Abstract
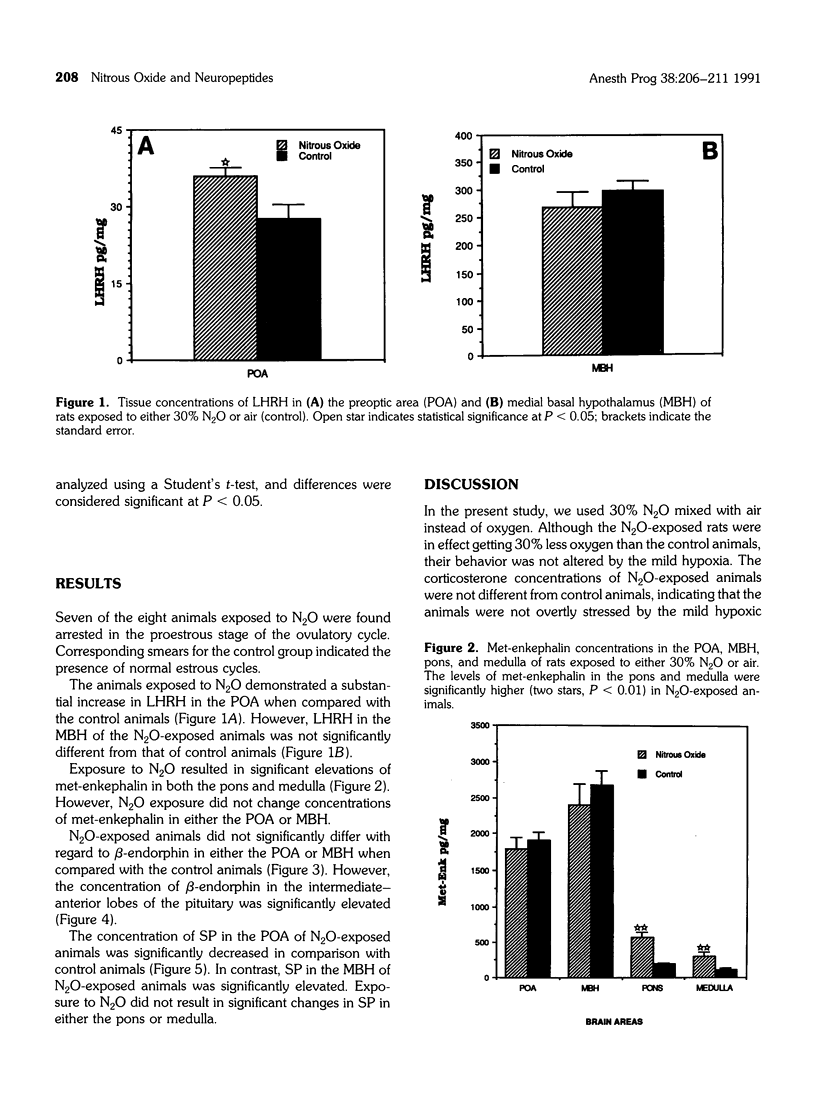
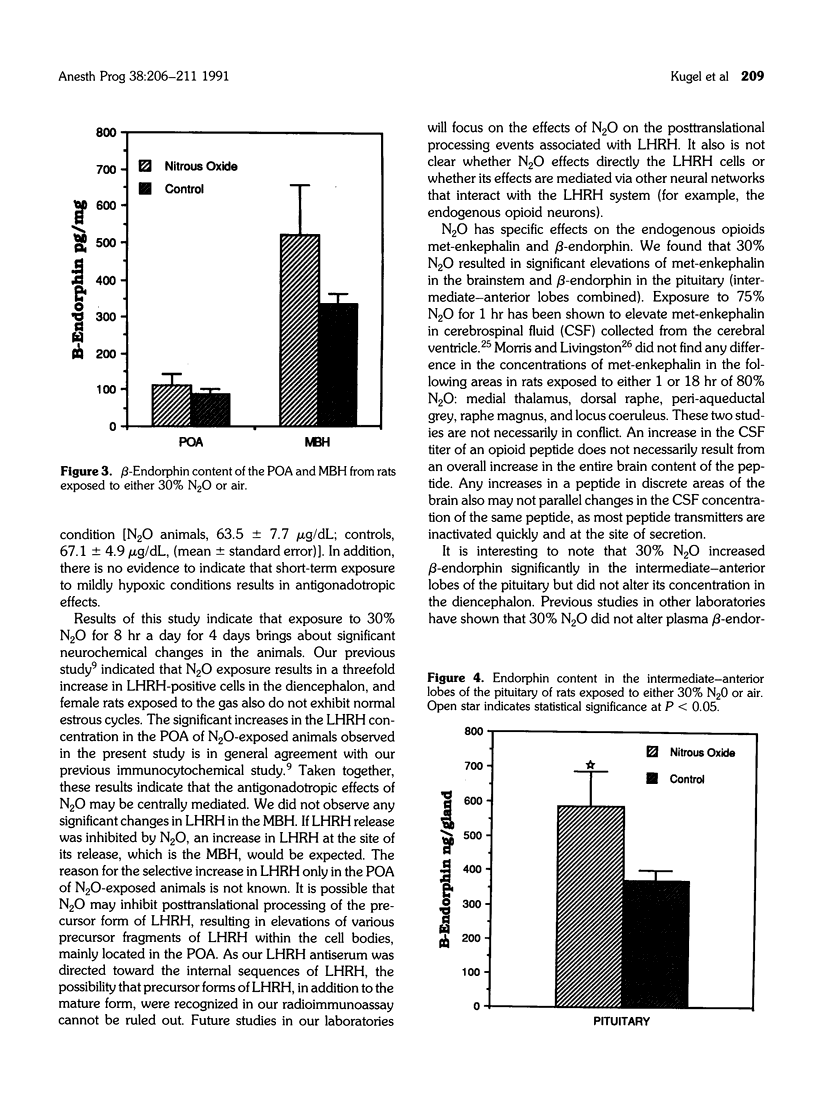
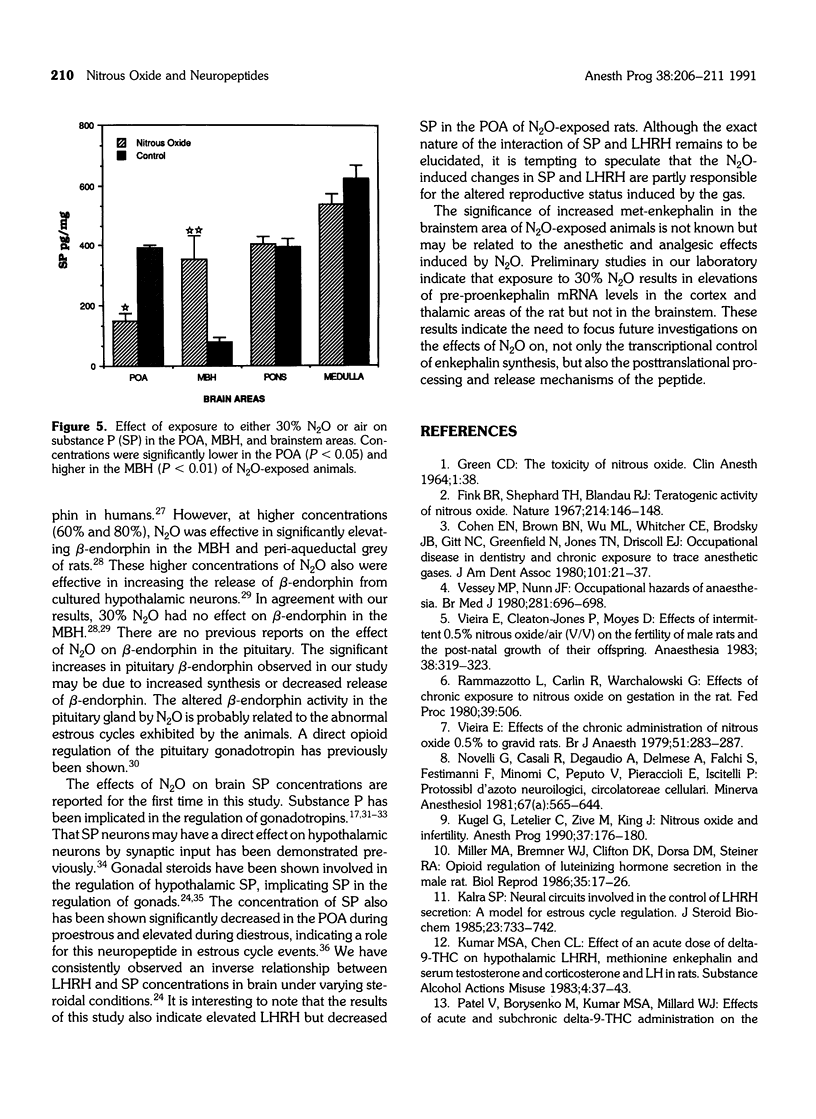
Many studies have indicated that nitrous oxide (N2O) exposure results in specific effects on the reproductive system, some of which are antigonadotropic. The neurochemical events regulating the pituitary-gonadal axis are probably influenced by N2O, but precise documentation is lacking. The effects of exposure to 30% N2O in air on the brain tissue concentrations of luteinizing hormone releasing hormone (LHRH), substance P (SP), met-enkephalin, and beta-endorphin and on beta-endorphin concentrations of the pituitary gland are described in this study. Female rats were exposed to either N2O or air for 8 hr a day over one estrous cycle, and the brain and pituitary tissues were collected and processed. Neuropeptide concentrations were measured by specific radioimmunoassays. Exposure to N2O resulted in significant elevation of LHRH in the preoptic area, with a concomitant decrease in SP. The SP concentration of the medial basal hypothalamus was significantly elevated in N2O-exposed animals. Exposure to N2O resulted in significant increases in met-enkephalin in the brainstem area and beta-endorphin in the pituitary. These results suggest that exposure to N2O alters the interactive neural system activity regulating gonadotropin secretion from the pituitary. The significance of increased met-enkephalin in the brainstem of N2O-exposed animals is not known.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arisawa M., De Palatis L., Ho R., Snyder G. D., Yu W. H., Pan G., McCann S. M. Stimulatory role of substance P on gonadotropin release in ovariectomized rats. Neuroendocrinology. 1990 May;51(5):523–529. doi: 10.1159/000125386. [DOI] [PubMed] [Google Scholar]
- Bergström L., Sakurada T., Terenius L. Substance P levels in various regions of the rat central nervous system after acute and chronic morphine treatment. Life Sci. 1984 Dec 3;35(23):2375–2382. doi: 10.1016/0024-3205(84)90530-7. [DOI] [PubMed] [Google Scholar]
