Even for those outcome indicators for which no statistically significant differences were found, the combination of MET/CBT and CM was consistently associated with the best outcomes overall. This thus supports our hypothesis that CM would be more effective when combined with MET/CBT. It is possible that CM may have enhanced MET/CBT by allowing more participants to sample the benefits of abstinence, to think more clearly about treatment goals, and to “own” the decision to reduce marijuana use, particularly when compared with DC, in which abstinence was not discussed as a choice. Moreover, although it was conceivable that CM (with its emphasis on providing extrinsic motivation for change via provision of incentives for abstinence and attendance) might work against MET/CBT (with its emphasis on enhancing intrinsic motivation; Deci, Koestner, & Ryan, 1999), there was little evidence, at least from the retention and marijuana use data, that this was the case in this study. Not only were outcomes consistently better in the MET/CBT plus CM combination, but in several cases they were significantly superior to the DC plus CM combination, suggesting that further work on identifying the most efficacious combinations of CM and various behavioral therapies may be promising.
In contrast to the strong and consistent effects for CM, there were comparatively few indications of a significant main effect for MET/CBT compared with DC alone during the active phase of treatment, with the exception of the ASI legal composite score. There was, however, evidence of continuing improvement for participants assigned to the MET/CBT condition during follow-up. Because the MET/CBT condition used here included significant attention to skills-building approaches, this finding is consistent with previous findings of durable, continuing improvement with cognitive–behavioral approaches (Carroll et al., 1994; Hollon, 2003; Rawson et al., 2002). Moreover, the MET/CBT plus CM combination consistently produced the best outcomes overall and outcomes that were markedly more positive compared with the condition intended to approximate standard treatment at the performance site (DC without CM). In fact, the 39% completion rate for the DC without CM condition is consistent with the typical retention rates at the clinic for this population (Sinha et al., 2003).
There are several limitations to this study. First, the duration of treatment was comparatively brief (8 weeks); a longer course of treatment may have allowed more participants to become abstinent or for other benefits of the study treatments to emerge. Second, because the MET/CBT treatment included elements of both motivation interviewing and cognitive–behavioral therapy, it is not possible to ascribe the treatment effects seen here to particular elements of either approach. Third, urine specimens were collected only once per week; more frequent collection would not necessarily have detected other instances of marijuana use not reported by the participants, given marijuana’s comparatively long half-life.
There were several other findings of note. These data support and extend those reported by Moore and Budney (2002), suggesting that an early marijuana-positive urine sample was strongly associated with outcome, thus linking the treatment outcome literature for marijuana dependence to that of cocaine. On the other hand, whether the first urine specimen was positive for marijuana was not significantly related to retention or treatment completion; this may reflect both the comparatively brief duration of the 8-week treatment as well as the marked effects of the MET/CBT plus CM combination on treatment completion.
Finally, the study sample was noteworthy in several respects, in that the participants were primarily young African American men with an average of five arrests by the age of 21, 43% met diagnostic criteria for antisocial personality disorder, and most were unemployed and had not completed high school. Although referred to treatment by the legal system and hence likely to incur significant consequences for continued drug use, none had completely stopped marijuana use at the time of their application for treatment. Thus, it is promising that (a) effect sizes for CM and for the MET/CBT plus CM condition were in the moderate range (.25–.47) for retention and marijuana use outcomes, and (b) the MET/CBT plus CM condition doubled the rate of individuals who completed treatment and submitted at least one marijuana-free urine specimen during treatment compared with the DC without CM condition (the latter approximated the rate associated with standard treatment at the clinic [46% vs. 21%]). Furthermore, previous clinic data on individuals from this population underlined that their retention in treatment was strikingly poor, even in the context of the implied consequences imposed by the criminal justice system (Sinha et al., 2003). Hence, the fact that more than half of the sample completed treatment and the combined MET/CBT plus CM approach evaluated here was associated with retention of 70% of participants through the end of treatment is of great potential significance regarding treatment effectiveness for this very challenging population.
