Abstract
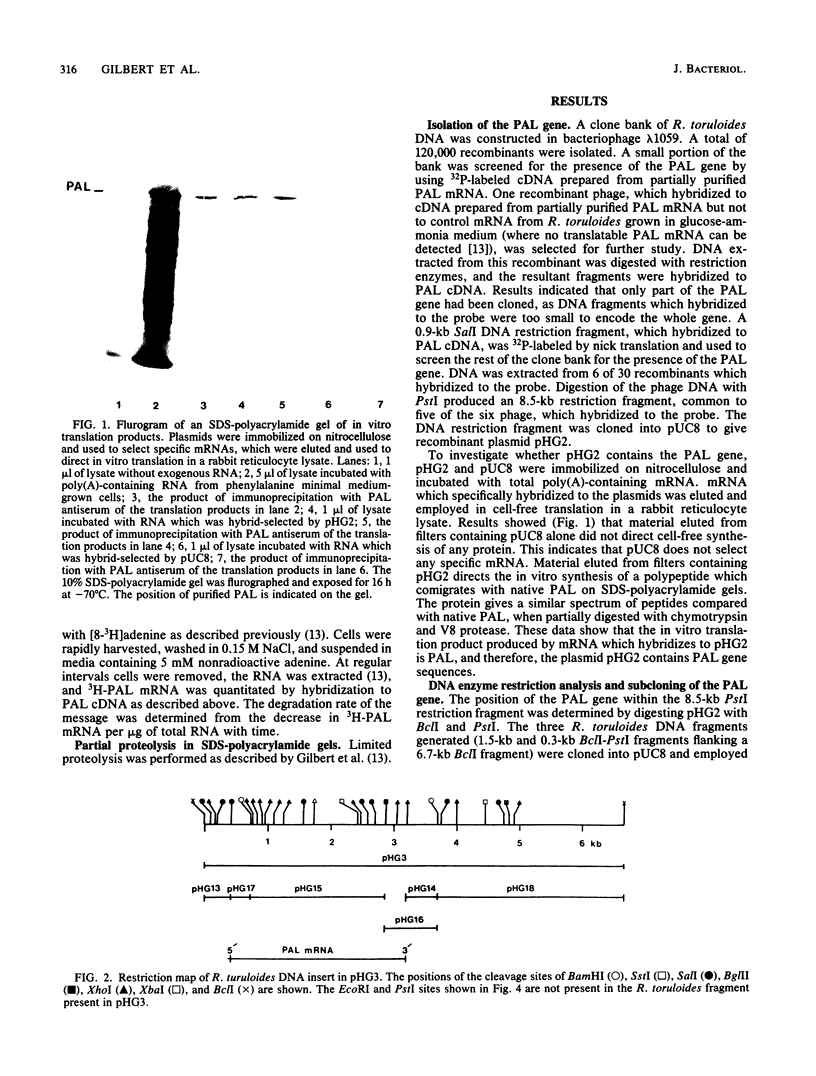
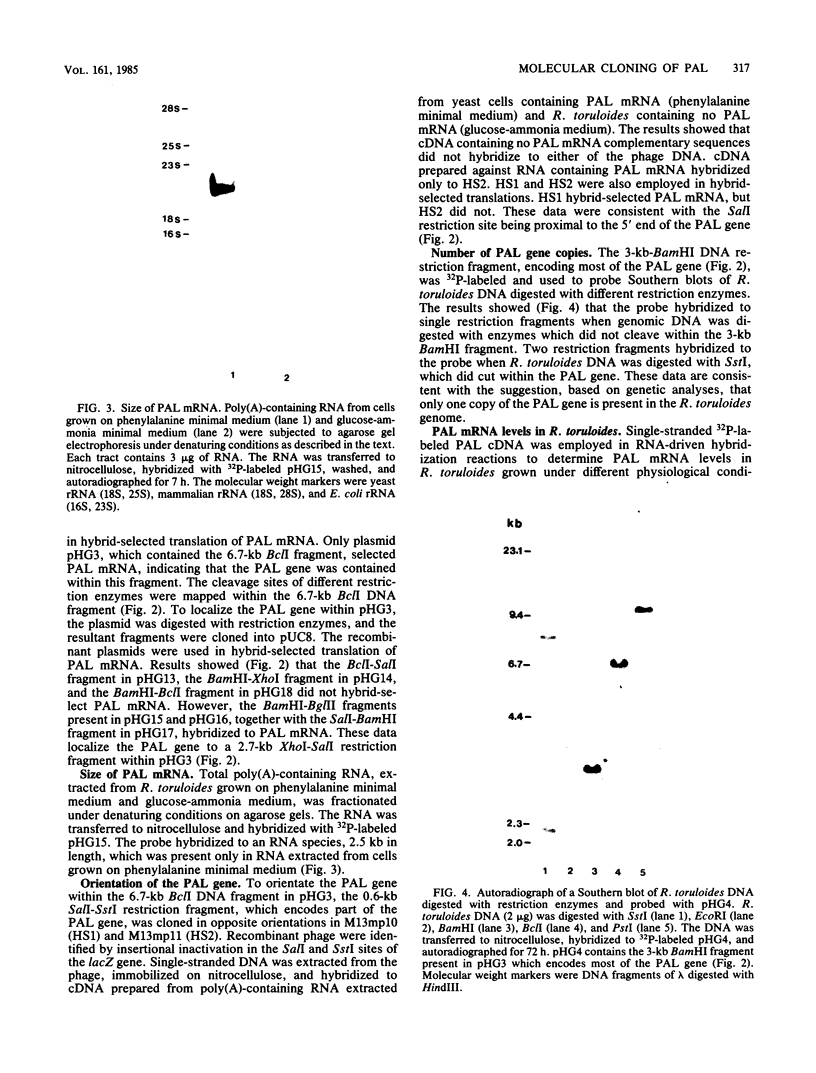
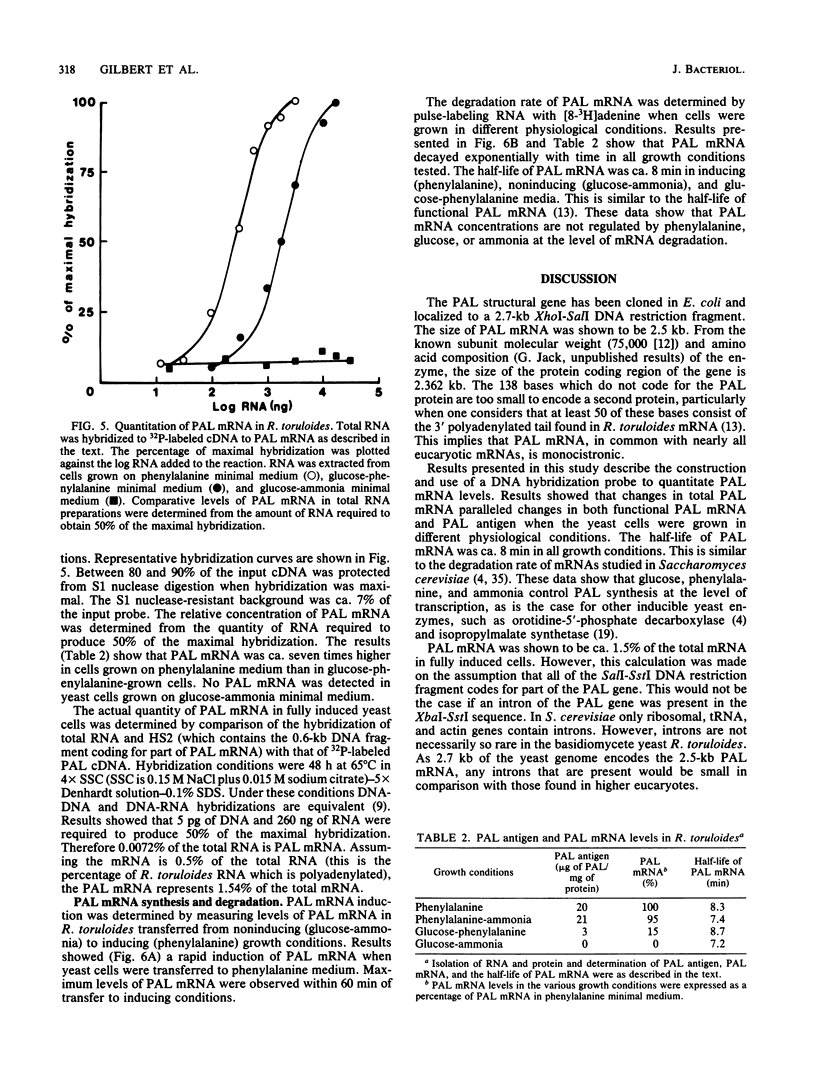
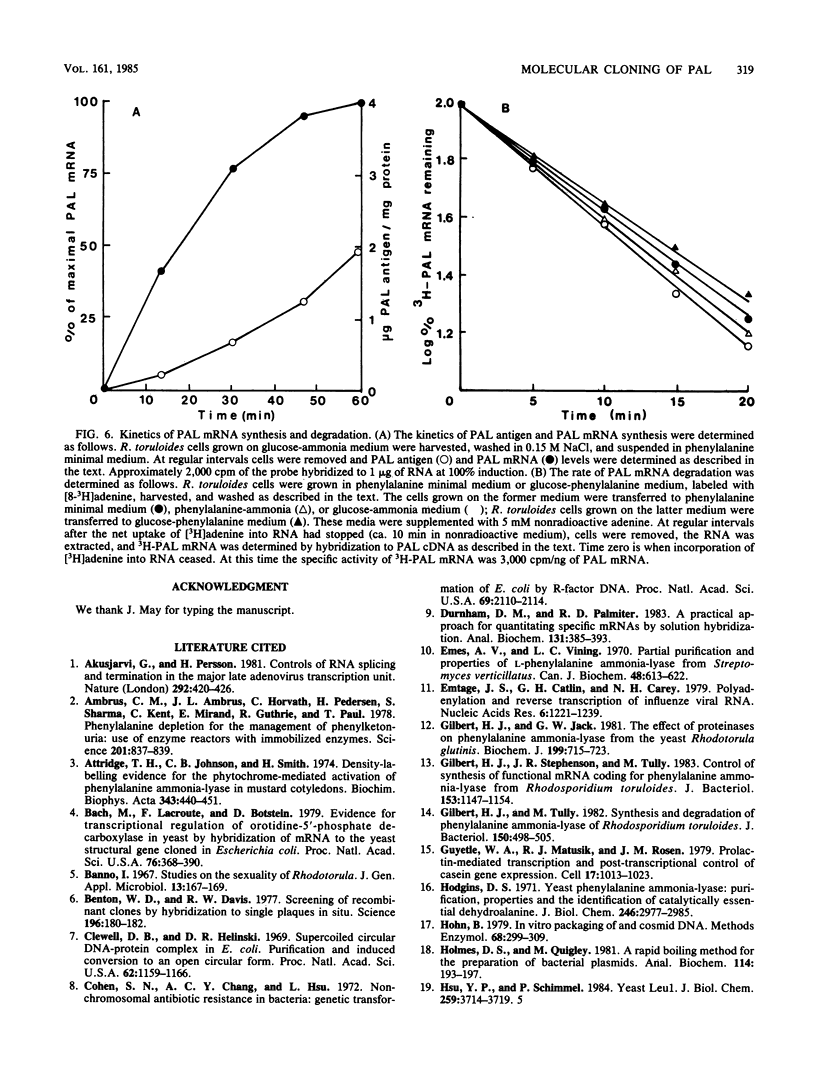
A genomic library of Rhodosporidium toruloides DNA was constructed in bacteriophage lambda 1059. Recombinant phage containing phenylalanine ammonia lyase (PAL) gene sequences were identified by using 32P-labeled cDNA to partially purified PAL mRNA. The PAL gene was subcloned on an 8.5-kilobase PstI DNA restriction fragment into pUC8 to generate the recombinant plasmid pHG2. A restriction map of the PAL gene, together with its flanking regions, was constructed. Northern hybridization analysis of R. toruloides RNA with a restriction fragment encoding part of the PAL gene indicates that PAL mRNA is 2.5 kilobases in length. A single-stranded DNA hybridization probe was constructed and used to quantitate PAL mRNA levels in R. toruloides grown under different physiological conditions. PAL mRNA levels paralleled changes in functional PAL mRNA and antigen. These data are consistent with control of PAL expression being at the level of transcription.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Persson H. Controls of RNA splicing and termination in the major late adenovirus transcription unit. Nature. 1981 Jul 30;292(5822):420–426. doi: 10.1038/292420a0. [DOI] [PubMed] [Google Scholar]
