Abstract
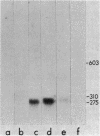
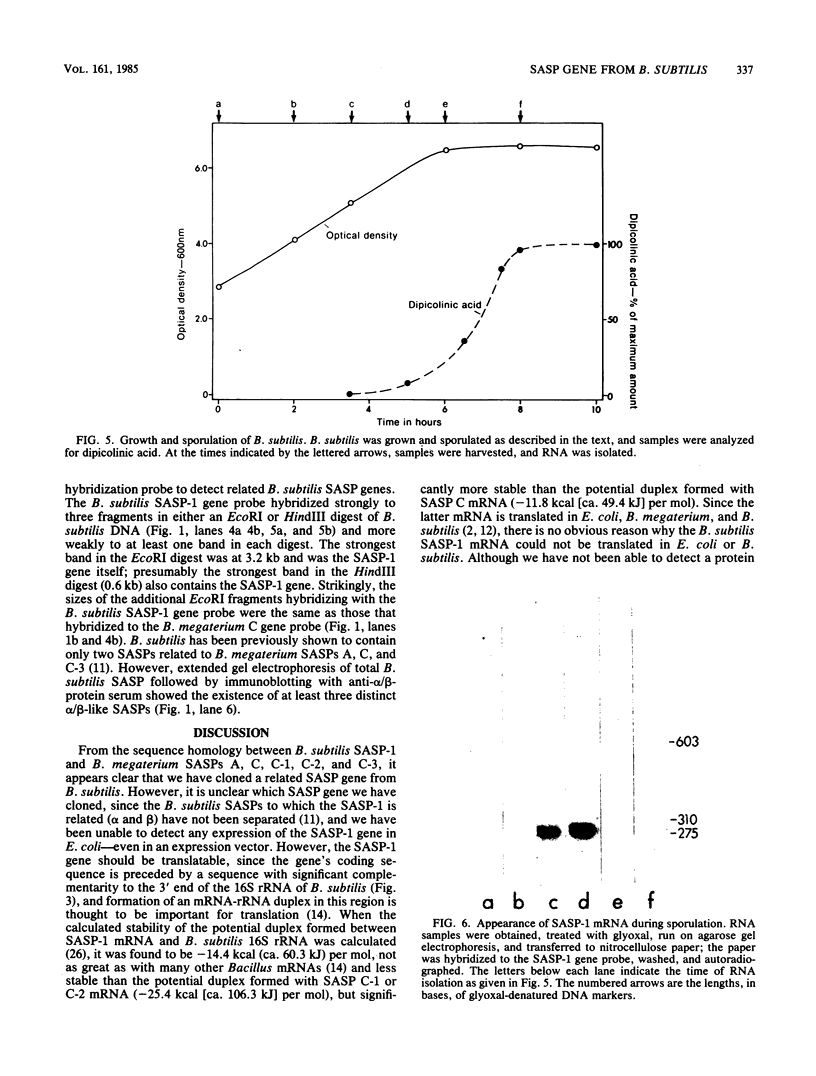
The first Bacillus subtilis small, acid-soluble spore protein (SASP) gene has been cloned by using previously cloned B. megaterium SASP genes as DNA-DNA hybridization probes. Determination of the DNA sequence of the B. subtilis SASP gene showed that it codes for a 72-residue protein (termed SASP-1) containing a single spore protease cleavage site as well as other sequences conserved in Bacillus megaterium SASPs A, C, C-1, C-2, and C-3. The B. subtilis SASP-1 genes's coding sequence is preceded by a potential Bacillus ribosome-binding site, and is followed by a sequence that could form a stem-and-loop structure characteristic of transcription termination sites. Upstream from the coding sequence there are no obvious homologies with other B. subtilis sporulation genes, but similarities with B. megaterium SASP genes are evident. SASP-1 mRNA (290 bases long) is absent from vegetative cells, but appears midway in sporulation and then disappears. The cloned SASP-1 gene hybridizes to three bands other than the SASP-1 gene itself in EcoRI or HindIII digests of B. subtilis DNA. Presumably these other bands represent SASP genes related to the SASP-1 gene, and we have been able to detect at least three such proteins in B. subtilis spores.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Curiel-Quesada E., Setlow B., Setlow P. Cloning of the gene for C protein, a low molecular weight spore-specific protein from Bacillus megaterium. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3250–3254. doi: 10.1073/pnas.80.11.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel-Quesada E., Setlow P. Cloning of a new low-molecular-weight spore-specific protein gene from Bacillus megaterium. J Bacteriol. 1984 Mar;157(3):751–757. doi: 10.1128/jb.157.3.751-757.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam S. S., Setlow P. In vivo and in vitro synthesis of the spore-specific proteins A and C of bacillus megaterium. J Biol Chem. 1980 Sep 25;255(18):8417–8423. [PubMed] [Google Scholar]
- Ferrari E., Henner D. J., Hoch J. A. Isolation of Bacillus subtilis genes from a charon 4A library. J Bacteriol. 1981 Apr;146(1):430–432. doi: 10.1128/jb.146.1.430-432.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari F. A., Nguyen A., Lang D., Hoch J. A. Construction and properties of an integrable plasmid for Bacillus subtilis. J Bacteriol. 1983 Jun;154(3):1513–1515. doi: 10.1128/jb.154.3.1513-1515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliss E. R., Setlow P. Complete nucleotide sequence and start sites for transcription and translation of the Bacillus megaterium protein C gene. J Bacteriol. 