Abstract
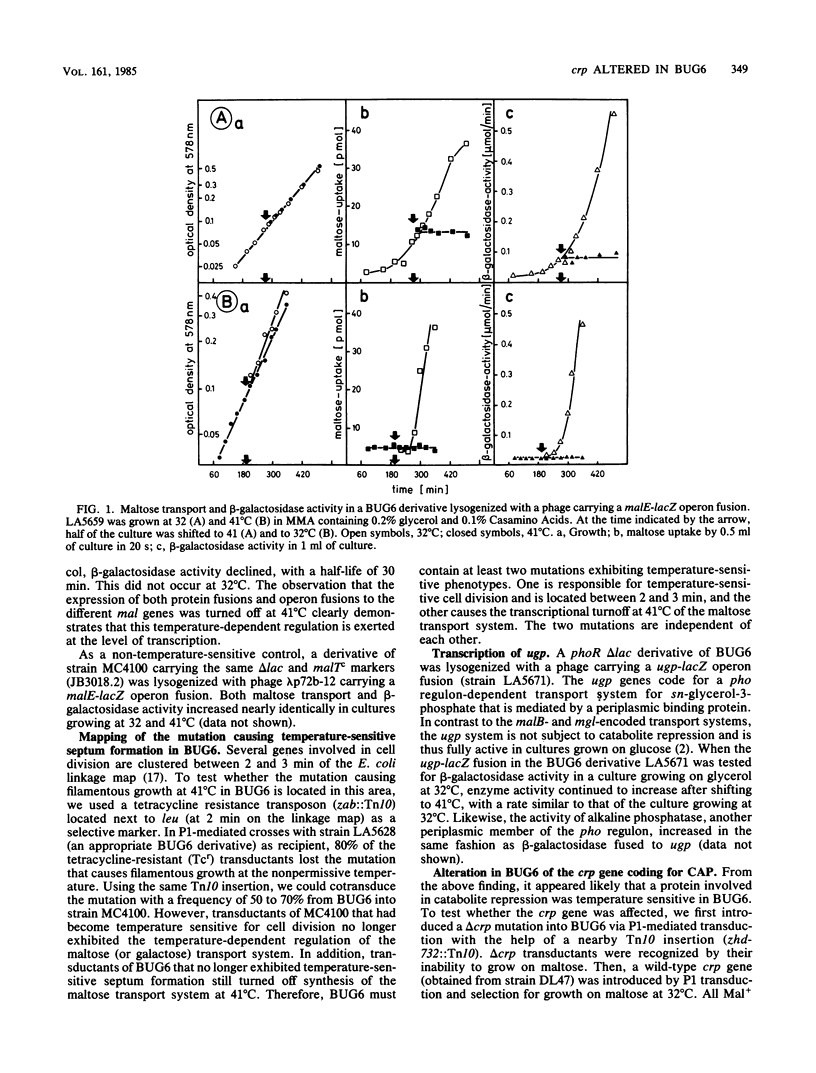
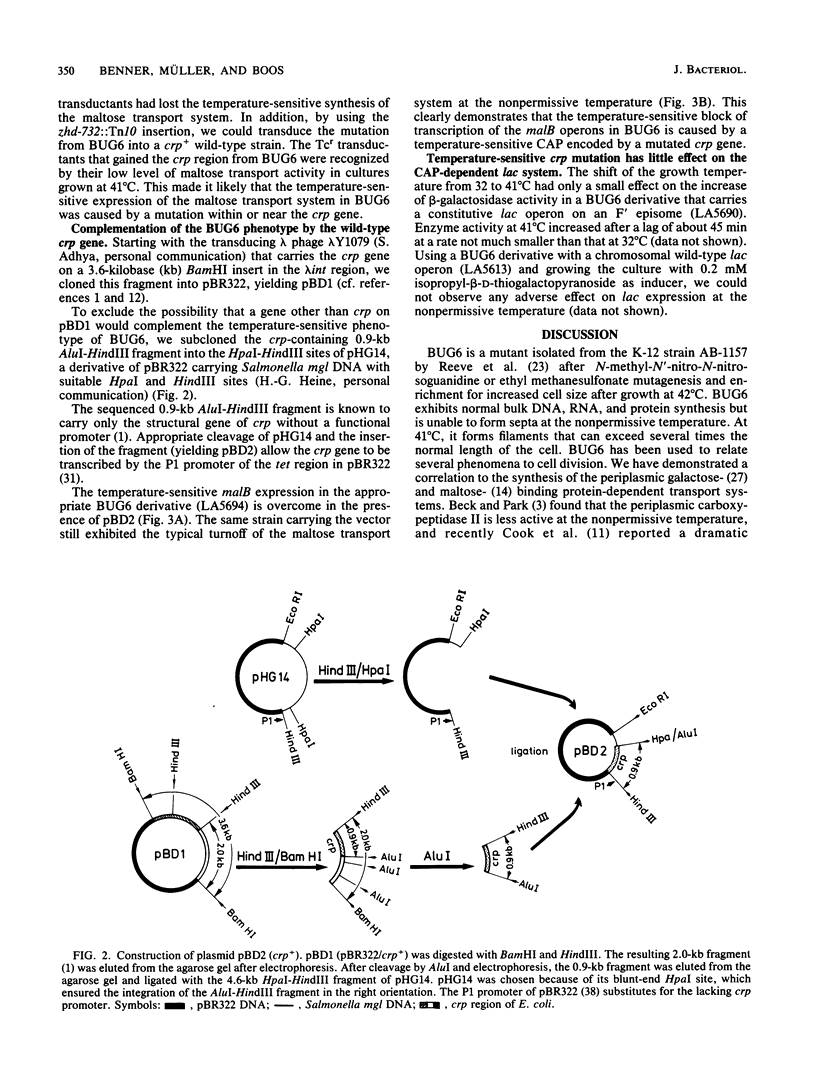
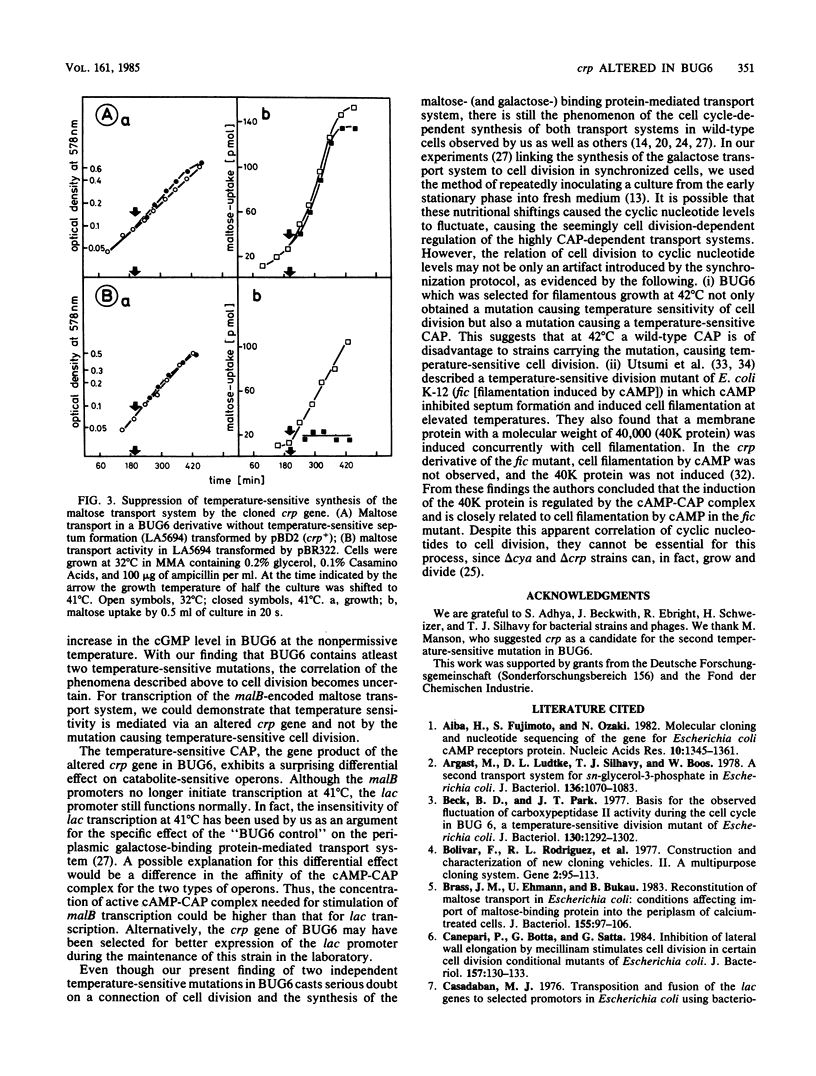
BUG6 is a temperature-sensitive cell division mutant which forms filaments at the nonpermissive temperature. Synthesis of the maltose- and galactose-binding protein-dependent transport systems is also temperature sensitive in BUG6. Using operon and protein fusions of the maltose transport genes to lacZ, we observed that the temperature-sensitive control of the maltose transport system in BUG6 occurs at the transcriptional level. By P1-mediated transductions, we found that BUG6 contains two independent temperature-sensitive mutations. One maps between 2 and 3 min on the Escherichia coli linkage map, in close proximity to the fts-envA region. This mutation is responsible for temperature-sensitive cell division. The other mutation maps at 73 min in crp, the structural gene of the catabolite activator protein. The latter could be complemented by a hybrid plasmid carrying the wild-type crp as the only gene on a 0.9-kilobase HindIII-AluI restriction fragment. The mutation in crp alone was found to be responsible for the temperature-sensitive synthesis of the maltose transport system. Although it causes a complete block of transcription of the maltose transport genes at 41 degrees C, this mutation had only a marginal effect on the transcription of the lac operon.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Fujimoto S., Ozaki N. Molecular cloning and nucleotide sequencing of the gene for E. coli cAMP receptor protein. Nucleic Acids Res. 1982 Feb 25;10(4):1345–1361. doi: 10.1093/nar/10.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argast M., Ludtke D., Silhavy T. J., Boos W. A second transport system for sn-glycerol-3-phosphate in Escherichia coli. J Bacteriol. 1978 Dec;136(3):1070–1083. doi: 10.1128/jb.136.3.1070-1083.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck B. D., Park J. T. Basis for the observed fluctuation of carboxypeptidase II activity during the cell cycle in BUG 6, a temperature-sensitive division mutant of Escherichia coli. J Bacteriol. 