- Brown E. R., Harlan R. E., Krause J. E. Gonadal steroid regulation of substance P (SP) and SP-encoding messenger ribonucleic acids in the rat anterior pituitary and hypothalamus. Endocrinology. 1990 Jan;126(1):330–340. doi: 10.1210/endo-126-1-330. [DOI] [PubMed] [Google Scholar]
- Chao C. C., Moss G. E., Malven P. V. Direct opioid regulation of pituitary release of bovine luteinizing hormone. Life Sci. 1986 Aug 11;39(6):527–534. doi: 10.1016/0024-3205(86)90509-6. [DOI] [PubMed] [Google Scholar]
- Clement-Jones V., Lowry P. J., Rees L. H., Besser G. M. Development of a specific extracted radioimmunoassay for methionine enkephalin in human plasma and cerebrospinal fluid. J Endocrinol. 1980 Aug;86(2):231–243. doi: 10.1677/joe.0.0860231. [DOI] [PubMed] [Google Scholar]
- Cohen E. N., Gift H. C., Brown B. W., Greenfield W., Wu M. L., Jones T. W., Whitcher C. E., Driscoll E. J., Brodsky J. B. Occupational disease in dentistry and chronic exposure to trace anesthetic gases. J Am Dent Assoc. 1980 Jul;101(1):21–31. doi: 10.14219/jada.archive.1980.0345. [DOI] [PubMed] [Google Scholar]
- Dees W. L., Skelley C. W., Kozlowski G. P. Central effects of an antagonist and an antiserum to substance P on serum gonadotropin and prolactin secretion. Life Sci. 1985 Oct 28;37(17):1627–1631. doi: 10.1016/0024-3205(85)90482-5. [DOI] [PubMed] [Google Scholar]
- Evans S. F., Stringer M., Bukht M. D., Thomas W. A., Tomlin S. J. Nitrous oxide inhalation does not influence plasma concentrations of beta-endorphin or Met-enkephalin-like immunoreactivity. Br J Anaesth. 1985 Jun;57(6):624–628. doi: 10.1093/bja/57.6.624. [DOI] [PubMed] [Google Scholar]
- Fink B. R., Shepard T. H., Blandau R. J. Teratogenic activity of nitrous oxide. Nature. 1967 Apr 8;214(5084):146–148. doi: 10.1038/214146a0. [DOI] [PubMed] [Google Scholar]
- Frankfurt M., Siegel R. A., Sim I., Wuttke W. Estrous cycle variations in cholecystokinin and substance P concentrations in discrete areas of the rat brain. Neuroendocrinology. 1986;42(3):226–231. doi: 10.1159/000124444. [DOI] [PubMed] [Google Scholar]
