Abstract
The present study was undertaken with controls using equal doses ID and IM plus the standard full dose IM to assess the role of route of vaccine in immunogenicity of inactivated influenza vaccine. The study was a prospective, randomized, active-controlled, open label clinical trial conducted in healthy young adult outpatients to compare the effect of route (IM vs ID) on antibody responses to influenza vaccine. Volunteers received 3, 6 or 9 μg of vaccine by ID or IM route; 15 μg IM was also studied. Low doses of vaccine given by either route were almost as immunogenic as the standard 15 μg IM dose of influenza vaccine. ID route was not superior to IM vaccine at inducing antibodies. ID vaccine induced significantly more local inflammatory response than IM vaccine.
Keywords: influenza, vaccine, route
2. Introduction
Routine yearly administration of influenza vaccine to adults is a long-standing recommendation, and in 2006 the recommendation was further extended to children 6 months to 5 years of age. Moreover, inactivated trivalent influenza vaccine (TIV) may be administered to anyone over 6 months of age wishing to reduce the risk of contracting influenza. Influenza vaccines have repeatedly been shown to be effective at reducing influenza related morbidity and mortality [3-6]. The extension of the influenza vaccine recommendations to include children and household contacts of high-risk persons has increased the number of influenza doses needed to be produced [7]. Currently, 180 million persons are recommended to receive vaccine. In recent years there has been a shortage of vaccine, particularly in the fall months of the year when most providers and patients seek influenza vaccine. We and others have conducted trials on lower doses of vaccine and other administration routes to try and stretch vaccine supply [1,2]. One possible method is the intradermal (ID) administration of partial doses of influenza vaccine.
Recently, we and others demonstrated that in healthy adults lower doses (3 μg or 6 μg) of TIV administered ID produced immune responses equivalent to a standard dose of TIV (15 μg per HA) administered IM [1,2]. While local reactions were more common in the group receiving TIV by ID injection, the systemic safety profile was similar in the group receiving ID injections as compared to IM. However, in these studies, rigorous controls (i.e. low dose IM) were not included in the design. The present study was undertaken with controls using equal doses ID and IM plus the standard full dose IM to assess the role of route of vaccine in immunogenicity.
3. Materials and Methods
3.1 Trial Design
The goals of the study were to compare the immunogenicity and safety of injection of TIV across different dose levels (3, 6, 9, and 15 μg/antigen/dose) and different routes of administration (IM versus ID). The study was a single-center, prospective, randomized, active-controlled, open label clinical trial. Approximately 31 subjects per group (217 in total) were to be enrolled to each of the following groups determined by dose and route of administration:
| Vaccine, Route | ||
|---|---|---|
| Dose | TIV, IM | TIV, ID |
| 15 μg | 0.5 mL | N/A |
| 9 μg | 0.3 mL | 0.1 mL × 3 |
| 6 μg | 0.2 mL | 0.1 mL × 2 |
| 3 μg | 0.1 mL | 0.1 mL × 1 |
Two sera samples were taken, one on Day 0 before vaccination (baseline) and one on approximately Day 28 post-vaccination, to assess immunogenicity.
3.2 Subjects
Healthy adults between the ages of 18 and 49 were recruited into the study after providing informed consent approved by the Saint Louis University Institutional Review Board. Subjects were excluded if they were breastfeeding or pregnant, had a history of receiving influenza vaccine in any of the three previous years, were allergic to eggs or other components in the vaccine, had a history of Guillain-Barré syndrome or immunosuppression or any condition that in the opinion of the investigator would interfere with the evaluation of antibody responses to the vaccine. Female subjects were tested by urine pregnancy test and had to be negative prior to vaccination. The subjects included 147 (68%) women and 70 (32%) men; 29 (13%) were black, 182 (84%) were white, and the remainder other. Details by group are given in the appendix.
Appendix Table 1.
Demographics by Group All Enrollees and ATP Cohort
| Vaccine Dose/Route All Enrollees ATP Cohert |
3 μg ID (n= 31) [n= 29] |
3 μg IM (n= 31) [n= 29] |
6 μg ID (n= 31) [n= 28] |
6 μg IM (n= 31) [n= 30] |
9 μg ID (n= 30) [n= 27] |
9 μg IM (n= 32) [n= 32] |
15 μg IM (n= 31) [n= 31] |
All Groups (N= 217) [N= 206] |
|---|---|---|---|---|---|---|---|---|
| Gender -- n(%) | ||||||||
| Male | 8 (26%) 8 (28%) |
7 (23%) 7 (24%) |
14 (45%) 12 (43%) |
10 (32%) 10 (33%) |
12 (40%) 10 (37%) |
13 (41%) 13 (41%) |
6 (19%) 6 (19%) |
70 (32%) 66 (32%) |
| Female | 23 (74%) 21 (72%) |
24 (77%) 22 (76%) |
17 (55%) 16 (57%) |
21 (68%) 20 (67%) |
18 (60%) 17 (63%) |
19 (59%) 19 (59%) |
25 (81%) 25 (81%) |
147 (68%) 140 (68%) |
| Ethnicity – n(%) | ||||||||