1977 Jun;130(3):1292–1302. doi: 10.1128/jb.130.3.1292-1302.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brass J. M., Ehmann U., Bukau B. Reconstitution of maltose transport in Escherichia coli: conditions affecting import of maltose-binding protein into the periplasm of calcium-treated cells. J Bacteriol. 1983 Jul;155(1):97–106. doi: 10.1128/jb.155.1.97-106.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canepari P., Botta G., Satta G. Inhibition of lateral wall elongation by mecillinam stimulates cell division in certain cell division conditional mutants of Escherichia coli. J Bacteriol. 1984 Jan;157(1):130–133. doi: 10.1128/jb.157.1.130-133.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapon C., Kolb A. Action of CAP on the malT promoter in vitro. J Bacteriol. 1983 Dec;156(3):1135–1143. doi: 10.1128/jb.156.3.1135-1143.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapon C. Role of the catabolite activator protein in the maltose regulon of Escherichia coli. J Bacteriol. 1982 May;150(2):722–729. doi: 10.1128/jb.150.2.722-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux M., Feutrier J., Lepelletier M., Touati-Schwartz D., Pascal M. C. Selection of recA+ recombinant cosmids: an easy method for making recA strains temporarily Rec+, permitting P1-mediated transduction in a recA background and transduction of a recA mutation. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1269–1271. doi: 10.1016/0006-291x(82)91249-9. [DOI] [PubMed] [Google Scholar]
- Cook W. R., Ringler N., Black B., Bernlohr R. W. Accumulation of cyclic GMP in filaments of Escherichia coli BUG6. J Bacteriol. 1983 Jul;155(1):69–73. doi: 10.1128/jb.155.1.69-73.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart P., Gicquel-Sanzey B. Cloning and sequence of the crp gene of Escherichia coli K 12. Nucleic Acids Res. 1982 Feb 25;10(4):1363–1378. doi: 10.1093/nar/10.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler R. G., Evans J. E. Synchronization of bacteria by a stationary-phase method. J Bacteriol. 1966 Feb;91(2):469–476. doi: 10.1128/jb.91.2.469-476.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzel I., Kolb V., Boos W. Pole cap formation in Escherichia coli following induction of the maltose-binding protein. Arch Microbiol. 1978 Aug 1;118(2):207–218. doi: 10.1007/BF00415731. [DOI] [PubMed] [Google Scholar]