This study also underscores the difficulty of applying standard definitions of clinical significance (Jacobson, Roberts, Berns, & McGlinchey, 1999; Jacobson & Truax, 1991; Kendall, Marrs-Garcia, Nath, & Sheldrick, 1999) to treatment studies with substance-using populations; these problems are heightened with nonnormative behaviors such as illegal drug use. Rather than well-normed psychological assessments with established psychometric properties (i.e., the Beck Depression Inventory), treatment outcome research in substance abuse relies on indices such as frequency and intensity of substance use, as determined through self-report or biological measures. These indices tend to be highly variable; thus, determining the proportion who met cutoffs such as change of 2 standard deviations (Jacobson & Truax, 1991), calculation of a Reliable Change Index (Jacobson et al., 1999), no longer meeting diagnostic criteria for substance dependence disorder (Kazdin, 1999), or even comparison with population norms (Kendall et al., 1999) would, in most studies, as it would have in this one, require a standard of abstinence from marijuana and other substances. The multiple problems with highly insensitive measures to evaluate complete abstinence as an outcome are well known (Babor et al., 1994; McLellan, McKay, Forman, Cacciola, & Kemp, 2005), and thus the measure used here (demonstration of some abstinence plus treatment retention) is less than ideal. Further work on this area is needed to facilitate outcome comparisons across different studies.
Acknowledgments
Support was provided by National Institute on Drug Abuse Grants K05-DA, 00457, P50-DA09241, and the U.S. Department of Veterans Affairs VISN 1 Mental Illness Research, Education and Clinical Center.
References
- Alterman AI, Kampman KM, Boardman C, Cacciola JS, Rutherford MJ, McKay JR, et al. A cocaine-positive baseline urine predicts outpatient treatment attrition and failure to attain initial abstinence. Drug and Alcohol Dependence. 1997;46:79–85. doi: 10.1016/s0376-8716(97)00049-5. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances and inhalants. Basic findings from the National Comorbidity Study. Experimental and Clinical Psychopharmacology. 1994;2:244–268. [Google Scholar]
- Babor TF, Longabaugh R, Zweben A, Fuller RK, Stout RL, Anton RF, et al. Issues in the definition and measurement of drinking outcomes in alcoholism treatment research. Journal of Studies on Alcohol. 1994;12(Suppl):83–90. doi: 10.15288/jsas.1994.s12.101. [DOI] [PubMed] [Google Scholar]
- Babor TF, Steinberg K, Anton RF, Del Boca FK. Talk is cheap: Measuring drinking outcomes in clinical trials. Journal of Studies on Alcohol. 2000;61:55–63. doi: 10.15288/jsa.2000.61.55. [DOI] [PubMed] [Google Scholar]
- Baker SM. Twelve step facilitation therapy for drug abuse and dependence. New Haven, CT: Yale University Professional Development Committee; 1998. [Google Scholar]
- Budney AJ, Higgins ST. A community reinforcement plus vouchers approach: Treating cocaine addiction. Rockville, MD: National Institute on Drug Abuse; 1998. [Google Scholar]
- Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. Journal of Consulting and Clinical Psychology. 2000;68:1051–1061. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Nich C, O’Connor PG, Eagan D, Frankforter TL, et al. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence: Efficacy of contingency management and significant other involvement. Archives of General Psychiatry. 2001;58:755–761. doi: 10.1001/archpsyc.58.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Connors GJ, Cooney NL, DiClemente CC, Donovan DM, Longabaugh RL, et al. Internal validity of Project MATCH treatments: Discriminability and integrity. Journal of Consulting and Clinical Psychology. 1998;66:290–303. doi: 10.1037//0022-006x.66.2.290. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA, McCance-Katz E, Rounsaville BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93:713–728. doi: 10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Sifry R, Frankforter T, Nuro KF, Ball SA, et al. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug and Alcohol Dependence. 