Abstract
The Gram-positive bacterium Metabacterium polyspora is an uncultivated symbiont of the guinea pig gastrointestinal tract. Here we present evidence that in M. polyspora vegetative cell division has taken on a minor, and apparently dispensable, role in propagation. Instead, this unusual bacterium has evolved the capacity to produce progeny in the form of multiple endospores. Endospore formation is coordinated with transit of the bacterium through the gastrointestinal tract of the guinea pig. For the majority of cells, sporulation is initiated in the ileum, whereas later stages of development take place in the cecum. We show that multiple endospores are generated both by asymmetric division at both poles of the cell and by symmetric division of the endospores at an early stage of their development. Our findings suggest that M. polyspora represents an intermediate step in the evolution of a novel mode of cellular propagation that originates with endospore-forming Bacillus and Clostridium spp., which reproduce by binary fission, and extends to Epulopiscium spp., which create multiple viviparous offspring by a process of internal reproduction.
Bacteria normally reproduce by division to generate vegetative offspring. We report an unusual mode of cellular reproduction in which a bacterium propagates itself by producing multiple progeny in the form of spores. In response to cues indicative of detrimental changes in the environment, many microbes halt reproduction and alter their growth, generating quiescent forms designed to endure adverse conditions. Certain kinds of low G+C Gram-positive bacteria, for example, possess a provisional developmental program that allows them to differentiate and produce a dormant, highly resistant cell known as an endospore. Here we present evidence that in the guinea pig symbiont Metabacterium polyspora vegetative cell division has taken on a minor, and apparently dispensable, role in propagation. Instead, this unusual bacterium produces multiple endospores and does so in a manner that appears to be an obligatory feature of its life cycle.
The remarkable ability of M. polyspora to produce multiple endospores was first described in 1913 (1), testimony to the prominence of the bacterium among the microbiota of the guinea pig. Generally, M. polyspora produces at least two endospores and as many as eight or nine (2–4). Its ability to produce more than two viable endospores is unusual among the known endospore-forming species of the low G+C Gram-positive bacteria (5). Endospore formation is generally thought of as a developmental program launched when adverse environmental conditions are sensed by the bacterium. Its primary function is to allow the bacterium to store its genome in a dormant cell until better conditions prevail. We became interested in why M. polyspora has gained the ability to regularly produce multiple endospores and how sporulation in M. polyspora is modified to allow for the production of multiple endospores.
In the case of the endospore-forming bacterium Bacillus subtilis, which produces only a single endospore, development occurs by a modification of the process of binary fission. Vegetative cells divide by the formation of a medially positioned ring of the tubulin-like protein FtsZ (6). The FtsZ ring, in turn, recruits additional cell division proteins that drive the formation of the septum. Endospore formation, in contrast, takes place by the formation of two rings of FtsZ, one near each pole of the cell (7). Normally, only one ring becomes functional and produces a single septum located near one pole of the cell. This asymmetrically positioned septum divides the developing cell (the sporangium) into two dissimilar sized progeny cells, the forespore (the smaller cell) and the mother cell. In certain (disporic) mutants of B. subtilis both FtsZ rings become functional and two polar septa are formed sequentially (7, 8). Initially, the forespore and the mother cell lie side-by-side in the sporangium, but later in development the forespore is wholly engulfed by, and pinched off within, the mother cell as a free protoplast, which will develop into the mature endospore.
Here we show that the life cycle of M. polyspora is coordinated with passage of the bacterium through the gastrointestinal tract of its guinea pig host. We found that binary fission is restricted to a brief period in the life cycle after spore germination when the bacterium is in the ileum. Often, binary fission is bypassed altogether in the M. polyspora life cycle. Instead, M. polyspora has gained the ability to produce multiple endospores, a process that commences in the ileum and is completed in the cecum. We conclude that the principal mode of propagation is the production of multiple internal progeny in the form of endospores. We also show that multiple endospores are generated both by asymmetric division as well as by the unusual capacity of nascent endospores to undergo a round of division after they have been engulfed by the mother cell. We hypothesize that multiple endospore formation in M. polyspora represents an evolutionary transition between a highly successful dormancy program and a novel means of propagation in which progeny are produced internally.