| Non-Hispanic | 31 (100%) 29 (100%) |
31 (100%) 29 (100%) |
31 (100%) 28 (100%) |
31 (100%) 30 (100%) |
30 (100%) 27 (100%) |
32 (100%) (32 (100%) |
31 (100%) (31 (100%) |
217 (100%) 206 (100%) |
| Hispanic | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Race -- n(%) | ||||||||
| American Indian/ Alaskan Native |
0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (3%) 1 (4%) |
0 (0%) | 0 (0%) | 1 (0%) |
| Asian | 0 (0%) | 1 (3%) | 0 (0%) | 0 (0%) | 1 (3%) 0 (0%) |
1 (3%) 1 (3%) |
1 (3%) 1 (3%) |
4 (2%) 3 (1%) |
| Hawaiian/Pacific Islander |
0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Black/African American |
8 (26%) 7 (24%) |
1 (3%) | 2 (6%) 2 (7%) |
3 (10%) | 7 (23%) 6 (22%) |
2 (6%) 2 (6%) |
6 (19%) 6 (19%) |
29 (13%) 27 (13%) |
| White | 23 (74%) 22 (76%) |
29 (94%) 27 (93%) |
29 (94%) 26 (93%) |
28 (90%) 27 (90%) |
21 (70%) 20 (74%) |
28 (88%) 28 (88%) |
24 (77%) 24 (77%) |
182 (84%) 174 (84%) |
| Multi-Racial | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (3%) 1 (3%) |
0 (0%) | 1 (0%) |
| Other/Unknown | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Age | ||||||||
|
Vaccine Dose/Route All Enrollees ATP Cohert |
3 μg ID (n= 31) [n= 29] |
3 μg IM (n= 31) [n= 29] |
6 μg ID (n= 31) [n= 28] |
6 μg IM (n= 31) [n= 30] |
9 μg ID (n= 30) [n= 27] |
9 μg IM (n= 32) [n= 32] |
15 μg IM (n= 31) [n= 31] |
All Groups (N= 217) [N= 206] |
| Mean (STD) | 28.5 (9.6) 29.1 (9.6) |
31.9(10.3) 32.2(10.6) |
31.5 (10.3) 32.4 (10.4) |
30.1(10.3) 30.3(10.4) |
28.3 (7.5) 28.4 (7.5) |
31.2 (9.4) 31.2 (9.4) |
31.5 (9.6) 31.5 (9.6) |
30.4 (9.6) 30.7 (9.7) |
| Median | 24.6 25.5 |
27.9 | 26.8 27.7 |
25.4 25.9 |
27.6 27.8 |
29.0 29.0 |
29.2 29.2 |
27.5 27.8 |
| Min,Max | (18.2, 48.5) | (18.3, 49.7) | (19.1, 49.3) | (18.1, 48.5) | (18.5, 47.5) | (18.6, 48.9) | (18.2, 49.6) | (18.1, 49.7) |
3.3 Vaccine and Vaccine Administration
Trivalent inactivated influenza vaccine (Fluzone®, Sanofipasteur, Swiftwater, PA) was used in the study. The Mantoux technique was used to administer .1 mL of vaccine into the non-dominant upper arm (deltoid region). One Mantoux injection was used for 3 μg; two Mantoux injections were used for 6 μg injection; and three Mantoux injections were used for 9 μg injections. All injections were given in the same arm and were separated by 5cm distance. Intramuscular TIV was given in the non-dominant arm; .1 mL, .2 mL, .3 mL or the standard .5 mL volume was administered corresponding to 3 μg, 6 μg, 9 μg or 15 μg of each HA antigen in the vaccine.
3.4 Reactogenicity
The safety endpoints assessed the frequency and severity of solicited local and systemic symptoms collected at 30 minutes post-vaccination, during the 7-day period following vaccination (reactogenicity), all unsolicited adverse events (AEs) through Day 28, and serious adverse events (SAEs) during the length of the study. The number and proportions of subjects in each group experiencing any injection site or systemic symptoms, and the proportions of subjects who experienced moderate-to-severe symptoms were determined for each vaccine dose and route.
After vaccination, subjects were provided with a memory aid (diary card), a digital thermometer and a flexible centimeter ruler, and were instructed how to record their reactogenicity responses on the day of vaccination and daily for the 7 days after vaccination. They recorded their maximum daily oral temperature (in degrees Fahrenheit), maximum daily erythema and swelling (in centimeters), maximum severity grade of all other solicited injection site and systemic reactions, any other adverse events, and any new medications or changes in medications. Volunteers who received more than one Mantoux injection were instructed to record the measurements at all of the sites. The maximum reaction was used for safety analysis. Subjects were contacted by telephone on Day 8-12 after vaccination to collect memory aid information and to assess AEs and SAEs.
Solicited injection site reactions and systemic reactions were defined and graded according to Tables 1 and 2.
Table 1.
Solicited Local Reactions: Definitions, Terminology and Severity Scales
| Injection site pain | Injection site erythema1 | Injection site swelling1 | |
|---|---|---|---|
|
Memory Aid term |
Pain | Redness | Swelling |
| Definition | See severity scale below for definitions |
Presence of redness including the approximate point of needle entry |
Swelling at or near the injection site* |
|
Severity scale |
Mild: Easily tolerated Moderate: Sufficiently discomforting to interfere with normal behavior or activities Severe: Incapacitating, unable to perform usual activities, may require medical care or absenteeism |
Small: <2.5 cm Medium: ≥2.5 to <5 Large: ≥5 cm |
Small: <2.5 cm Medium: ≥2.5 to <5 Large: ≥5 cm |
Swelling or edema is caused by a fluid infiltration in tissue or cavity and, depending on the space available for the fluid to disperse, swelling may be either soft (typically) or firm (less typical) to touch and thus can be best described by looking at the size of the swelling
Table 2.
