Abstract
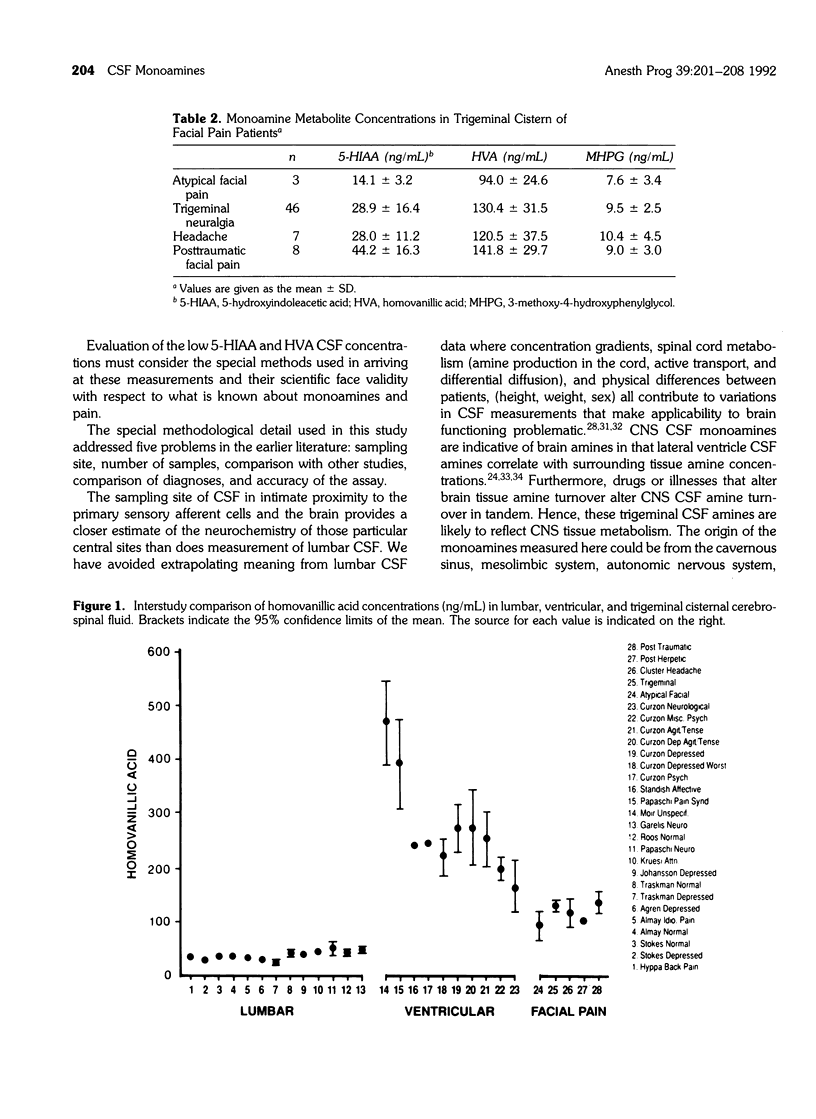
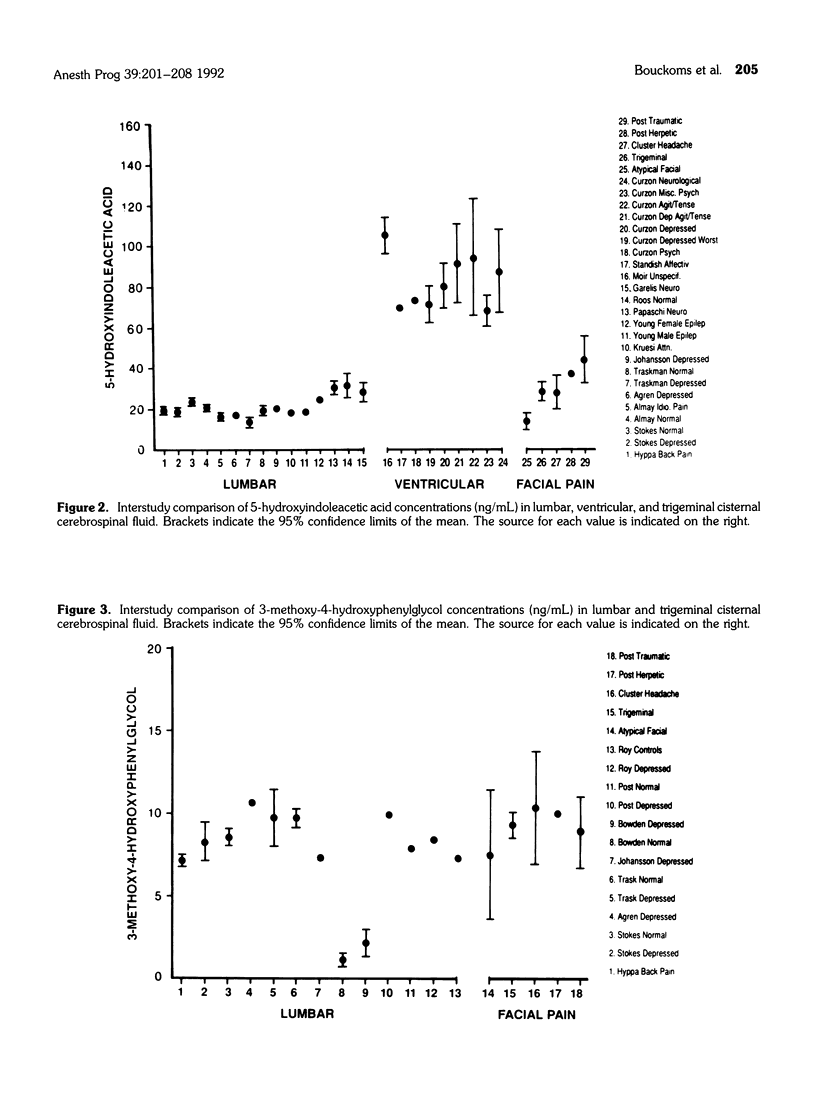
The purpose of the study was to assay monoamines in cerebrospinal fluid (CSF) obtained from the trigeminal cistern of 64 patients with intractable facial pain. The CSF was analyzed for homovanillic acid (HVA), 5-hydroxyindoleacetic acid (5-HIAA), and 3-methoxy-4-hydroxyphenylglycol (MHPG), end-product markers of activity for the dopamine, serotonin, and norepinephrine systems, respectively. HVA averaged 121 ng/mL in these facial pain patients, compared to 150 to 550 ng/mL in 10 studies of ventricular brain CSF in assorted psychiatric and pain patients. 5-HIAA averaged 29 to ng/mL in our facial pain patients compared to 60 to 120 ng/mL in nine studies of ventricular brain CSF in assorted psychiatric and neurological patients. Trigeminal cistern CSF MHPG averaged 9 ng/mL, similar to the range of 13 studies of lumbar CSF of assorted psychiatric and pain diagnoses. These results indicate that (1) the electrochemical detection method provides a unique way of accurately measuring nanogram concentrations of multiple monoamines in a little as 0.25 mL of CSF; (2) trigeminal cistern and posterior fossa brain CSF monoamine metabolites reflect a different profile of dopaminergic and serotonergic functioning in these facial pain patients from that previously reported with lumbar CSF measurements of other patients; and (3) trigeminal sensory ganglion or brain dopamine and serotonin systems may be concomitantly dysfunctional in intractable facial pain.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akil H., Liebeskind J. C. Monoaminergic mechanisms of stimulation-produced analgesia. Brain Res. 1975 Aug 29;94(2):279–296. doi: 10.1016/0006-8993(75)90062-1. [DOI] [PubMed] [Google Scholar]
- Almay B. G., Häggendal J., von Knorring L., Oreland L. 5-HIAA and HVA in CSF in patients with idiopathic pain disorders. Biol Psychiatry. 1987 Apr;22(4):403–412. doi: 10.1016/0006-3223(87)90162-4. [DOI] [PubMed] [Google Scholar]
- Almay B. G., Johansson F., von Knorring L., Sedvall G., Terenius L. Relationships between CSF levels of endorphins and monoamine metabolites in chronic pain patients. Psychopharmacology (Berl) 1980 Feb;67(2):139–142. doi: 10.1007/BF00431968. [DOI] [PubMed] [Google Scholar]