MATERIALS AND METHODS
Bacterial Strains.
Clostridium propionicum (ATCC 25522) and Clostridium lentocellum (ATCC 49066) were purchased from the American Type Culture Collection (Manassas, VA) and grown at 37°C and 25°C, respectively, in the media recommended by the supplier except for the following modification. Medium for C. lentocellum was supplemented with 1% sucrose while the cellulose was excluded. Clostridia were grown in stoppered serum bottles where the medium was overlaid with nitrogen (grade 5.5, WESCO).
Samples containing Epulopiscium sp. morphotype B cells were provided by Kendall Clements (University of Auckland, New Zealand). The cells were collected from the surgeonfish Naso tuberosus, fixed with 80% ethanol, and stored at −20°C (9). Samples containing M. polyspora were collected from guinea pigs, Cavia porcellus, and fixed for microscopy as described below.
Cloning ftsZ Homologues.
Obtaining a complete genomic clone of either the C. lentocellum or the C. propionicum ftsZ homologue in a plasmid vector in Escherichia coli was not possible. As an alternative approach, fragments of the gene were PCR-amplified from genomic DNA of C. lentocellum and C. propionicum by using degenerate primers. Inverse PCR (10) was used to obtain the 5′ and 3′ ends of the genes and flanking regions. Based on these gene sequences the amplification primers were further refined and used to amplify a portion of the gene from cell lysates of Epulopiscium sp. morphotype B. This bacterium cannot be grown in pure culture, so a lysate of isolated cells was used in amplification reactions, as described (9).
PCR and inverse PCR products were cloned into the pCRII vector (Invitrogen). At least two independent clones of each homologue were characterized. The sequences were determined manually using a dideoxynucleotide chain-termination method with Sequenase version 2.0 (Amersham). Sequence variation between clones from a single strain was seen for homologues obtained from C. lentocellum and Epulopiscium. In both cases the predicted amino acid sequence of each variant was identical.
Rabbit Polyclonal Anti-FtsZ Serum.
Strain EA5 was generated by cloning the partial Epulopiscium ftsZ gene as an EcoRV/KpnI fragment into the pRSETC vector and transforming Escherichia coli strain BL21 (Novagen) with this plasmid. BL21 contains isopropyl β-d-thiogalactoside (IPTG)-inducible T7 RNA polymerase. An EA5 culture was grown in Luria–Bertani supplemented with ampicillin (200 μg/ml) to an OD600 of 0.9, induced by the addition of IPTG to 2 mM, and harvested 2 hr after induction. The six-histidine-tagged polypeptide was isolated by using a Ni-nitrilotriacetate (NTA) column, following protocol 7 in the QIAexpress Manual (Qiagen, Chatsworth, CA). The protein preparation was dialyzed to reduce the amount of urea in the solution to 0.5 M and then used to generate antibodies in a rabbit (Covance, Denver, PA). On Western blot analysis this antiserum binds with high specificity to the purified six-histidine-tagged Epulopiscium FtsZ fragment. It also cross-reacts with purified B. subtilis FtsZ and polypeptides of an apparent molecular weight expected for FtsZ in whole cell lysates of C. propionicum and C. lentocellum (data not shown).
Immunofluorescence Microscopy.
Whole rabbit antiserum raised against a fragment of the Epulopiscium FtsZ, which specifically recognized FtsZ rings in C. lentocellum and C. propionicum cells (data not shown), was used for immunolocalization of FtsZ in M. polyspora cells. Immunolocalization of FtsZ was performed as described (11, 12) with the following modifications. Guinea pig cecum contents were diluted with PBS and fixed in 2.6% paraformaldehyde and 0.004% or 0.008% glutaraldehyde for ≈15 min at room temperature and 30 min on ice. The cells were washed twice with PBS and once with GTE, pH 6.3 (50 mM glucose/20 mM Tris⋅HCl, pH 6.3/10 mM EDTA), then resuspended in GTE (pH 6.3) and stored on ice. During the wash, multiple rounds of low-speed spins (3,000 × g, 30 sec) followed by discarding the supernatant and resuspending the pellet in wash buffer, removed many of the other smaller symbionts from the sample. Samples were filtered through loosely packed glass wool to remove large vegetal matter.