Solicited Systemic Reactions: Definitions, Terminology and Severity Scales
| Fever | Headache | Malaise | Myalgia | |
|---|---|---|---|---|
|
Memory Aid term |
Temperature | Headache | Feeling unwell | Muscle aches and pains |
| Definition | Fever is defined by an oral temperature of ≥37.5°C |
A headache is pain or discomfort in the head or scalp. Does not include migraine. |
General ill feeling* | Muscle aches and pains are common and can involve more than one muscle at the same time# |
|
Severity scale |
Mild: ≥37.5°C − 38.0°C Oral Moderate: ≥38.1°C − 39.0°C Oral Severe: ≥39.1°C Oral |
Mild: Noticeable but does not interfere with daily activities Moderate: Interferes with daily activities Severe: Prevents daily activities |
Mild: Noticeable but does not interfere with daily activities Moderate: Interferes with daily activities Severe: Prevents daily activities |
Mild: Noticeable but does not interfere with daily activities Moderate: Interferes with daily activities Severe: Prevents daily activities |
Important notes for the accurate assessment of fever
Oral temperature should be accurately measured in the clinic and at home. Tympanic thermometry must not be used.
Malaise is a generalized feeling of discomfort, illness or lack of well-being that can be associated with a disease state. It can be accompanied by a sensation of exhaustion or inadequate energy to accomplish usual activities
Muscle pain can also involve the soft tissues that surround muscles. These structures, which are often referred to as connective tissues, include ligaments, tendons, and fascia (thick bands of tendons). It does not apply to muscle pain at the injection site, which should be reported as injection site pain.
3.5 Immune Responses to Vaccine
Immunogenicity was evaluated using the hemagglutination-inhibition assay (HAI) on serum samples collected prior to vaccination and at day 28 (± 3 days) post vaccination. The assessment of the immune response to the vaccine included the following: 1) the geometric mean titer (GMT) of serum HAI antibody measured against each of the 3 vaccine antigens; 2) the proportion of subjects in each group who achieved a serum HAI antibody titer of at least 1:32 for each of the 3 vaccine antigens after vaccination; and 3) the proportion of subjects achieving at least a 4-fold increase in serum HAI antibody titer between pre-immunization and post-immunization serum samples. Paired serum samples were tested by HAI against all three strains of virus (influenza A/H1N1/, influenza A/H3N2, and influenza B) using turkey red blood cells [8]. The antigens used in the assay were comparable to the strains of virus in the TIV.
3.6 Statistics
The study sample size specified an accrual goal of 31 subjects, and assumed that after modest attrition, 30 subjects would be evaluable for the immunogenicity endpoints. A randomization list without stratification and using random blocks of size 7 or 14 was prepared by the Data Coordinating Center (The EMMES Corporation). Eligible subjects were registered to the trial via the online enrollment module of The EMMES Corporation's Internet Data Entry System. Since sham injections were not utilized, the ID groups were easily identified by the number of injections received, and the study was conducted open-label.
The study was restricted to only those subjects who had not received influenza vaccine during the 2003-2004, 2004-2005 or 2005-2006 season. Within these constraints, the study was designed with the following goals: 1) to detect a dose-response trend, and 2) to detect an additive effect of ID versus IM administration, rather than to characterize with precision the entire dose-response curve. Note that the study power calculations presented below assumed that the trend effect or route effect would be tested in models fit separately to each of antigens A/H1N1, A/H3N2 and B, without adjustment for multiple comparisons.
The sample size of 30 per group was chosen to confer power to detect a trend in response with increasing dose group. For example, examining the proportion of subjects with reactogenicity in the 3 μg, 6 μg, 9 μg, and 15 μg IM dose groups, there is 90% power for a test to detect an alternative of 0.1, 0.2, 0.3, 0.4, and 78% power to detect 0.075, 0.15, 0.225, 0.30. Further, this sample size confers power of 82% with a level 0.05 test to detect a difference in mean titer level of 0.75 in the log2 mean antibody titer when comparing the IM to the ID route, while controlling for dose level. With respect to reactogenicity and safety endpoints, if no serious adverse events are observed in a dose-route group of 30 subjects, the 95% exact confidence interval for the event rate extends from 0.00 to 0.12.
This power calculation for detecting the route assumed that the log2 titer measurements follow parallel (but not necessarily linear or monotone) curves for the ID and IM groups, separated by an additive effect of route. For example, under the null hypothesis titer measurements had mean of 4, 6 and 5 in the log2 mean antibody titer in the 3 μg, 6 μg, and 9 μg IM groups, and under the alternative hypothesis, mean of 4.75, 6.75 and 5.75 in the log2 mean antibody titer in the ID group. The standard deviation of the HAI response was assumed to be 1.7 in the log 2 mean antibody titer, based on data from the previous trial.
Except for one subject in the 3 μg ID group, all subjects reported reactogenicity data from the memory aid for each of the 7 days post vaccination, and were included in the safety analysis. Local symptoms included pain, redness and swelling. For ID recipients of multiple administrations, the maximum local symptom across all injections sites was taken. Systemic symptoms included fever, headache, malaise and myalgia. Symptoms were summarized by taking the maximum severity over each of the first seven days post vaccination, as available. The denominators used in calculating incidence are based on the number of subjects who reported information, and are displayed in the summary table provided in the results section. The assessment of immune response to the vaccine was performed in the According to Protocol (ATP) cohort of subjects who were successfully vaccinated, and for whom both pre-vaccination and approximately 28 days post-vaccination sera samples were available.