- Asberg M., Thorén P., Träskman L., Bertilsson L., Ringberger V. "Serotonin depression"--a biochemical subgroup within the affective disorders? Science. 1976 Feb 6;191(4226):478–480. doi: 10.1126/science.1246632. [DOI] [PubMed] [Google Scholar]
- Ashcroft G. W., Crawford T. B., Eccleston D., Sharman D. F., MacDougall E. J., Stanton J. B., Binns J. K. 5-hydroxyindole compounds in the cerebrospinal fluid of patients with psychiatric or neurological diseases. Lancet. 1966 Nov 12;2(7472):1049–1052. doi: 10.1016/s0140-6736(66)92028-9. [DOI] [PubMed] [Google Scholar]
- Curzon G., Gumpert E. J., Sharpe D. M. Amine metabolites in the lumbar cerebrospinal fluid of humans with restricted flow of cerebrospinal fluid. Nat New Biol. 1971 Jun 9;231(23):189–191. doi: 10.1038/newbio231189a0. [DOI] [PubMed] [Google Scholar]
- Curzon G., Kantamaneni B. D., Bartlett J. R., Bridges P. K. Transmitter precursors and metabolites in human ventricular cerebrospinal fluid. J Neurochem. 1976 Mar;26(3):613–615. doi: 10.1111/j.1471-4159.1976.tb01520.x. [DOI] [PubMed] [Google Scholar]
- Curzon G., Kantamaneni B. D., Van Boxel P., Gillman P. K., Bartlett J. R., Bridges P. K. Substances related to 5-hydroxytryptamine in plasma and in lumbar and ventricular fluids of psychiatric patients. Acta Psychiatr Scand Suppl. 1980;280:3–20. [PubMed] [Google Scholar]
- France R. D., Urban B. J., Pelton S., Kilts C. D., Hong J. S., Nemeroff C. B. CSF monoamine metabolites in chronic pain. Pain. 1987 Nov;31(2):189–198. doi: 10.1016/0304-3959(87)90035-2. [DOI] [PubMed] [Google Scholar]
- Garattini S., Samanin R. Biochemical hypotheses on antidepressant drugs: a guide for clinicians or a toy for pharmacologists? Psychol Med. 1988 May;18(2):287–304. doi: 10.1017/s0033291700007844. [DOI] [PubMed] [Google Scholar]
- Garelis E., Sourkes T. L. Sites of origin in the central nervous system of monoamine metabolites measured in human cerebrospinal fluid. J Neurol Neurosurg Psychiatry. 1973 Aug;36(4):625–629. doi: 10.1136/jnnp.36.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghia J. N., Mueller R. A., Duncan G. H., Scott D. S., Mao W. Serotonergic activity in man as a function of pain, pain mechanisms, and depression. Anesth Analg. 1981 Dec;60(12):854–861. [PubMed] [Google Scholar]