Metabacterium polyspora cell walls are resistant to lysozyme digestion. Therefore fixed cells were permeabilized by incubating for 2 hr at 37°C in GTE (pH 6.3) containing 500 units/μl mutanolysin (Sigma). The cells were washed once with GTE (pH 7.4) then incubated for 2 hr at 37°C in GTE (pH 7.4) containing lysozyme at 5 mg/ml. Cells were applied to poly-l-lysine-coated slides or coverslips and hybridized as described (11, 12) except slides were not exposed to methanol or acetone treatment. Samples were incubated overnight at 4°C in a 1:10,000 dilution of the Epulopiscium FtsZ antiserum.
To delineate membranes in M. polyspora, fixed cells were incubated for 5 min in a lipophilic fluorescent dye, either FM 4–64 or FM 1–43 (Molecular Probes), 1.5 μg/ml in PBS. Cells were simultaneously stained with the DNA-specific dye 4′,6-diamidino-2-phenylindole (DAPI), 2 μg/ml. Cells were applied to poly-l-lysine-coated slides and washed 5–10 times with PBS. We found that the lipophilic compounds also have an affinity for spore coats.
Image Acquisition and Deconvolution.
Photomicroscopy was performed using an Olympus BX 60 microscope as described (11) with the following modifications. Fluorescent and differential interference contrast (DIC) images were captured using a MicroMax (Princeton Instruments, Trenton, NJ) cooled charge-coupled device camera driven by the metamorph software package (version 3.0, Universal Imaging, Media, PA). For deconvolution, a stack of image planes with z axis spacing between planes of 0.1 μm was acquired using a piezoelectric device (Physik Instruments, Auburn, MA) driven by metamorph. For deconvolution an exhaustive photon reassignment program (Scanalytics, Billerica, MA) was applied to the image stack. Three-dimensional reconstruction of the processed image stack was performed using metamorph. Phase-contrast images were captured on Kodak Ektachrome ASA 100 film by using a Leica DMRD microscope equipped with a 35-mm camera. Images from 35-mm slides were digitized with a Nikon LS-1000 film scanner. Final figures were assembled using Adobe Systems (Mountain View, CA) photoshop 4.0 and canvas 5.0 (Deneba, Miami, FL).
RESULTS AND DISCUSSION
Previous studies of M. polyspora have focused on cells harvested from the cecum, a large, blind sac located at the junction between the ileum and colon (1–5, 13, 14). We now report that germination, binary fission, and the initiation of the next round of sporulation are coordinated with transit of M. polyspora through the upper gastrointestinal tract of the guinea pig (Fig. 1). Only mature endospores survive passage through the mouth and stomach and, upon entering the small intestine, all spores germinate. From the ileum, bacteria are deposited in the cecum. In guinea pigs, the cecum is well developed and densely populated with a magnificent array of microorganisms. More advanced stages of sporulation are observed in the cecum. The bacteria pass out of the animal via the colon. Fully fruited endospores, still contained within mother cells, are found in the animal’s feces. Guinea pigs are coprophagous, and this behavior allows for the reintroduction of M. polyspora to the guinea pig gastrointestinal tract. Mastication of feces and deterioration of the mother cell leads to release of mature endospores, thus completing the cycle. This suggests a reliance on endospore formation for dispersal and for reintroduction of the bacterium into its host.
Figure 1.