The immunogenicity assessment included the following: 1) the geometric mean titer (GMT) of serum HAI antibody measured against each of the 3 vaccine antigens; 2) the proportion of subjects in each group who achieved a serum HAI antibody titer of at least 1:32 for each of the 3 vaccine antigens after vaccination (seroresponse); and 3) the proportion of subjects achieving at least a 4-fold increase in serum HAI antibody titer between pre-immunization and post-immunization serum samples (seroconversion).
For computational purposes, any pre-vaccination or post-vaccination titer reported as below the lower limit of detection (LOD) was converted to a value of 0.5 LOD in calculating the GMTs. When fold rise was calculated, any pre-vaccination value reported as less than the LOD was converted to LOD, and any post-vaccination titer reported as less than the LOD was converted to a titer of 0.5 LOD when only one of either numerator or denominator was less than the LOD. If both numerator and denominator were less than LOD, then the fold rise was defined as one.
A Gaussian distribution was assumed for the log 2 transformed titers of HAI response in calculating point and interval estimates (GMT and 95% confidence interval), and in fitting models to estimate dose-response trend and additive effect of route. Confidence intervals for the GMT were calculated using standard normal theory and the T-distribution. Exact (Clopper-Pearson) intervals were constructed for the proportion of responders, under the assumption of a binomial distribution for the number of subjects achieving seroresponse or seroconversion. Note that with the exception of the analysis of four-fold rise, the analyses were not adjusted for differing baseline levels of immune response. Calculations were performed in SAS version 8.2 and StatXact version 6.1 with graphical summaries provided.
4. Results
Two-hundred seventeen subjects were randomized to the 7 groups (N = 30, 31 or 32 per group). Thirty-one were allocated to each dose-route group, except that 30 were allocated to the 9 μg ID group, and 32 to the 9 μg IM group. Demographics of participants are summarized in the appendix Table 2. The mean age was 30 years, and the majority were female (68%), 84% were white, and 16% were from minorities. Two-hundred nine of the 217 participants completed the study and had both serum samples obtained. At most, two subjects in each group withdrew, but none due to serious adverse events associated with the vaccine. Eleven vaccinees were excluded from the ATP cohort: 8 subjects were lost to follow-up; 1 subject in the 3 μg IM group who completed follow-up had sera samples unavailable for assays; and 2 vaccinees in the 6 μg ID group were not successfully vaccinated, i.e., failed to exhibit a wheal for at least one site of Mantoux injection. As reported previously in other studies, local adverse reactions were more common after intradermal immunization than after intramuscular immunization (Figure 1). Maximum local reactogenicity including either redness or swelling or pain was significantly more likely to occur after intradermal immunization (Figure 1).
Appendix Table 2.
Maximum Local and Systemic Reactogenicity by Dose Group
| Local Reactogenicity | Systemic Reactogenicity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | N | Severity | Pain | Redness | Swelling | Maximum Local |
Elevated Oral Temp |
Malaise | Myalgia | Headache | Maximum Systemic |
| 3 μg ID |
30 | None | 20 (67) |
2 (7) | 0 (0) | 0 (0) | 30 (100) |
19 (63) | 22 (73) | 15 (50) | 12 (40) |
| Mild | 8 (27) | 7 (23) | 26 (87) | 9 (30) | 0 (0) | 8 (27) | 7 (23) | 9 (30) | 10 (33) | ||
| Moderate | 2 (7) | 14 (47) | 4 (13) | 14 (47) | 0 (0) | 2 (7) | 1 (3) | 6 (20) | 7 (23) | ||
| Severe | 0 (0) | 7 (23) | 0 (0) | 7 (23) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 1 (3) | ||
| 3 μg IM |
31 | None | 16 (52) |
22 (71) | 24 (77) | 10 (32) | 29 (94) | 28 (90) | 24 (77) | 23 (74) | 17 (55) |
| Mild | 14 (45) |
9 (29) | 7 (23) | 20 (65) | 2 (6) | 3 (10) | 7 (23) | 7 (23) | 13 (42) | ||
| Moderate | 1 (3) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 1 (3) | ||
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| 6 μg ID |
31 | None | 20 (65) | 0 (0) | 0 (0) | 0 (0) | 31 (100) | 21 (68) | 19 (61) | 21 (68) | 13 (42) |
| Mild | 10 (32) |
3 (10) | 24 (77) | 2 (6) | 0 (0) | 9 (29) | 12 (39) | 10 (32) | 17 (55) | ||
| Moderate | 1 (3) | 20 (65) | 6 (19) | 20 (65) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 1 (3) | ||
| Severe | 0 (0) | 8 (26) | 1 (3) | 9 (29) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| 6 μg IM |
31 | None | 17 (55) |
22 (71) | 27 (87) | 14 (45) | 31 (100) | 24 (77) | 22 (71) | 22 (71) | 17 (55) |
| Mild | 13 (42) | 9 (29) | 4 (13) | 16 (52) | 0 (0) | 6 (19) | 8 (26) | 7 (23) | 11 (35) | ||
| Moderate | 1 (3) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 1 (3) | 0 (0) | 2 (6) | 2 (6) | ||
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 1 (3) | ||
| 9 μg ID |
30 | None | 17 (57) |
1 (3) | 0 (0) | 0 (0) | 30 (100) | 21 (70) | 22 (73) | 22 (73) | 15 (50) |
| Mild | 12 (40) |
5 (17) | 25 (83) | 6 (20) | 0 (0) | 8 (27) | 6 (20) | 5 (17) | 10 (33) | ||
| Moderate | 1 (3) | 18 (60) | 4 (13) | 18 (60) | 0 (0) | 1 (3) | 2 (7) | 3 (10) | 5 (17) | ||
| Severe | 0 (0) | 6 (20) | 1 (3) | 6 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| 9 μg IM |
31 | None | 21 (66) |
21 (66) | 28 (88) | 12 (38) | 31 (97) | 24 (75) | 26 (81) | 26 (81) | 18 (56) |
| Mild | 11 (34) |
11 (34) | 4 (13) | 20 (63) | 1 (3) | 5 (16) | 4 (13) | 3 (9) | 9 (28) | ||
| Moderate | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (9) | 2 (6) | 3 (9) | 5 (16) | ||
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| 15 μg IM |
31 | None | 16 (52) |
23 (74) | 24 (77) | 10 (32) | 30 (97) | 23 (74) | 21 (68) | 16 (52) | 11 (35) |
| Mild | 12(39) | 8 (26) | 5 (16) | 17 (55) | 1 (3) | 7 (23) | 10 (32) | 10 (32) | 15 (48) | ||
| Moderate | 3 (10) | 0 (0) | 1 (3) | 3 (10) | 0 (0) | 1 (3) | 0 (0) | 4 (13) | 4 (13) | ||
| Severe | 0 (0) | 0 (0) | 1 (3) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 1 (3) | ||
Figure 1.