- Gottfries C. G., Gottfries I., Roos B. E. Homovanillic acid and 5-hydroxyindoleacetic acid in the cerebrospinal fluid of patients with senile dementia, presenile dementia and parkinsonism. J Neurochem. 1969 Sep;16(9):1341–1345. doi: 10.1111/j.1471-4159.1969.tb05984.x. [DOI] [PubMed] [Google Scholar]
- Hyyppä M. T., Scheinin H., Alaranta H., Hurme M., Lahtela K., Scheinin M. Neurotransmission and the experience of low back pain; no association between CSF monoamine metabolites and pain. Pain. 1985 Jan;21(1):57–65. doi: 10.1016/0304-3959(85)90077-6. [DOI] [PubMed] [Google Scholar]
- Johansson B., Roos B. E. 5-hydroxyindoleacetic and homovanillic acid levels in the cerebrospinal fluid of healthy volunteers and patients with Parkinson's syndrome. Life Sci. 1967 Jul 1;6(13):1449–1454. doi: 10.1016/0024-3205(67)90193-2. [DOI] [PubMed] [Google Scholar]
- Kruesi M. J., Swedo S. E., Hamburger S. D., Potter W. Z., Rapoport J. L. Concentration gradient of CSF monoamine metabolites in children and adolescents. Biol Psychiatry. 1988 Sep;24(5):507–514. doi: 10.1016/0006-3223(88)90161-8. [DOI] [PubMed] [Google Scholar]
- Lechin F., van der Dijs B., Lechin M. E., Amat J., Lechin A. E., Cabrera A., Gómez F., Acosta E., Arocha L., Villa S. Pimozide therapy for trigeminal neuralgia. Arch Neurol. 1989 Sep;46(9):960–963. doi: 10.1001/archneur.1989.00520450030015. [DOI] [PubMed] [Google Scholar]
- Liebeskind J. C., Guilbaud G., Besson J. M., Oliveras J. L. Analgesia from electrical stimulation of the periaqueductal gray matter in the cat: behavioral observations and inhibitory effects on spinal cord interneurons. Brain Res. 1973 Feb 28;50(2):441–446. doi: 10.1016/0006-8993(73)90748-8. [DOI] [PubMed] [Google Scholar]
- Matson W. R., Langlais P., Volicer L., Gamache P. H., Bird E., Mark K. A. n-Electrode three-dimensional liquid chromatography with electrochemical detection for determination of neurotransmitters. Clin Chem. 1984 Sep;30(9):1477–1488. [PubMed] [Google Scholar]
- Meek J. L., Neff N. H. Is cerebrospinal fluid the major avenue for the removal of 5-hydroxyindoleacetic acid from the brain? Neuropharmacology. 1973 May;12(5):497–499. doi: 10.1016/0028-3908(73)90067-1. [DOI] [PubMed] [Google Scholar]
- Moir A. T., Ashcroft G. W., Crawford T. B., Eccleston D., Guldberg H. C. Cerebral metabolites in cerebrospinal fluid as a biochemical approach to the brain. Brain. 1970;93(2):357–368. doi: 10.1093/brain/93.2.357. [DOI] [PubMed] [Google Scholar]