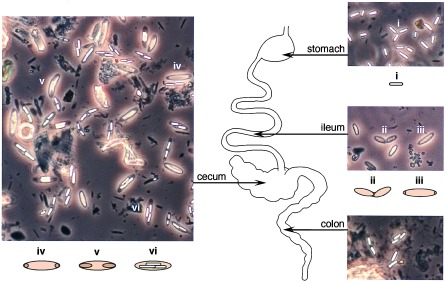
The life cycle of M. polyspora is coordinated with its passage through the gastrointestinal tract of the guinea pig (Cavia porcellus). The stomach contains only free, mature endospores (i). The ileum contains cells undergoing binary fission (ii) and cells with asymmetrically positioned septa (iii). [Cells undergoing binary fission (ii) are surrounded by a spore coat, which is best seen under higher magnification and by DIC microscopy as in Fig. 2h. Likewise, the presence of polar septa in cells (iii) is more clearly seen at higher magnification and by use of a fluorescent membrane stain as in Fig. 2a.] The majority of cells in the cecum contain either developing forespores, seen as phase-dark bodies within the cell (iv and v) or phase-bright, mature endospores (vi). Panels show phase-contrast micrographs of samples of contents taken from the organ indicated.
But why has M. polyspora gained the ability to produce multiple endospores? The answer seems to be that M. polyspora undergoes binary fission infrequently. In populations from two guinea pigs examined extensively, we observed negligible binary fission in the cecum (<0.1% of the cells) and only ≈4 (18/473 cells) to 8 (33/415) percent of the cells in the ileum exhibited any evidence of binary fission. Furthermore, in every instance, such cells were still contained within a spore coat, a finding that indicates that binary fission is restricted to a time just after the start of germination (Fig. 2h). We conclude that the low rate of binary fission and its restricted manifestation could provide only a modest increase in population size. How then is M. polyspora able to propagate itself? The answer seems to lie in its propensity to produce multiple endospores (Fig. 2 a–f). In two independent populations of M. polyspora, we found that ≈56% (231/415) to 66% (312/473) of the cells in the ileum were undergoing sporulation and a high proportion of these cells contained multiple nascent spores. Moreover, germinating spores in the ileum were frequently observed to enter into sporulation directly, as indicated by the presence of asymmetrically positioned septa (Fig. 2g).
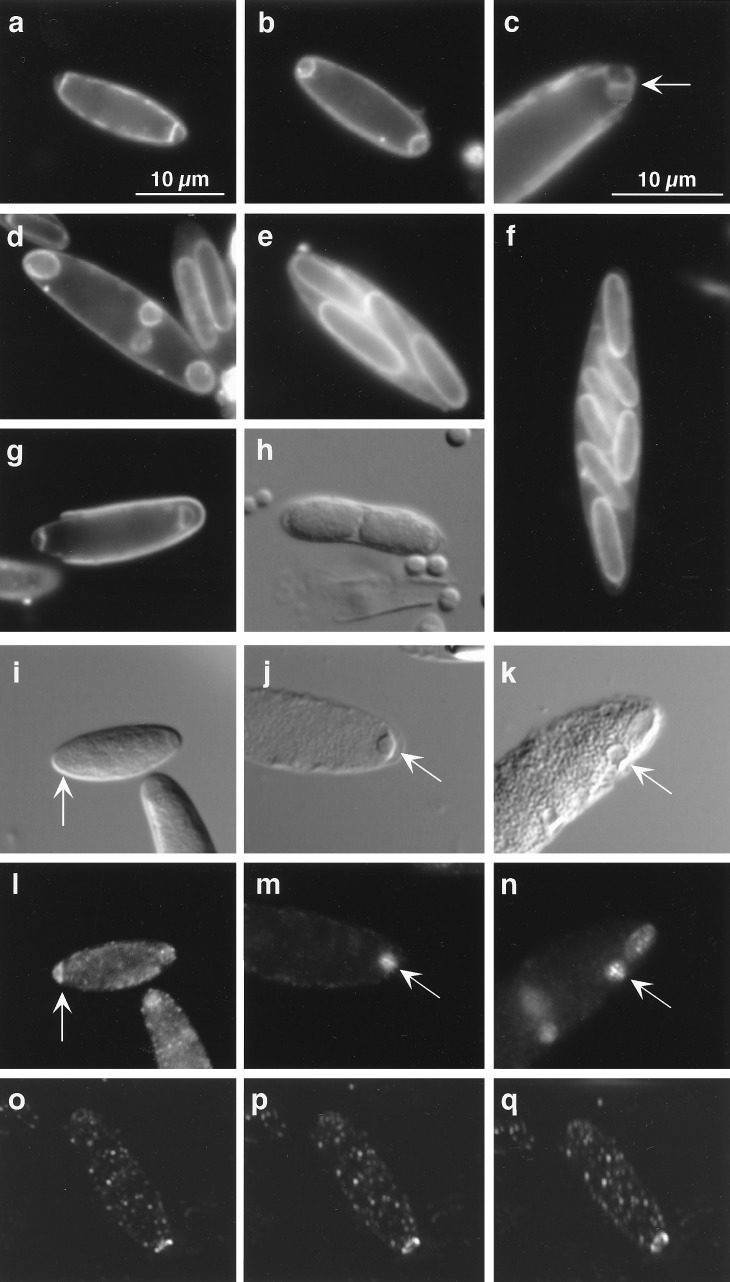
Figure 2.
