Abstract
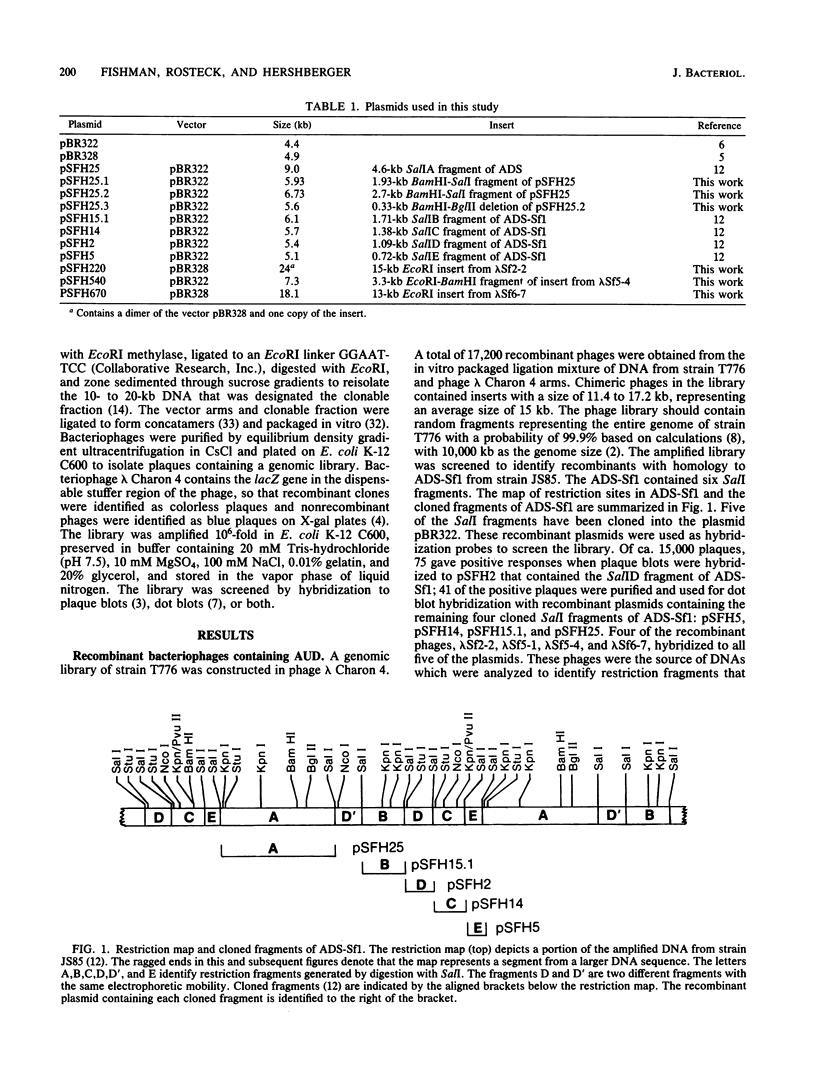
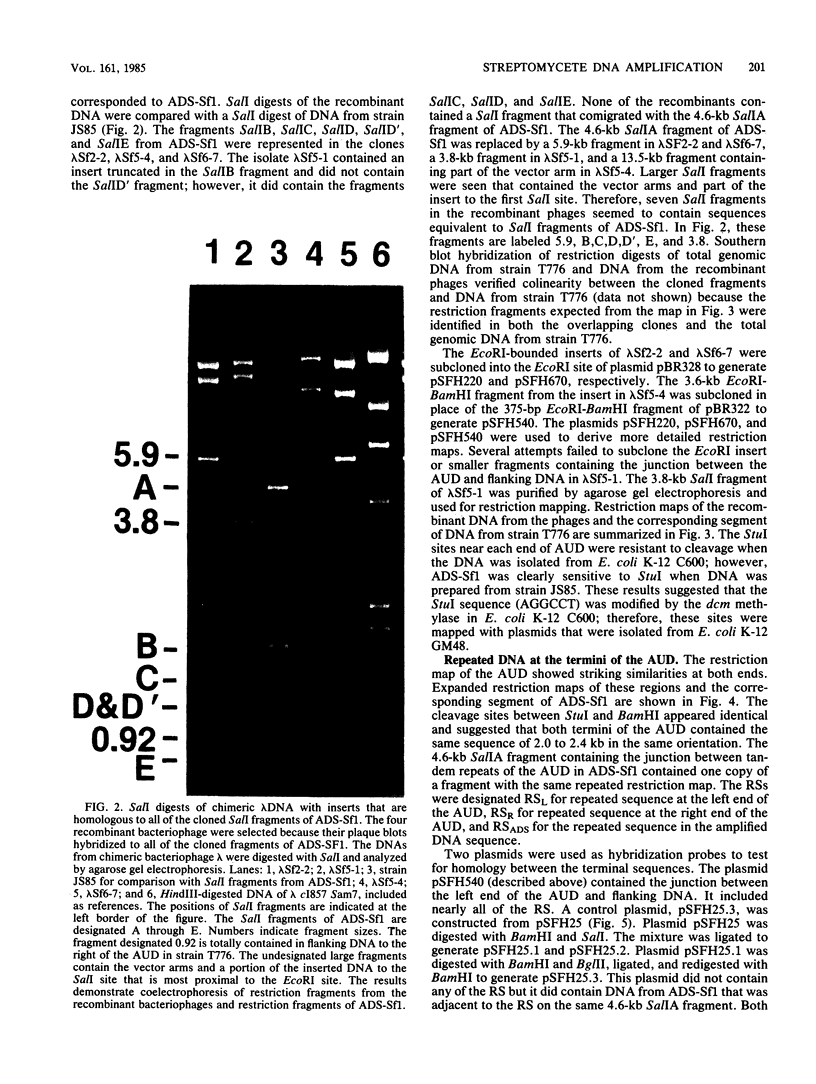
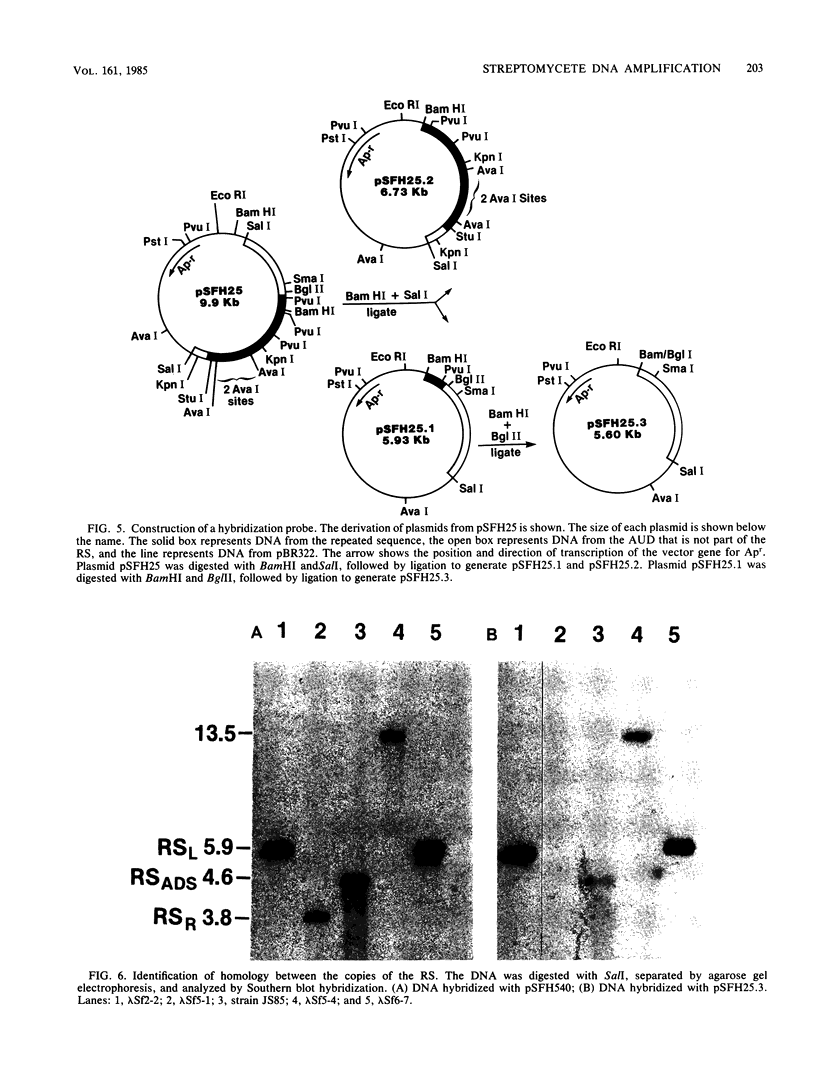
We have previously identified a 10.5-kilobase DNA sequence which is highly amplified and tandemly repeated in the mutant Streptomyces fradiae JS85. A library of DNA was prepared from S. fradiae T776, which does not contain amplified DNA. The library was screened by plaque hybridization to identify phage clones containing the unamplified 10.5-kilobase DNA sequence. Four phage isolates were identified which contained DNA homology to the amplified DNA sequence. This sequence was designated the amplifiable unit of DNA. None of the clones carried an entire amplifiable unit of DNA, and so overlapping regions were aligned to create a map of the entire region. Detailed restriction mapping identified a 2.2-kilobase direct repeat at the ends of the amplifiable unit of DNA. Analysis by Southern hybridization confirmed that the direct repeats were homologous to each other. The DNA of S. fradiae contained at least two additional copies of DNA that was homologous to the repeat sequence.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltz R. H. Genetic recombination in Streptomyces fradiae by protoplast fusion and cell regeneration. J Gen Microbiol. 1978 Jul;107(1):93–102. doi: 10.1099/00221287-107-1-93. [DOI] [PubMed] [Google Scholar]
- Benigni R., Petrov P. A., Carere A. Estimate of the genome size by renaturation studies in Streptomyces. Appl Microbiol. 1975 Aug;30(2):324–326. doi: 10.1128/am.30.2.324-326.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brandsma J., Miller G. Nucleic acid spot hybridization: rapid quantitative screening of lymphoid cell lines for Epstein-Barr viral DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6851–6855. doi: 10.1073/pnas.77.11.