- Ambrus C. M., Ambrus J. L., Horvath C., Pedersen H., Sharma S., Kant C., Mirand E., Guthrie R., Paul T. Phenylalanine depletion for the management of phenylketonuria: use of enzyme reactors with immobilized enzymes. Science. 1978 Sep 1;201(4358):837–839. doi: 10.1126/science.567372. [DOI] [PubMed] [Google Scholar]
- Attridge T. H., Johnson C. B., Smith H. Density-labelling evidence for the phytochrome-mediated activation of phenylalanine ammonia-lyase in mustard cotyledons. Biochim Biophys Acta. 1974 May 24;343(3):440–451. doi: 10.1016/0304-4165(74)90261-x. [DOI] [PubMed] [Google Scholar]
- Bach M. L., Lacroute F., Botstein D. Evidence for transcriptional regulation of orotidine-5'-phosphate decarboxylase in yeast by hybridization of mRNA to the yeast structural gene cloned in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):386–390. doi: 10.1073/pnas.76.1.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. A practical approach for quantitating specific mRNAs by solution hybridization. Anal Biochem. 1983 Jun;131(2):385–393. doi: 10.1016/0003-2697(83)90188-4. [DOI] [PubMed] [Google Scholar]
- Emes A. V., Vining L. C. Partial purification and properties of L-phenylalanine ammonia-lyase from Streptomyces verticillatus. Can J Biochem. 1970 May;48(5):613–622. doi: 10.1139/o70-099. [DOI] [PubMed] [Google Scholar]