- Fowler A. V. High-level production of -galactosidase by Escherichia coli merodiploids. J Bacteriol. 1972 Nov;112(2):856–860. doi: 10.1128/jb.112.2.856-860.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J. F. Coupling of DNA replication and cell division: sulB is an allele of ftsZ. J Bacteriol. 1983 Jun;154(3):1339–1346. doi: 10.1128/jb.154.3.1339-1346.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki M. Correlation between metabolism of phosphatidylglycerol and membrane synthesis in Escherichia coli. J Mol Biol. 1972 Jul 21;68(2):249–264. doi: 10.1016/0022-2836(72)90212-4. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Roa M., Braun-Breton C., Schwartz M. Structure of the malB region in Escherichia coli K12. I. Genetic map of the malK-lamB operon. Mol Gen Genet. 1979 Jul 24;174(3):241–248. doi: 10.1007/BF00267796. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Schwartz M. Restriction map of the Escherichia coli malA region and identification of the malT product. J Bacteriol. 1980 Aug;143(2):761–771. doi: 10.1128/jb.143.2.761-771.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Groves D. J., Clark D. J. Regulation of Cell Division in Escherichia coli: Characterization of Temperature-Sensitive Division Mutants. J Bacteriol. 1970 Dec;104(3):1052–1064. doi: 10.1128/jb.104.3.1052-1064.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A., Shuman H., Schwartz M. Intergration of the receptor for bacteriophage lambda in the outer membrane of Escherichia coli: coupling with cell division. J Bacteriol. 1975 Apr;122(1):295–301. doi: 10.1128/jb.122.1.295-301.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabourin D., Beckwith J. Deletion of the Escherichia coli crp gene. J Bacteriol. 1975 Apr;122(1):338–340. doi: 10.1128/jb.122.1.338-340.