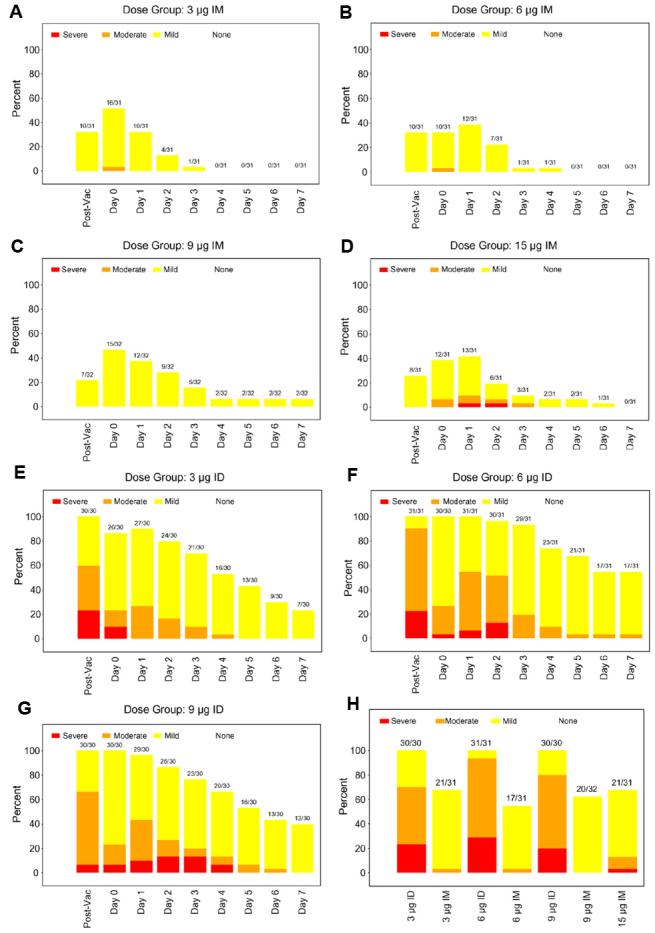
Panels A through H. There is evidence of a strong effect of route (ID vs. IM) on maximum severity of local reactions in the 7 days after vaccination. Panels 1A, 1B, 1C, 1D vs Panel 1E, 1F, 1G illustrate the local reactions, pain, erythema and swelling by day post vaccine and route. This formal comparison utilized the 7-day maximum of severity of all local reactions, dichotomizing it as none/mild versus moderate/severe, and adjusted for antigen dosage (p < 0.001, by an exact test of association between route and moderate or greater maximum severity, stratified by dosage). Panel 1H illustrates the maximum local reactogenicity by dose.
Tables 3, 4 and 5 show the GMT of HAI antibody titers, and proportion of subjects who were seropositive or seroconverted by dose and route of administration, for each antigen contained in the vaccine. There is a mild dose effect, with higher doses stimulating more antibody than lower doses, but this trend in dose is not significant at the .05 level when tested separately within each route of administration, and for each antigen response. However, the 3 μg dose was quite immunogenic and stimulated antibody titers ≥ 1:32 in the majority of subjects. For H1, H3 and B, respectively, 65%, 83% and 79% of volunteers exhibited post vaccine antibodies ≥ 1:32 at the 3 μg ID dose-route group.
Table 3.