- Emtage J. S., Catlin G. H., Carey N. H. Polyadenylation and reverse transcription of influenza viral RNA. Nucleic Acids Res. 1979 Apr;6(4):1221–1239. doi: 10.1093/nar/6.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert H. J., Jack G. W. The effect of proteinases on phenylalanine ammonia-lyase from the yeast Rhodotorula glutinis. Biochem J. 1981 Dec 1;199(3):715–723. doi: 10.1042/bj1990715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert H. J., Stephenson J. R., Tully M. Control of synthesis of functional mRNA coding for phenylalanine ammonia-lyase from Rhodosporidium toruloides. J Bacteriol. 1983 Mar;153(3):1147–1154. doi: 10.1128/jb.153.3.1147-1154.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Hodgins D. S. Yeast phenylalanine ammonia-lyase. Purification, properties, and the identification of catalytically essential dehydroalanine. J Biol Chem. 1971 May 10;246(9):2977–2985. [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hsu Y. P., Schimmel P. Yeast LEU1. Repression of mRNA levels by leucine and relationship of 5'-noncoding region to that of LEU2. J Biol Chem. 1984 Mar 25;259(6):3714–3719. [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L., Cesareni G. Novel bacteriophage lambda cloning vector. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5172–5176. doi: 10.1073/pnas.77.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marusich W. C., Jensen R. A., Zamir L. O. Induction of L-phenylalanine ammonia-lyase during utilization of phenylalanine as a carbon or nitrogen source in Rhodotorula glutinis. J Bacteriol. 