- Papeschi R., McClure D. J. Homovanillic and 5-hydroxyindoleacetic acid in cerebrospinal fluid of depressed patients. Arch Gen Psychiatry. 1971 Oct;25(4):354–358. doi: 10.1001/archpsyc.1971.01750160066012. [DOI] [PubMed] [Google Scholar]
- Roy A., Agren H., Pickar D., Linnoila M., Doran A. R., Cutler N. R., Paul S. M. Reduced CSF concentrations of homovanillic acid and homovanillic acid to 5-hydroxyindoleacetic acid ratios in depressed patients: relationship to suicidal behavior and dexamethasone nonsuppression. Am J Psychiatry. 1986 Dec;143(12):1539–1545. doi: 10.1176/ajp.143.12.1539. [DOI] [PubMed] [Google Scholar]
- Roy A., Pickar D., De Jong J., Karoum F., Linnoila M. Norepinephrine and its metabolites in cerebrospinal fluid, plasma, and urine. Relationship to hypothalamic-pituitary-adrenal axis function in depression. Arch Gen Psychiatry. 1988 Sep;45(9):849–857. doi: 10.1001/archpsyc.1988.01800330081010. [DOI] [PubMed] [Google Scholar]
- Sagen J., Winker M. A., Proudfit H. K. Hypoalgesia induced by the local injection of phentolamine in the nucleus raphe magnus: blockade by depletion of spinal cord monoamines. Pain. 1983 Jul;16(3):253–263. doi: 10.1016/0304-3959(83)90113-6. [DOI] [PubMed] [Google Scholar]
- Siever L., Kraemer H., Sack R., Angwin P., Berger P., Zarcone V., Barchas J., Brodie H. K. Gradients of biogenic amine metabolites in cerebrospinal fluid. Dis Nerv Syst. 1975 Jan;36(1):13–16. [PubMed] [Google Scholar]
- Standish-Barry H. M., Bouras N., Hale A. S., Bridges P. K., Bartlett J. R. Ventricular size and CSF transmitter metabolite concentrations in severe endogenous depression. Br J Psychiatry. 1986 Apr;148:386–392. doi: 10.1192/bjp.148.4.386. [DOI] [PubMed] [Google Scholar]
- Stokes P. E., Maas J. W., Davis J. M., Koslow S. H., Casper R. C., Stoll P. M. Biogenic amine and metabolite levels in depressed patients with high versus normal hypothalamic-pituitary-adrenocortical activity. Am J Psychiatry. 1987 Jul;144(7):868–872. doi: 10.1176/ajp.144.7.868. [DOI] [PubMed] [Google Scholar]
- Träskman L., Asberg M., Bertilsson L., Sjöstrand L. Monoamine metabolites in CSF and suicidal behavior. Arch Gen Psychiatry. 1981 Jun;38(6):631–636. doi: 10.1001/archpsyc.1981.01780310031002. [DOI] [PubMed] [Google Scholar]