2000;57:225–238. doi: 10.1016/s0376-8716(99)00049-6. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Onken LS. Behavioral therapies for drug abuse. American Journal of Psychiatry. 2005;162:1452–1460. doi: 10.1176/appi.ajp.162.8.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin FH. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence: Delayed emergence of psychotherapy effects. Archives of General Psychiatry. 1994;51:989–997. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Sinha R, Nich C, Babuscio T, Rounsaville BJ. Contingency management to enhance naltrexone treatment of opioid dependence: A randomized clinical trial of reinforcement magnitude. Experimental and Clinical Psychopharmacology. 2002;10:54–63. doi: 10.1037//1064-1297.10.1.54. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. American Journal of Public Health. 1995;85:41–47. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler RA, Kowalchulk RK, Saunders SM, Zweben A, Trinh HQ. Applying clinical significance methodology to alcoholism treatment trials: Determining recovery outcome status with individual-and population-based measures. Alcoholism: Clinical and Experimental Research. 2005;29:1991–2000. doi: 10.1097/01.alc.0000187159.75424.77. [DOI] [PubMed] [Google Scholar]
- Commission on Adolescent Substance and Alcohol Abuse. Treatment of substance use disorders. In: O’Brien CP, editor. Treating and preventing adolescent mental health disorders: What we know and what we don’t know. New York: Oxford University Press; 2005. pp. 391–410. [Google Scholar]
- Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991–1992 and 2001–2002. Journal of the American Medical Association. 2004;291:2114–2121. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- Crits-Christoph P, Siqueland L, Blaine JD, Frank A, Luborsky L, Onken LS, et al. Psychosocial treatments for cocaine dependence: Results of the National Institute on Drug Abuse collaborative cocaine study. Archives of General Psychiatry. 1999;56:495–502. doi: 10.1001/archpsyc.56.6.493. [DOI] [PubMed] [Google Scholar]
- Deas D, Thomas SE. An overview of controlled studies of adolescent substance abuse treatment. The American Journal on Addictions. 2001;10:178–189. doi: 10.1080/105504901750227822. [DOI] [PubMed] [Google Scholar]
- Deci FL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychological Bulletin. 1999;128:627–668. doi: 10.1037/0033-2909.125.6.627. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Cornish JW. Results of a baseline urine test predict levels of cocaine use during treatment. Drug and Alcohol Dependence. 2001;62:1–7. doi: 10.1016/s0376-8716(00)00137-x. [DOI] [PubMed] [Google Scholar]
- Ellickson PL, Martino SC, Collins RL. Marijuana use from adolescence to young adulthood: Multiple developmental trajectories and their associated outcomes. Health Psychology. 2004;23:299–307. doi: 10.1037/0278-6133.23.3.299. [DOI] [PubMed] [Google Scholar]
- Epstein DE, Hawkins WE, Covi L, Umbricht A, Preston KL. Cognitive behavioral therapy plus contingency management for cocaine use: Findings during treatment and across 12-month follow-up. Psychology of Addictive Behaviors. 2003;17:73–82. doi: 10.1037/0893-164X.17.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The Timeline Followback reports of psychoactive substance use by drug-abusing patients: Psychometric properties. Journal of Consulting and Clinical Psychology. 2000;68:134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM–IV, patient edition. Washington, DC: American Psychiatric Publishing; 1995. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Hedeker D, Elkin I, Waternaux C, Kraemer HC, Greenhouse JB, et al. Some conceptual and statistical issues in analyses of longitudinal psychiatric data. Archives of General Psychiatry. 1993;50:739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- Hawks RL, Chiang CN. Urine testing for drugs of abuse. Rockville, MD: National Institute on Drug Abuse; 1986. [Google Scholar]