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer H., Boos W. Cloning of the ugp region containing the structural genes for the pho regulon-dependent sn-glycerol-3-phosphate transport system of Escherichia coli. Mol Gen Genet. 1983;192(1-2):177–186. doi: 10.1007/BF00327664. [DOI] [PubMed] [Google Scholar]
- Shen B. H., Boos W. Regulation of the -methylgalactoside transport system and the galatose-binding protein by the cell cycle of Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1481–1485. doi: 10.1073/pnas.70.5.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Brickman E., Bassford P. J., Jr, Casadaban M. J., Shuman H. A., Schwartz V., Guarente L., Schwartz M., Beckwith J. R. Structure of the malB region in Escherichia coli K12. II. Genetic map of the malE,F,G operon. Mol Gen Genet. 1979 Jul 24;174(3):249–259. doi: 10.1007/BF00267797. [DOI] [PubMed] [Google Scholar]
- Silhavy T. J., Shuman H. A., Beckwith J., Schwartz M. Use of gene fusions to study outer membrane protein localization in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5411–5415. doi: 10.1073/pnas.74.12.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi R., Kawamukai M., Obata K., Morita J., Himeno M., Komano T. Identification of a membrane protein induced concurrently with cell filamentation by cyclic AMP in an Escherichia coli K-12 fic mutant. J Bacteriol. 1983 Jul;155(1):398–401. doi: 10.1128/jb.155.1.398-401.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi R., Nakamoto Y., Kawamukai M., Himeno M., Komano T. Involvement of cyclic AMP and its receptor protein in filamentation of an Escherichia coli fic mutant. J Bacteriol. 1982 Aug;151(2):807–812. doi: 10.1128/jb.151.2.807-812.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi R., Tanabe H., Nakamoto Y., Kawamukai M., Sakai H., Himeno M., Komano T., Hirota Y. Inhibitory effect of adenosine 3',5'-phosphate on cell division of Escherichia coli K-12 mutant derivatives. J Bacteriol. 1981 Sep;147(3):1105–1109. doi: 10.1128/jb.147.3.1105-1109.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]