Comparison of HAI Antibody Titers, Percent With Titer ≥1:32 and Percent With 4 Fold Rises to H1N1 Antigen According to Route and Dose
|
Vaccine Dose/Route Group |
GMT (95% CI*) Pre-Vaccination |
GMT (95% CI*) Post-Vaccination |
N(%) (95% CI‡) HAI ≥ 1:32 Pre-Vaccination |
n(%) (95% CI‡) HAI ≥ 1:32 Post-Vaccination |
n(%) (95% CI‡) With 4-Fold or Greater Rise |
|---|---|---|---|---|---|
| 3 μg ID | 5.86 (4.5, 7.7) | 44.72 (23.7, 84.5) | 2 (6.9), (0.8, 22.8) | 19 (65.5), (45.7, 82.1) | 17 (58.6), (38.9, 76.5) |
| 3 μg IM | 7.81 (5.1, 12.0) | 46.91 (26.6, 82.6) | 6 (20.7), (8.0, 39.7) | 21 (72.4), (52.8, 87.3) | 18 (62.1), (42.3, 79.3) |
| 6 μg ID | 12.01 (6.5, 22.2) | 110.90 (64.4, 190.9) | 8 (27.6), (12.7, 47.2) | 24 (82.8), (64.2, 94.2) | 18 (62.1), (42.3, 79.3) |
| 6 μg IM | 10.08 (6.2, 16.3) | 62.54 (37.9, 103.3) | 8 (26.7), (12.3, 45.9) | 24 (80.0), (61.4, 92.3) | 18 (60.0), (40.6, 77.3) |
| 9 μg ID | 10.50 (6.7, 16.5) | 70.66 (36.5, 136.9) | 8 (28.6), (13.2, 48.7) | 21 (75.0), (55.1, 89.3) | 15 (53.6), (33.9, 72.5) |
| 9 μg IM | 9.85 (6.3, 15.4) | 73.52 (43.7, 123.6) | 7 (23.3), (9.9, 42.3) | 25 (83.3), (65.3, 94.4) | 20 (66.7), (47.2, 82.7) |
| 15 μg IM | 8.95 (6.1, 13.2) | 91.53 (48.5, 172.9) | 7 (22.6), (9.6, 41.1) | 24 (77.4), (58.9, 90.4) | 21 (67.7), (48.6, 83.3) |
CIs for GMTs are calculated assuming a normal approximation for the distribution of log2 GMT
CIs for proportions are calculated using the exact method of Clopper and Pearson (binomial assumption for n)
Table 4.
Comparison of HAI Antibody Titers, Percent With Titer ≥1:32 and Percent With 4 Fold Rises to H3N2 Antigen According to Route and Dose
|
Vaccine Dose/Route Group |
GMT (95% CI*) Pre-Vaccination |
GMT (95% CI*) Post-Vaccination |
n(%) (95% CI‡) HAI ≥ 1:32 Pre-Vaccination |
n(%) (95% CI‡) HAI ≥ 1:32 Post-Vaccination |
n(%) (95% CI‡) With 4-Fold or Greater Rise |
|---|---|---|---|---|---|
| 3 μg ID | 10.66 (6.8, 16.7) | 105.72 (61.8, 180.7) | 8 (27.6), (12.7, 47.2) | 24 (82.8), (64.2, 94.2) | 20 (69.0), (49.2, 84.7) |
| 3 μg IM | 9.92 (6.7, 14.7) | 52.86 (32.3, 86.5) | 7 (24.1), (10.3, 43.5) | 23 (79.3), (60.3, 92.0) | 17 (58.6), (38.9, 76.5) |
| 6 μg ID | 9.92 (6.1, 16.2) | 103.23 (64.6, 164.8) | 6 (20.7), (8.0, 39.7) | 26 (89.7), (72.6, 97.8) | 22 (75.9), (56.5, 89.7) |
| 6 μg IM | 11.31 (7.6, 16.9) | 88.44 (51.0, 153.5) | 8 (26.7), (12.3, 45.9) | 24 (80.0), (61.4, 92.3) | 18 (60.0), (40.6, 77.3) |
| 9 μg ID | 14.14 (9.7, 20.5) | 128.00 (73.0, 224.5) | 10 (35.7), (18.6, 55.9) | 25 (89.3), (71.8, 97.7) | 21 (75.0), (55.1, 89.3) |
| 9 μg IM | 8.57 (6.1, 12.1) | 108.89 (70.2, 168.9) | 7 (23.3), (9.9, 42.3) | 28 (93.3), (77.9, 99.2) | 27 (90.0), (73.5, 97.9) |
| 15 μg IM | 9.36 (6.6, 13.3) | 167.39 (117.8, 237.9) | 4 (12.9), (3.6, 29.8) | 31 (100.0), (88.8, 100.0) | 29 (93.5), (78.6, 99.2) |
Note: Both proportional endpoints in the last 2 columns (p ≥ 32 post vacc, 4-fold or greater rise) provide evidence of a trend for the H3N2 antigen (p < 0.01 by an exact permutation test using dosage-based scores, stratified by route).
CIs for GMTs are calculated assuming a normal approximation for the distribution of log2 GMT
CIs for proportions are calculated using the exact method of Clopper and Pearson (binomial assumption for n)
Table 5.