Sporulation, binary fission, and FtsZ localization. a–e show a series of cells illustrating successive stages of sporulation in M. polyspora. (a) This cell contains two asymmetrically positioned septa, indicative of the start of sporulation. (b) The phagocytic process of forespore engulfment can be seen in this cell. (c) The arrow indicates a membrane septum that has divided a single forespore protoplast in two. (d) Four immature forespores are visible within this sporangium. (e) The outline of four mature endospores can be seen within this sporangium. (f) A large cell with seven visible endospores. (g) In this germinating spore, asymmetrically positioned septa are visible even as the cell emerges from its spore coat. (h) This DIC micrograph shows cells just after binary fission and still contained within a single spore coat. Cells (a–g) were treated with a fluorescent membrane stain, either FM 4–64 or FM 1–43 (which we found also have an affinity for spore coats). i–q summarize the results of the FtsZ localization experiments. Pairs of panels show cells of the same field viewed with DIC (i–k) and fluorescence (l–n) microscopy. Arrows indicate the position of the FtsZ ring. In i and l, FtsZ is localized to the cell pole. j and m show an FtsZ ring bisecting a newly engulfed forespore. k and n show a forespore at a more lateral position undergoing symmetric division. (o–q) 0°, 15°, and 30° vertical plane rotations, respectively, of a three-dimensional reconstruction of a cell with FtsZ localized in a ring-like structure near one cell pole. All panels except d are represented at the same magnification. The scale bar for these images is shown in a.
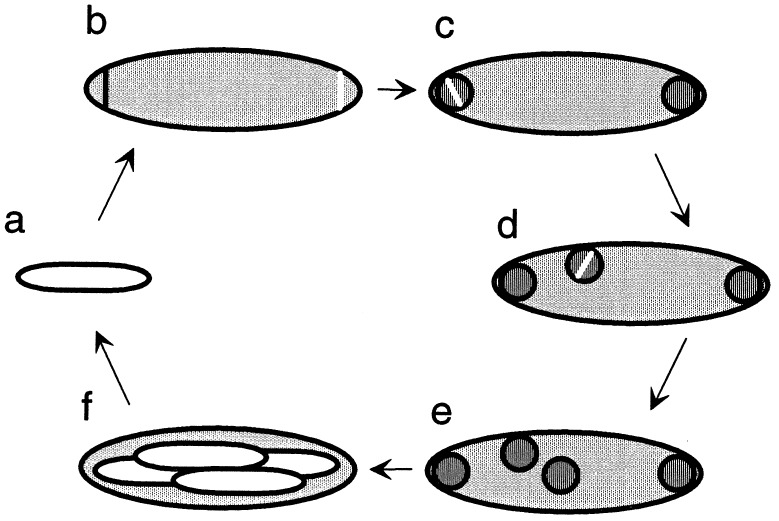
In the endospore-forming bacteria to which M. polyspora is related, forespores are produced by division near one pole of the cell (15). The sporulating cell contains two nucleoids, one of which is packaged into the forespore in a highly condensed state, while the other remains in the mother cell (16, 17). Next, the forespore is wholly surrounded by the mother cell in a phagocytic-like process known as engulfment. In most Bacillus and Clostridium spp., only one forespore is produced and hence only one endospore. In striking contrast, M. polyspora produce two forespores by division near both poles (Fig. 2 a, b, and g). Remarkably, after engulfment these forespores frequently undergo fission to generate additional progeny protoplasts (see, for example, Fig. 2c). As a consequence, sporangia are readily observed that have multiple forespore protoplasts (Fig. 2d). These in turn mature into multiple cigar-shaped endospores (Fig. 2 e and f). Thus, a mechanism that is ordinarily employed in other species for the production of a single dormant cell has evidently been adapted as a means of internal reproduction in which multiple dormant progeny are produced in each cycle of sporulation (Fig. 4).