- Hoffman G. E. Organization of LHRH cells: differential apposition of neurotensin, substance P and catecholamine axons. Peptides. 1985 May-Jun;6(3):439–461. doi: 10.1016/0196-9781(85)90110-x. [DOI] [PubMed] [Google Scholar]
- Kalra S. P. Neural circuits involved in the control of LHRH secretion: a model for estrous cycle regulation. J Steroid Biochem. 1985 Nov;23(5B):733–742. doi: 10.1016/s0022-4731(85)80009-1. [DOI] [PubMed] [Google Scholar]
- Kream R. M., Schoenfeld T. A., Mancuso R., Clancy A. N., el-Bermani W., Macrides F. Precursor forms of substance P (SP) in nervous tissue: detection with antisera to SP, SP-Gly, and SP-Gly-Lys. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4832–4836. doi: 10.1073/pnas.82.14.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugel G., Letelier C., Zive M. A., King J. C. Nitrous oxide and infertility. Anesth Prog. 1990 Jul;37(4):176–180. [PMC free article] [PubMed] [Google Scholar]
- Kumar A. M., Haney M., Becker T., Thompson M. L., Kream R. M., Miczek K. Effect of early exposure to delta-9-tetrahydrocannabinol on the levels of opioid peptides, gonadotropin-releasing hormone and substance P in the adult male rat brain. Brain Res. 1990 Aug 13;525(1):78–83. doi: 10.1016/0006-8993(90)91322-8. [DOI] [PubMed] [Google Scholar]
- Kumar A. M., Solomon J., Patel V., Kream R. M., Drieze J. M., Millard W. J. Early exposure to delta 9-tetrahydrocannabinol influences neuroendocrine and reproductive functions in female rats. Neuroendocrinology. 1986;44(2):260–264. doi: 10.1159/000124654. [DOI] [PubMed] [Google Scholar]
- Kumar M. S., Chen C. L. Effect of an acute dose of delta 9-THC on hypothalamic luteinizing hormone releasing hormone and met-enkephalin content and serum levels of testosterone and corticosterone in rats. Subst Alcohol Actions Misuse. 1983;4(1):37–43. [PubMed] [Google Scholar]