Comparison of HAI Antibody Titers, Percent With Titer ≥1:32 and Percent With 4 Fold Rises to Influenza B Antigen According to Route and Dose
|
Vaccine Dose/Route Group |
GMT (95% CI*) Pre-Vaccination |
GMT (95% CI*) Post-Vaccination |
n(%) (95% CI‡) HAI ≥ 1:32 Pre-Vaccination |
n(%) (95% CI‡) HAI ≥ 1:32 Post-Vaccination |
n(%) (95% CI‡) With 4-Fold or Greater Rise |
|---|---|---|---|---|---|
| 3 μg ID | 17.61 (11.7, 26.5) | 89.43 (53.4, 149.8) | 11 (37.9), (20.7, 57.7) | 23 (79.3), (60.3, 92.0) | 16 (55.2), (35.7, 73.6) |
| 3 μg IM | 20.81 (12.7, 34.0) | 81.28 (53.8, 122.8) | 12 (41.4), (23.5, 61.1) | 26 (89.7), (72.6, 97.8) | 15 (51.7), (32.5, 70.6) |
| 6 μg ID | 24.02 (14.9, 38.8) | 128.00 (79.0, 207.5) | 16 (55.2), (35.7, 73.6) | 27 (93.1), (77.2, 99.2) | 19 (65.5), (45.7, 82.1) |
| 6 μg IM | 16.37 (10.0, 26.9) | 67.03 (42.5, 105.7) | 11 (36.7), (19.9, 56.1) | 26 (86.7), (69.3, 96.2) | 21 (70.0), (50.6, 85.3) |
| 9 μg ID | 24.98 (15.9, 39.3) | 115.93 (73.1, 183.8) | 13 (46.4), (27.5, 66.1) | 24 (85.7), (67.3, 96.0) | 17 (60.7), (40.6, 78.5) |
| 9 μg IM | 22.11 (13.8, 35.4) | 128.00 (87.1, 188.0) | 15 (50.0), (31.3, 68.7) | 28 (93.3), (77.9, 99.2) | 20 (66.7), (47.2, 82.7) |
| 15 μg IM | 17.50 (11.4, 26.7) | 130.89 (82.9, 206.7) | 12 (38.7), (21.8, 57.8) | 28 (90.3), (74.2, 98.0) | 21 (67.7), (48.6, 83.3) |
CIs for GMTs are calculated assuming a normal approximation for the distribution of log2 GMT
CIs for proportions are calculated using the exact method of Clopper and Pearson (binomial assumption for n)
As expected, prevaccine geometric mean antibody to each of the antigens in the vaccine were generally low since the study population was restricted to subjects who had not been vaccinated in the previous three years. In any case, the analysis of 4-fold rise in post vaccine GMT, which adjusts for potential differences in baseline titers, showed that seroconversion was observed in more than half of the subjects regardless of the dose and antigen examined (Tables 3, 4 and 5). The antibody responses to the H3N2 antigen were stronger than to the H1N1 and B antigens. The confidence intervals for the response levels are wide and overlapping. With the exception of the 3 μg ID response, which was significantly inferior to that of the standard 15 μg IM group, there are no significant comparisons between pairs of dose groups.
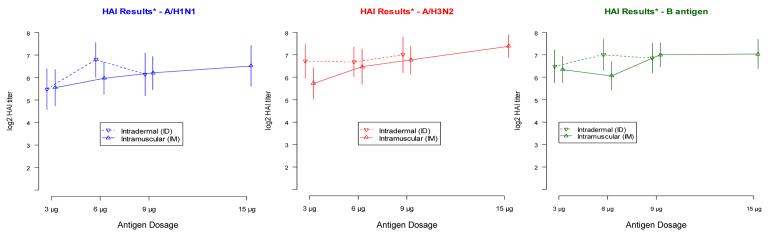
Figure 2 shows the log2 titer responses for each antigen by dose and route of administration, for each antigen contained in the vaccine. To estimate the additive effect of route from the observed data, we fit the following dose response model:
This model contains an intercept and two indicator terms for dose level, permitting the response to vary in a general fashion by dose level, but only a single indicator term for route, forcing a constant additive effect of ID administration across all three dose levels. The point estimate and 95% confidence intervals for the route effect for each antigen are as follows: H1N1: 0.237 (−0.418,0.892), H3N2: 0.484 (−0.064,1.032), B: 0.313 (−0.199,0.825). None of the estimates are statistically significant at the .05 level.
Figure 2.
Geometric mean and standard deviation of post vaccine HAI antibody titers for H1N1, H3N2 and influenza B antigens according to route and dose of vaccine.
Results <8 were assigned a titer value of 4 (log2 titer = 2).
We note that the hypothesized difference of 0.75 in the log2 mean antibody titer was not observed consistently across all dose levels. Further, the observed standard deviation of log2 titer response was 1.7 log2 units, as assumed in the power calculations, for the baseline titer measurements, but was 2.3 log2 units among the post vaccination responses. In sum, the magnitude of the effect was less than anticipated, and the heterogeneity in the data was more than expected.
5. Discussion
Investigations into strategies to reduce the dose of inactivated influenza vaccines have an extensive history. As early as 1948, Weller, et al, noted that low doses of influenza antigens given intradermally induced localized redness and swelling in 90% of subjects and four fold antibody responses in the majority of subjects [9]. During the pandemic of 1957 “Asian influenza” (the H2N2 pandemic), two groups, Boger and Liu [10] and McCarroll and Kilbourne [11], investigated the possible dose sparing ability of intradermal vaccine. Boger et al, demonstrated that in naive subjects (i.e. adults not yet infected with the novel or pandemic strain of influenza A/H2N2), 0.1 ml of commercial influenza vaccine given intradermally did not stimulate equal immune responses to 1.0 ml of vaccine given subcutaneously. McCarroll and Kilbourne noted the shortage of vaccine during the pandemic and demonstrated that two low doses, i.e. priming and boosting, could be dose sparing but the route (ID vs subcutaneous) did not influence the immune responses. The priming by previous natural infection was noted by McCarroll and Kilbourne as the most likely reason observed for low doses of ID vaccine stimulating antibody in some persons, but in naive individuals, this did not work. The present report recapitulates the finding that primed individuals respond to low doses of IM or ID influenza vaccine. A large study (N = 1009) of 1/2 dose IM vaccine vs full dose IM vaccine in young adults was conducted by the NIH [12] and the results were similar to the dose response shown in the present report; full dose was slightly better, but the dose response curve is quite flat at the doses evaluated.