- Hedeker D, Gibbons RD. Mixreg: A computer program for mixed effect regression analysis with autocorrelated errors. Computer Methods and Programs. 1996;49:229–252. doi: 10.1016/0169-2607(96)01723-3. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Delany DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, et al. A behavioral approach to achieving initial cocaine abstinence. American Journal of Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Wong CJ, Badger GJ, Haug-Ogden DE, Dantona RL. Contingent reinforcement increases cocaine abstinence during outpatient treatment and one-year follow-up. Journal of Consulting and Clinical Psychology. 2000;68:64–72. doi: 10.1037//0022-006x.68.1.64. [DOI] [PubMed] [Google Scholar]
- Hollon SD. Does cognitive therapy have an enduring effect? Cognitive Therapy and Research. 2003;27:71–75. [Google Scholar]
- Jacobson NS, Roberts LJ, Berns SB, McGlinchey JB. Methods for defining and determining the clinical significance of treatment effects: Description, application, and alternatives. Journal of Consulting and Clinical Psychology. 1999;67:300–307. doi: 10.1037//0022-006x.67.3.300. [DOI] [PubMed] [Google Scholar]
- Jacobson NS, Truax PA. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Alterman AI, Volpicelli JR, Maany I, Muller ES, Luce DD, et al. Cocaine withdrawal symptoms and initial urine toxicology results predict treatment attrition in outpatient cocaine dependence treatment. Psychology of Addictive Behaviors. 2001;15:52–59. doi: 10.1037/0893-164x.15.1.52. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Chen K, Warner LA, Kessler RC, Grant B. Prevalence and demographic correlates of symptoms of last year dependence on alcohol, nicotine, marijuana, and cocaine in the U.S. population. Drug and Alcohol Dependence. 1997;44:11–29. doi: 10.1016/s0376-8716(96)01315-4. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Faust R. Sequence and stages in patterns of adolescent drug use. Archives of General Psychiatry. 1975;32:923–932. doi: 10.1001/archpsyc.1975.01760250115013. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood. Journal of Studies on Alcohol. 1992;53:447–457. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- Kazdin AE. The meaning and measurement of clinical significance. Journal of Consulting and Clinical Psychology. 1999;67:332–339. doi: 10.1037//0022-006x.67.3.332. [DOI] [PubMed] [Google Scholar]
- Kendall PC, Marrs-Garcia A, Nath SR, Sheldrick RC. Normative comparisons for the evaluation of clinical significance. Journal of Consulting and Clinical Psychology. 1999;67:285–299. doi: 10.1037//0022-006x.67.3.285. [DOI] [PubMed] [Google Scholar]
- Maisto SA, Kaczynski NA, Ammerman RT. Analysis of change in adolescents involved in alcohol and drug treatment. Experimental and Clinical Psychopharmacology. 1996;4:322–329. [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McLellan AT, McKay JR, Forman R, Cacciola JS, Kemp J. Reconsidering the evaluation of addiction treatment: From retrospective follow-up to concurrent recovery monitoring. Addiction. 2005;100:447–458. doi: 10.1111/j.1360-0443.2005.01012.x. [DOI] [PubMed] [Google Scholar]
- Mercer DE, Woody GE. An individual drug counseling approach to treat cocaine addiction: The collaborative cocaine treatment study model. Rockville, MD: National Institute on Drug Abuse; 1999. [Google Scholar]
- Miller WR, Del Boca FK. Measurement of drinking behavior using the Form 90 family of instruments. Journal of Studies on Alcohol. 1994;12(Suppl):112–117. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. 2. New York: Guilford Press; 2002. [Google Scholar]
- Moore BA, Budney AJ. Abstinence at intake for marijuana dependence treatment predicts response. Drug and Alcohol Dependence. 2002;67:249–257. doi: 10.1016/s0376-8716(02)00079-0. [DOI] [PubMed] [Google Scholar]
- MTP Research Group. Brief treatments for cannabis dependence: Findings from a randomized multisite trial. Journal of Consulting and Clinical Psychology. 2004;72:455–466. doi: 10.1037/0022-006X.72.3.455. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. Epidemiologic trends in drug abuse: Proceedings of the Community Epidemiology Work Group. Vol. 1. Bethesda, MD: Author; 2003. [Google Scholar]