Figure 4.
Sporulation and the life cycle of M. polyspora. As an endospore (a) germinates, the emerging bacterium often shows signs of the initiation of the next round of sporulation. (b) Two forespores are formed by the production of two asymmetrically positioned septa, one near each cell pole. This septation is mediated by the cell division protein FtsZ, shown here as a white line spanning a membrane-bound compartment (see also Fig. 2). (c) A newly engulfed forespore may undergo fission. (d) Forespore fission can also occur later, after a forespore has migrated to a lateral position within the mother cell. (e) The forespores enlarge and develop into mature, phase-bright endospores (f).
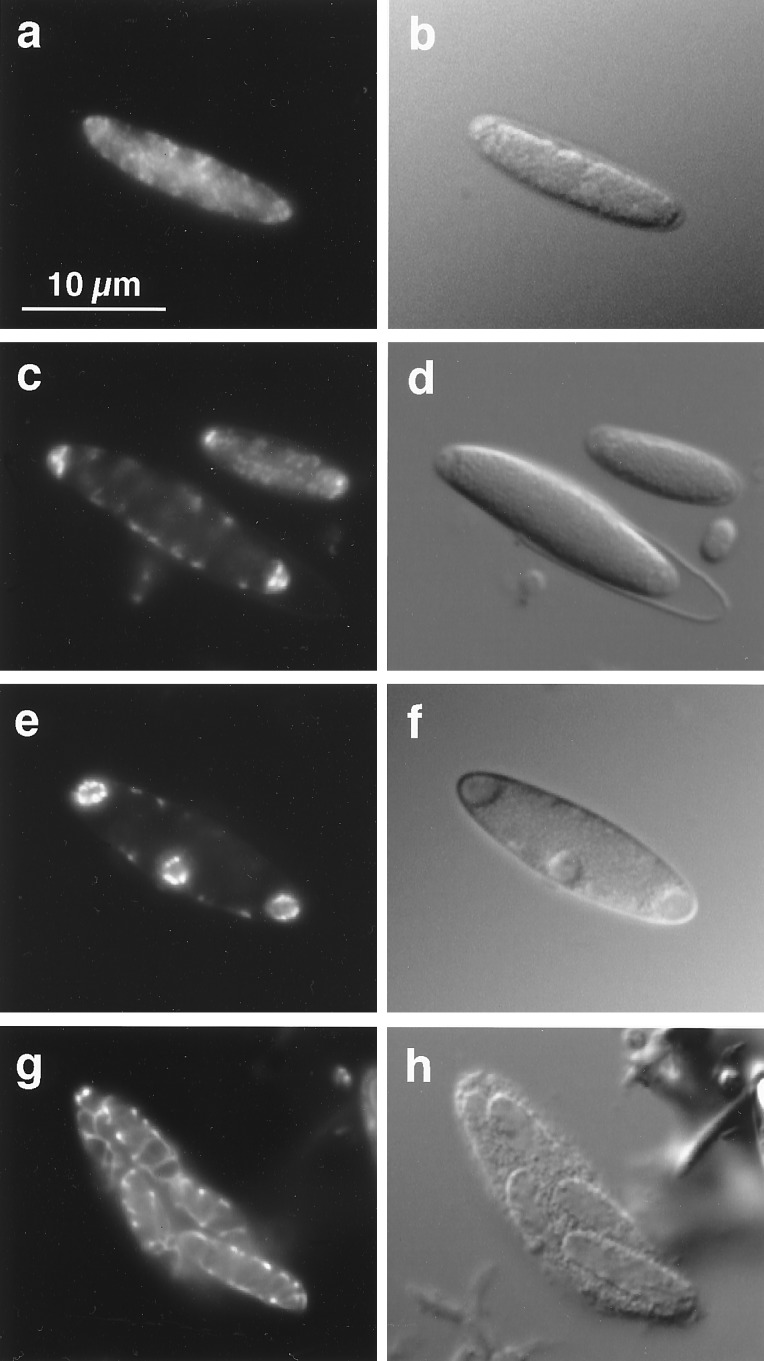
To support the production of multiple forespore progeny, each differentiating M. polyspora cell appears to contain multiple nucleoids (Fig. 3). Nascent forespores contain highly condensed DNA (Fig. 3c), probably composed of multiple nucleoids that are distributed as forespores divide (Fig. 3e). Eventually the DNA assumes an ordered, peripheral arrangement as the forespores enlarge and mature (Fig. 3g).