1984 Jun;158(3):809–813. doi: 10.1128/jb.158.3.809-813.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrick S., Setlow P. Expression of a Bacillus megaterium sporulation-specific gene during sporulation of Bacillus subtilis. J Bacteriol. 1983 Sep;155(3):1459–1462. doi: 10.1128/jb.155.3.1459-1462.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldenwang W. G., Banner C. D., Ollington J. F., Losick R., Hoch J. A., O'Connor M. B., Sonenshein A. L. Mapping a cloned gene under sporulation control by inserttion of a drug resistance marker into the Bacillus subtilis chromosome. J Bacteriol. 1980 Apr;142(1):90–98. doi: 10.1128/jb.142.1.90-98.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. C., Tipper D. J. Acid-soluble spore proteins of Bacillus subtilis. J Bacteriol. 1981 Jun;146(3):972–982. doi: 10.1128/jb.146.3.972-982.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. M., Setlow P. Expression of Bacillus megaterium and Bacillus subtilis small acid-soluble spore protein genes during stationary-phase growth of asporogenous B. subtilis mutants. J Bacteriol. 1984 Mar;157(3):931–933. doi: 10.1128/jb.157.3.931-933.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Localization of low-molecular-weight basic proteins in Bacillus megaterium spores by cross-linking with ultraviolet light. J Bacteriol. 1979 Aug;139(2):486–494. doi: 10.1128/jb.139.2.486-494.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Ozols J. Covalent structure of protein A. A low molecular weight protein degraded during germination of Bacillus megaterium spores. J Biol Chem. 1979 Dec 10;254(23):11938–11942. [PubMed] [Google Scholar]
- Setlow P., Ozols J. Covalent structure of protein C. A second major low molecular weight protein degraded during germination of Bacillus megaterium spores. J Biol Chem. 1980 Sep 25;255(18):8413–8416. [PubMed] [Google Scholar]
- Setlow P., Ozols J. The complete covalent structure of protein B. The third major protein degraded during germination of Bacillus megaterium spores. J Biol Chem. 1980 Nov 10;255(21):10445–10450. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary P. S., Garbe J. C., Franzen M., Frampton E. W. MP13, a generalized transducing bacteriophage for Bacillus megaterium. J Bacteriol. 1982 Mar;149(3):1112–1119. doi: 10.1128/jb.149.3.1112-1119.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K., Johnson W. C., Tipper D. J., Setlow P. Comparison of various properties of low-molecular-weight proteins from dormant spores of several Bacillus species. J Bacteriol. 1981 Jun;146(3):965–971. doi: 10.1128/jb.146.3.965-971.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]