1981 Jun;146(3):1013–1019. doi: 10.1128/jb.146.3.1013-1019.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricca G. A., Hamilton R. W., McLean J. W., Conn A., Kalinyak J. E., Taylor J. M. Rat alpha 1-acid glycoprotein mRNA. Cloning of double-stranded cDNA and kinetics of induction of mRNA levels following acute inflammation. J Biol Chem. 1981 Oct 25;256(20):10362–10368. [PubMed] [Google Scholar]
- Schröder J., Kreuzaler F., Schäfer E., Hahlbrock K. Concomitant induction of phenylalanine ammonia-lyase and flavanone synthase mRNAs in irradiated plant cells. J Biol Chem. 1979 Jan 10;254(1):57–65. [PubMed] [Google Scholar]
- Sikora L. A., Marzluf G. A. Regulation of L-phenylalanine ammonia-lyase by L-phenylalanine and nitrogen in Neurospora crassa. J Bacteriol. 1982 Jun;150(3):1287–1291. doi: 10.1128/jb.150.3.1287-1291.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S., Nabe K., Izuo N., Nakamichi K., Chibata I. Production of l-Phenylalanine from trans-Cinnamic Acid with Rhodotorula glutinis Containing l-Phenylalanine Ammonia-Lyase Activity. Appl Environ Microbiol. 1981 Nov;42(5):773–778. doi: 10.1128/aem.42.5.773-778.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitomer R. S., Montgomery D. L., Nichols D. L., Hall B. D. Transcriptional regulation of the yeast cytochrome c gene. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3627–3631. doi: 10.1073/pnas.76.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]