- Macrides F., Schoenfeld T. A., Clancy A. N., Kumar M. S., Kream R. M. Hormones and neurotransmitters in the mammalian olfactory system. Ann N Y Acad Sci. 1987;519:150–164. doi: 10.1111/j.1749-6632.1987.tb36294.x. [DOI] [PubMed] [Google Scholar]
- Marchand J. E., Hershman K., Kumar M. S., Thompson M. L., Kream R. M. Disulfiram administration affects substance P-like immunoreactive and monoaminergic neural systems in rodent brain. J Biol Chem. 1990 Jan 5;265(1):264–273. [PubMed] [Google Scholar]
- Miller M. A., Bremner W. J., Clifton D. K., Dorsa D. M., Steiner R. A. Opioid regulation of luteinizing hormone secretion in the male rat. Biol Reprod. 1986 Aug;35(1):17–26. doi: 10.1095/biolreprod35.1.17. [DOI] [PubMed] [Google Scholar]
- Morris B., Livingston A. Effects of nitrous oxide exposure on Met-enkephalin levels in discrete areas of rat brain. Neurosci Lett. 1984 Mar 9;45(1):11–14. doi: 10.1016/0304-3940(84)90321-5. [DOI] [PubMed] [Google Scholar]
- Novelli G. P., Casali R., De Gaudio A. R., Del Mese A., Falchi S., Festimanni F., Minoni C., Peduto V. A., Pieraccioli E., Piscitelli P. Il protossido d'azoto. Effetti neurologici, circolatori e cellulari. Minerva Anestesiol. 1981 Sep;47(9):565–644. [PubMed] [Google Scholar]
- Ohtsuka S., Miyake A., Nishizaki T., Tasaka K., Aono T., Tanizawa O. Substance P stimulates gonadotropin-releasing hormone release from rat hypothalamus in vitro with involvement of oestrogen. Acta Endocrinol (Copenh) 1987 Jun;115(2):247–252. doi: 10.1530/acta.0.1150247. [DOI] [PubMed] [Google Scholar]
- Patel V., Borysenko M., Kumar M. S., Millard W. J. Effects of acute and subchronic delta 9-tetrahydrocannabinol administration on the plasma catecholamine, beta-endorphin, and corticosterone levels and splenic natural killer cell activity in rats. Proc Soc Exp Biol Med. 1985 Nov;180(2):400–404. doi: 10.3181/00379727-180-42195. [DOI] [PubMed] [Google Scholar]
- Picanço-Diniz D. L., Valença M. M., Franci C. R., Antunes-Rodrigues J. Role of substance P in the medial preoptic area in the regulation of gonadotropin and prolactin secretion in normal or orchidectomized rats. Neuroendocrinology. 1990 Jun;51(6):675–682. doi: 10.1159/000125409. [DOI] [PubMed] [Google Scholar]