Figure 3.
Distribution of DNA during sporulation in M. polyspora as seen in cells stained with the DNA-specific dye 4′,6-diamidino-2-phenylindole (DAPI). This figure shows pairs of micrographs of the same cell with 4′,6-diamidino-2-phenylindole (DAPI) fluorescence on the left and DIC on the right. (a and b) Prior to septation DNA is seen dispersed throughout the cell. (c and d) After septation highly condensed DNA is found in each forespore while DNA remaining in the mother cell is arranged about the cell periphery. This pattern of DNA distribution is similar to that observed in Epulopiscium sp. early in daughter cell development (23). Note that the next round of sporulation has been initiated in the larger cell shown here even before it has emerged completely from its spore coat. (e and f) As forespores enlarge the DNA takes on a peripheral arrangement. (g and h) In large forespores the DNA assumes a highly ordered pattern.
The pattern of localization of the cell division protein FtsZ is consistent with this description of the sporulation process. FtsZ initially localizes to the poles of M. polyspora cells and is responsible for the formation of one or two asymmetrically positioned septa (Fig. 2 i, l, and o–q). After engulfment, additional septa are not formed at the cell poles. Instead, FtsZ was found to assemble at the midline of engulfed forespores (Fig. 2 j, k, m, and n). Presumably, this is a precursor of forespore division. We used deconvolution microscopy and images of a rotated three-dimensional reconstruction of M. polyspora to illustrate the ring-like localization pattern of FtsZ (Fig. 2 o–q). Fig. 4 summarizes the FtsZ localization results and illustrates the contribution of asymmetric and symmetric division to the life cycle of M. polyspora.
Both mechanisms for generating forespores have been observed in distantly related, endospore-forming species that naturally produce two endospores per cell. Electron micrographs of disporic C. oceanicum cells show forespores initiated at each cell pole (18). In contrast, the segmented filamentous bacteria found in the intestinal tract of rodents, produce two endospores per cell but this is accomplished by division of the forespore protoplast immediately following engulfment (19).
Our findings may explain the failure of previous attempts to cultivate M. polyspora in the laboratory (13, 14). A population of M. polyspora can significantly increase in numbers only by multiple rounds of germination, binary fission and sporulation, with each round entrained by passage of the bacteria through the host.
Viewed in a broader evolutionary scheme M. polyspora may represent an intermediate step in the development of a mode of cellular propagation. Relatives of M. polyspora in the genera Bacillus and Clostridium, propagate by binary fission but also have the provisional capacity to produce within each cell a single endospore (15). In contrast, M. polyspora undergoes binary fission only during a restricted portion of its life cycle and then only occasionally. Instead, it has acquired the capacity to produce multiple endospores, which represents its principal mode of propagation. Epulopiscium spp., which are also closely related to M. polyspora (5), have evolved a step further. Instead of cycling through the surgeonfish gastrointestinal tract, these enormous bacteria (9) form a stable association with their host (20–22). In Epulopiscium, multiple progeny cells arise internally as in endospore formation by M. polyspora, but these progeny are viviparous and have lost the capacity to differentiate into dormant cells. Therefore, if our evolutionary scheme is correct, the provisional capacity of certain bacteria to produce endospores has given rise in step-wise progression to a novel mode of cellular propagation in which vegetative offspring are generated by internal reproduction.