- Petry NM. A comprehensive guide to the application of contingency management procedures in clinical settings. Drug and Alcohol Dependence. 2000;58:9–25. doi: 10.1016/s0376-8716(99)00071-x. [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Umbricht A, DeJesus A, Montoya ID, Schuster CR. Improvement in naltrexone treatment compliance with contingency management. Drug and Alcohol Dependence. 1999;54:127–135. doi: 10.1016/s0376-8716(98)00152-5. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Huber A, McCann MJ, Shoptaw S, Farabee D, Reiber C, et al. A comparison of contingency management and cognitive–behavioral approaches during methadone maintenance for cocaine dependence. Archives of General Psychiatry. 2002;59:817–824. doi: 10.1001/archpsyc.59.9.817. [DOI] [PubMed] [Google Scholar]
- Santisteban DA, Coatsworth JD, Perez-Vidal A, Mitrani V, Jean-Gilles M, Szapocznik J. Engaging behavior problem/drug abusing youth and their families in treatment: A replication and further exploration of the factors that contribute to differential effectiveness. Journal of Family Psychology. 1996;10:35–44. [Google Scholar]
- Schwartz RH. Urine testing in the detection of drugs of abuse. Archives of Internal Medicine. 1988;148:2407–2412. [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;86:420–429. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Simpson DD, Joe GW, Broome KM. A national 5-year follow-up of treatment outcomes for cocaine dependence. Archives of General Psychiatry. 2002;59:538–544. doi: 10.1001/archpsyc.59.6.538. [DOI] [PubMed] [Google Scholar]
- Simpson DD, Joe GW, Brown BS. Treatment retention and follow-up outcomes in the Drug Abuse Treatment Outcome Study (DATOS) Psychology of Addictive Behaviors. 1997;11:294–307. [Google Scholar]
- Sinha R, Easton C, Kemp K. Substance abuse treatment characteristics of probation-referred young adults in a community-based outpatient program. American Journal of Drug and Alcohol Abuse. 2003;29:585–597. doi: 10.1081/ada-120023460. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Followback: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen J, editors. Measuring alcohol consumption: Psychosocial and biological methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Sofuoglu M, Gonzalez G, Poling J, Kosten TR. Prediction of treatment outcome by baseline urine cocaine results and self-reported cocaine use for cocaine and opioid dependence. American Journal of Drug and Alcohol Abuse. 2003;29:713–727. doi: 10.1081/ada-120026256. [DOI] [PubMed] [Google Scholar]
- Steinberg K, Roffman RA, Carroll KM, McRee B, Babor TF, Miller M, et al. Brief counseling for marijuana dependence: A manual for treating adults. Rockville, MD: Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; 2005. [Google Scholar]
- Stephens RS, Babor TF, Kadden R, Miller M MTP Research Group. The Marijuana Treatment Project: Rationale, design, and participant characteristics. Addiction. 2002;97:109–124. doi: 10.1046/j.1360-0443.97.s01.6.x. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. Journal of Consulting and Clinical Psychology. 2000;68:898–908. [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Simpson EE. Treating adult marijuana dependence: A test of the relapse prevention model. Journal of Consulting and Clinical Psychology. 1994;62:92–99. doi: 10.1037//0022-006x.62.1.92. [DOI] [PubMed] [Google Scholar]
- Szapocznik J, Perez-Vidal A, Brickman AL, Foote FH, Santisteban DA, Hervis O, et al. Engaging adolescent drug abusers and their families in treatment: A strategic structural systems approach. Journal of Consulting and Clinical Psychology. 1988;56:552–557. doi: 10.1037//0022-006x.56.4.552. [DOI] [PubMed] [Google Scholar]
- Weisner C, Schmidt LA. Expanding the frame of health services research in the drug abuse field. Health Services Research. 1995;30:707–726. [PMC free article] [PubMed] [Google Scholar]
- Windle M, Wiesner M. Trajectories of marijuana use from adolescence to young adulthood: Predictors and outcomes. Development and Psychopathology. 2004;16:1007–1027. doi: 10.1017/s0954579404040118. [DOI] [PubMed] [Google Scholar]
- Zanis DA, McLellan AT, Randall M. Can you trust patient self-reports of drug use during treatment? Drug and Alcohol Dependence. 1994;35:127–132. doi: 10.1016/0376-8716(94)90119-8. [DOI] [PubMed] [Google Scholar]