While the results of preliminary clinical trials were intriguing using low dose vaccine given intradermally [1,2], when the rigorous controls were used, i.e. intramuscular doses that matched the intradermal doses, it was observed that low dose intramuscular vaccine was just as immunogenic as low dose intradermal vaccine in young healthy persons. Young healthy adults who have previous infections with influenza are clearly primed for a secondary antibody response to very low antigen concentrations. The dose response curves over the range of vaccine tested (3 to 15 μg for IM and 3 to 9 μg for ID) were relatively flat (less than 2 fold difference from lowest to highest dose for H1 and B antigens, and 3 fold difference for H3). All doses and either route were immunogenic and induced 4 fold rises in antibody in the majority of vaccinees. One potentially successful strategy in dealing with a shortage of influenza vaccine is to divide the population by age and give young healthy persons low dose vaccine by either IM or ID route and reserve standard doses (or higher doses) of vaccine for the elderly and for high risk persons who may not respond to the low doses used in the present study.
Clearly ID vaccine is immunogenic in young persons; however, so is low dose IM vaccine. The increased local reactions to ID vaccine did not translate into improved antibody responses versus IM vaccine at the same dose level. Cellular immune responses were not assessed and this could be the subject of a future comparison to evaluate the ID route. Biopsy and characterization of the local inflammatory response seen after ID vaccine would also be of interest to characterize the inflammation associated with ID vaccine.
6. Acknowledgments
The data have been presented at the PAHO conference on influenza vaccines in Washington DC, June 4, 2007.
All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Information: Funding for this research was provided by N01-AI-25464.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- 1.Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, Van Hoecke C, et al. Serum Antibody Responses After Intradermal Vaccination against Influenza. New Engl J Med. 2004;351(22):2286–2294. doi: 10.1056/NEJMoa043555. [PMID: 15525713] [DOI] [PubMed] [Google Scholar]
- 2.Kenney RT, Frech SA, Muenz RL, Villar CP, Glenn GM. Dose Sparing with Intradermal Injection of Influenza Vaccine. N Engl J Med. 2004;351(22):2295–2301. doi: 10.1056/NEJMoa043540. [PMID: 15525714] [DOI] [PubMed] [Google Scholar]
- 3.Bridges CB, Thompson WW, Meltzer MI, Reeve GR, Talamonti WJ, Cox NJ, et al. Effectiveness and cost-benefit of influenza vaccination of healthy working adults. A randomized controlled trial. JAMA. 2000;284(13):1655–1663. doi: 10.1001/jama.284.13.1655. [PMID: 11015795] [DOI] [PubMed] [Google Scholar]
- 4.Nichol LK, Lind A, Margolis KI, Murdoch M, McFadden R, Hauge M, et al. The effectiveness of vaccination against influenza in healthy, working adults. N Engl J Med. 1995;333(14):889–893. doi: 10.1056/NEJM199510053331401. [PMID: 7666874] [DOI] [PubMed] [Google Scholar]
- 5.Hak E, Nordin J, Wei F, Mullooly J, Poblete S, Strikas R, et al. Influence of high-risk medical conditions on the effectiveness of influenza vaccination among elderly members of 3 large managed-care organizations. Clin Infect Dis. 2002;35(4):370–377. doi: 10.1086/341403. [PMID: 12145718] [DOI] [PubMed] [Google Scholar]
- 6.Nichol KI, Wuorenma J, Von Sternberg T. Benefits of influenza vaccination for low-intermediate-, and high-risk senior citizens. Arch Intern Med. 1998;158(16):1769–1776. doi: 10.1001/archinte.158.16.1769. [PMID: 9738606] [DOI] [PubMed] [Google Scholar]
- 7.Advisory Committee on Immunization Practices. Smith NM, Bresee JS, Shay DK, Uyeki TM, Cox NJ, Strikas RA. Prevention and Control of Influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). [erratum appears in MMWR 2006;55:800] MMWR. 2006;55(RR10):1–42. [PMID: 16874296] [PubMed] [Google Scholar]
- 8.Schmidt NJ, Emmons RW. Diagnostic Procedures for Viral, Rickettsial, and Chlamydial Infections. 6th edition. American Public Health Association, Inc.; Washington, D.C.: 1989. [ISBN: 0875531555] [Google Scholar]
- 9.Weller TH, Cheever FS, Enders JF. Immunologic reactions following the intradermal inoculation of influenza A and B vaccine. Proc Soc Exp Biol Med. 1948;67:96–101. doi: 10.3181/00379727-67-16216. [DOI] [PubMed] [Google Scholar]
- 10.Boger WP, Liu OC. Subcutaneous and intradermal vaccination with Asian influenza vaccine. JAMA. 1957;165(13):1687–1689. doi: 10.1001/jama.1957.72980310001010. [DOI] [PubMed] [Google Scholar]
- 11.McCarroll JR, Kilbourne ED. Immunization with Asian-strain influenza vaccine. New Engl J Med. 1958;259(13):618–621. doi: 10.1056/NEJM195809252591304. [PMID: 13590412] [DOI] [PubMed] [Google Scholar]
- 12.Treanor J, Keitel W, Belshe R, Campbell J, Schiff G, Zangill K, et al. Evaluation of a single dose of half strength inactivated influenza vaccine in healthy adults. Vaccine. 2002;20(78):1099–1105. doi: 10.1016/s0264-410x(01)00440-6. [PMID: 11803070] [DOI] [PubMed] [Google Scholar]