Acknowledgments
E.R.A. thanks members of the Losick lab, particularly A. Hofmeister and P. Levin, for many helpful discussions. We thank K. Pogliano for introducing us to FM 4-64. E.R.A. is a postdoctoral fellow of the Jane Coffin Childs Memorial Medical Research Foundation. This work was supported by National Institutes of Health Grant GM18568 to R.L.
ABBREVIATION
- DIC
differential interference contrast
Footnotes
References
- 1.Chatton É, Pérard C. C R Hebd Soc Biol (Paris) 1913;74:1232–1234. [Google Scholar]
- 2.Robinow C F. J Gen Microbiol. 1951;5:439–457. doi: 10.1099/00221287-5-3-439. [DOI] [PubMed] [Google Scholar]
- 3.Robinow C F. Z Tropenmed Parasitol. 1957;8:225–227. [PubMed] [Google Scholar]
- 4.Stünkel S, Alves J, Kunstyr I. Folia Microbiol. 1993;38:171–175. doi: 10.1007/BF02814372. [DOI] [PubMed] [Google Scholar]
- 5.Angert E R, Brooks A E, Pace N R. J Bacteriol. 1996;178:1451–1456. doi: 10.1128/jb.178.5.1451-1456.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lutkenhaus J, Addinall S G. Annu Rev Biochem. 1997;66:93–116. doi: 10.1146/annurev.biochem.66.1.93. [DOI] [PubMed] [Google Scholar]
- 7.Levin P A, Losick R. Genes Dev. 1996;10:478–488. doi: 10.1101/gad.10.4.478. [DOI] [PubMed] [Google Scholar]
- 8.Errington J. Microbiol Rev. 1993;57:1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angert E R, Clements K D, Pace N R. Nature (London) 1993;362:239–241. doi: 10.1038/362239a0. [DOI] [PubMed] [Google Scholar]
- 10.Ochman H, Gerber A S, Hartl D L. Genetics. 1988;120:621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harry E J, Pogliano K, Losick R. J Bacteriol. 1995;177:3386–3393. doi: 10.1128/jb.177.12.3386-3393.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pogliano K, Harry E J, Losick R. Mol Microbiol. 1995;18:459–470. doi: 10.1111/j.1365-2958.1995.mmi_18030459.x. [DOI] [PubMed] [Google Scholar]
- 13.Warth A D. Appl Environ Microbiol. 1979;38:1029–1033. doi: 10.1128/aem.38.6.1029-1033.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunstyr I, Schiel R, Kaup F J, Uhr G, Kirchoff H. Naturwissenschaften. 1988;75:525–527. doi: 10.1007/BF00361293. [DOI] [PubMed] [Google Scholar]
- 15.Piggot P J, Coote J G. Bacteriol Rev. 1976;40:908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Setlow B, Magill N, Febbroriello P, Nakhimovsky L, Koppel D E, Setlow P. J Bacteriol. 1991;173:6270–6278. doi: 10.1128/jb.173.19.6270-6278.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu L J, Lewis P J, Allmansberger R, Hauser P M, Errington J. Genes Dev. 1995;9:1316–1326. doi: 10.1101/gad.9.11.1316. [DOI] [PubMed] [Google Scholar]
- 18.Smith L D. J Bacteriol. 1970;103:811–813. doi: 10.1128/jb.103.3.811-813.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson D J P, Birch-Andersen A. Acta Pathol Microbiol Scand Sect B. 1979;87:247–252. doi: 10.1111/j.1699-0463.1979.tb02434.x. [DOI] [PubMed] [Google Scholar]
- 20.Fishelson L, Montgomery W L, Myrberg A A., Jr Science. 1985;229:49–51. [Google Scholar]
- 21.Montgomery W L, Pollak P E. J Protozool. 1988;35:565–569. [Google Scholar]
- 22.Clements K D, Sutton D C, Choat J H. Mar Biol (Berlin) 1989;102:403–412. [Google Scholar]
- 23.Robinow, C. & Angert, E. R. (1998) Arch. Microbiol., in press. [DOI] [PubMed]