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugaiczyk A., Boyer H. W., Goodman H. M. Ligation of EcoRI endonuclease-generated DNA fragments into linear and circular structures. J Mol Biol. 1975 Jul 25;96(1):171–184. doi: 10.1016/0022-2836(75)90189-8. [DOI] [PubMed] [Google Scholar]
- Ellwood M., Nomura M. Chromosomal locations of the genes for rRNA in Escherichia coli K-12. J Bacteriol. 1982 Feb;149(2):458–468. doi: 10.1128/jb.149.2.458-468.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman S. E., Hershberger C. L. Amplified DNA in Streptomyces fradiae. J Bacteriol. 1983 Aug;155(2):459–466. doi: 10.1128/jb.155.2.459-466.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf H. Optimization of conditions for the in vitro formation of hybrid DNA molecules by DNA ligase. Biochim Biophys Acta. 1979 Sep 27;564(2):225–234. doi: 10.1016/0005-2787(79)90221-1. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Morrison D. A. Transformation in Escherichia coli: cryogenic preservation of competent cells. J Bacteriol. 1977 Oct;132(1):349–351. doi: 10.1128/jb.132.1.349-351.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono H., Hintermann G., Crameri R., Wallis G., Hütter R. Reiterated DNA sequences in a mutant strain of Streptomyces glaucescens and cloning of the sequence in Escherichia coli. Mol Gen Genet. 1982;186(1):106–110. doi: 10.1007/BF00422920. [DOI] [PubMed] [Google Scholar]
- Orlova V. A., Danilenko V. N. Mul'tiplikatsiia fragmenta DNK u Streptomyces antibioticus--produtsent oleandomitsina. Antibiotiki. 1983 Mar;28(3):163–167. [PubMed] [Google Scholar]