- Quock R. M., Kouchich F. J., Tseng L. F. Does nitrous oxide induce release of brain opioid peptides? Pharmacology. 1985;30(2):95–99. doi: 10.1159/000138056. [DOI] [PubMed] [Google Scholar]
- Tsuruo Y., Hisano S., Nakanishi J., Katoh S., Daikoku S. Immunohistochemical studies on the roles of substance P in the rat hypothalamus: possible implication in the hypothalamic-hypophysial-gonadal axis. Neuroendocrinology. 1987 May;45(5):389–401. doi: 10.1159/000124764. [DOI] [PubMed] [Google Scholar]
- Vessey M. P., Nunn J. F. Occupational hazards of anesthesia. Br Med J. 1980 Sep 13;281(6242):696–698. doi: 10.1136/bmj.281.6242.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira E., Cleaton-Jones P., Moyes D. Effects of intermittent 0.5% nitrous oxide/air (v/v) on the fertility of male rats and the post-natal growth of their offspring. Anaesthesia. 1983 Apr;38(4):319–323. doi: 10.1111/j.1365-2044.1983.tb10452.x. [DOI] [PubMed] [Google Scholar]
- Vieira E. Effect of the chronic administration of nitrous oxide 0.5% to gravid rats. Br J Anaesth. 1979 Apr;51(4):283–287. doi: 10.1093/bja/51.4.283. [DOI] [PubMed] [Google Scholar]
- Vijayan E., McCann S. M. In vivo and in vitro effects of substance P and neurotensin on gonadotropin and prolactin release. Endocrinology. 1979 Jul;105(1):64–68. doi: 10.1210/endo-105-1-64. [DOI] [PubMed] [Google Scholar]
- Zuniga J. R., Joseph S. A., Knigge K. M. The effects of nitrous oxide on the secretory activity of pro-opiomelanocortin peptides from basal hypothalamic cells attached to cytodex beads in a superfusion in vitro system. Brain Res. 1987 Sep 8;420(1):66–72. doi: 10.1016/0006-8993(87)90240-x. [DOI] [PubMed] [Google Scholar]
- Zuniga J. R., Knigge K. K., Joseph S. A. Central beta-endorphin release and recovery after exposure to nitrous oxide in rats. J Oral Maxillofac Surg. 1986 Sep;44(9):714–718. [PubMed] [Google Scholar]