- Peterson B. C., Rownd R. H. Homologous sequences other than insertion elements can serve as recombination sites in plasmid drug resistance gene amplification. J Bacteriol. 1983 Oct;156(1):177–185. doi: 10.1128/jb.156.1.177-185.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robinson M., Lewis E., Napier E. Occurrence of reiterated DNA sequences in strains of Streptomyces produced by an interspecific protoplast fusion. Mol Gen Genet. 1981;182(2):336–340. doi: 10.1007/BF00269680. [DOI] [PubMed] [Google Scholar]
- Rosteck P. R., Jr, Hershberger C. L. Selective retention of recombinant plasmids coding for human insulin. Gene. 1983 Nov;25(1):29–38. doi: 10.1016/0378-1119(83)90164-6. [DOI] [PubMed] [Google Scholar]
- Schrempf H. Deletion and amplification of DNA sequences in melanin-negative variants of Streptomyces reticuli. Mol Gen Genet. 1983;189(3):501–505. doi: 10.1007/BF00325917. [DOI] [PubMed] [Google Scholar]
- Schrempf H. Plasmid loss and changes within the chromosomal DNA of Streptomyces reticuli. J Bacteriol. 1982 Aug;151(2):701–707. doi: 10.1128/jb.151.2.701-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B., Parker R. C., Davidson N. Representation of DNA sequences in recombinant DNA libraries prepared by restriction enzyme partial digestion. Gene. 1982 Sep;19(2):201–209. doi: 10.1016/0378-1119(82)90007-5. [DOI] [PubMed] [Google Scholar]
- Seed B. Theoretical study of the fraction of a long-chain DNA that can be incorporated in a recombinant DNA partial-digest library. Biopolymers. 1982 Sep;21(9):1793–1810. doi: 10.1002/bip.360210909. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Tiemeier D., Enquist L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene. 1977 May;1(3-4):255–280. doi: 10.1016/0378-1119(77)90049-x. [DOI] [PubMed] [Google Scholar]