Abstract
Macroglial cells express ionotropic glutamate receptors. In the adult optic nerve, reverse transcription–PCR showed that the native receptors are formed by subunits belonging to the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate classes. Because activation of AMPA and kainate receptors can be toxic to oligodendrocytes in vitro, I examined the nature of the damage caused by kainate, an agonist of both receptor classes, applied directly onto the optic nerve. Acute infusion or chronic slow delivery of the agonist caused massive nerve damage that was more extensive in the latter. Interestingly, chronic delivery also produced inflammation and demyelination in well circumscribed areas of the nerve, together with other pathological features that closely resemble those observed in multiple sclerosis patients. Acute and chronic kainate lesions were both prevented by the non-N-methyl-d-aspartate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione. However, GYKI53655, a specific AMPA receptor antagonist, did not significantly reduce the size of the lesion, suggesting that the kainate toxicity was mainly mediated through activation of kainate-preferring glutamate receptors. These observations suggest that alterations in glutamate signaling may be detrimental to oligodendrocytes and may be involved in the pathogenesis of multiple sclerosis and other demyelinating diseases.
Keywords: kainate receptors/oligodendrocytes/cell death/multiple sclerosis
Nerve cells may die during development and in degenerative diseases. The mechanisms triggering cell death have been the subject of intense investigation over the last decade. It is now widely acknowledged that alterations in glutamate signaling may cause neurotoxicity, a feature that is regarded as a potential major component of abnormally occurring neuronal cell death in the adult central nervous system (1–3).
In addition to neurons, glial cells also have glutamate receptors (4). In particular, oligodendrocytes, the myelinating cells of central nervous system axons, express functional ionotropic glutamate receptors that are primarily of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate types (4). Moreover, excessive glutamate receptor activation is known to be toxic to oligodendrocytes (5, 6) suggesting that this mechanism might be involved in demyelinating disorders (5).
Multiple sclerosis (MS) is a chronic and progressive inflammatory disease that leads to the destruction of oligodendrocytes and myelin (7). Epidemiological evidence suggests that it is an acquired, possibly viral disease, for which a genetic predisposition exists (7, 8). This notion is strengthened by the fact that several animal models of experimental central nervous system demyelination have been developed, including models based on the use of toxins, autoimmune stimulation and virus infection (reviewed in refs. 9 and 10). These models reproduce some of the main neuropathological features of MS. However, the endogenous agents that trigger the disease have not been as yet determined.
To test whether excitotoxic insults result in tissue alterations that recapitulate the neuropathology of demyelinating diseases, I infused optic nerves with the glutamate receptor agonist kainate and analyzed the resulting histological damage. The results indicate that the extent and type of damage depends on whether the glutamate receptor agonist is applied acutely or chronically, and that in the latter case, optic nerve lesions resemble closely those occurring in MS.
MATERIALS AND METHODS
Reverse Transcription–PCR.
Total RNA was extracted from adult rat brains, including cerebellum, and optic nerves by using the guanidinium/phenol/chloroform method (11). Specific oligonucleotide primers, reverse transcription into cDNA, as well as PCR procedures used for each AMPA, kainate, and N-methyl-d-aspartate (NMDA) receptor subunit were as described earlier (5). Amplified products were analyzed by electrophoresis in 1.8% agarose gels and viewed with ethidium bromide staining. A φX174 HaeIII digest was used as a size standard. The specificity of the amplified PCR products was assessed by DNA sequencing by using the dideoxy chain termination method (12). The sequencing reactions were analyzed using a 377 automated DNA sequencer (Applied Biosystems).
Surgery and Drug Application.
Surgical procedures were as previously described (13). Drugs were delivered onto the optic nerve either from a Hamilton syringe (2 μl/min over 10 min) or from osmotic pumps (delivery rate 0.5–1 μl/h over 2–6 days; Alzet, Alza). Kainate (100 μM) was applied alone or together with 6-cyano-7-nitroquinoxaline-2,3-dione (30 μM) or GYKI53655 (100 μM), whereas glutamate was used together with cyclothiazide (both at 100 μM). Drugs were purchased from Tocris Neuramin (Bristol, U.K.) except for GYKI53655 that was kindly provided by D. Leander (Lilly Research, Indianapolis). Before surgery, the pumps were filled with drugs, all diluted in sterile saline (145 mM NaCl in 10 mM Na-phosphate, pH 7.2), attached to a catheter and equilibrated overnight at 37°C. Subsequently, they were implanted into adult male New Zealand White rabbits (1.5–2.5 kg) under deep anesthesia (ketamine·HCl, and tiazine-HCl, 50 and 10 mg/kg body weight, respectively, i.m.). Animals (n = 35) were reanesthetized and killed 2 days to 5 months after operation, and the optic nerves were carefully dissected out. Tissues were either snap frozen in isopentane chilled to −40°C or fixed by transcardial perfusion with 4% paraformaldehyde in PBS.
Histological Analysis.
Nerves were cut longitudinally on a cryostat (10 μm thick), mounted onto gelatinized slides and incubated with mouse mAbs to neurofilaments (2 μg/ml), or to myelin basic protein (0.8 μg/ml), both purchased from Boehringer Mannheim, or to 2′,3′-cyclic nucleotide 3′-phosphodiesterase (2 μg/ml; Sigma) following standard procedures. Binding of the antibodies was revealed by using the avidin-biotin-peroxidase complex as indicated by the supplier (Vector Laboratories). Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL) was carried out as indicated by the supplier (Boehringer Mannheim). In each experiment nearby sections were stained with toluidine blue solution (Nissl staining).
RESULTS
Expression of Ionotropic Glutamate Receptor Subunits in the Adult Optic Nerve.
The primer pairs and reverse transcription–PCR conditions used were appropriate to amplify efficiently each of the receptor subunit transcripts in control samples of whole brain of the adult rat (Fig. 1), where there are known to be expressed (1). In contrast, in the adult rat optic nerve, the main ionotropic glutamate receptor subunits found were the AMPA-selective glutamate receptor subunits 1, 3, 4 and the kainate-selective glutamate receptor subunits 5, 6, 7 and KA1, 2 (Fig. 1). Amplification of NMDA receptor subunits NR2A and NR2B, but not of NR1, was also observed (Fig. 1). Because native functional NMDA receptors are heterologous in nature and are formed by NR1 and NR2 subunits (14), these results indicate that their expression in the optic nerve is very low as demonstrated earlier in white matter areas of the brain (15, 16). In all instances, the sizes and sequences of the amplified subunits corresponded to those theoretically defined by the specific primers employed.
Figure 1.
The adult rat optic nerve expresses AMPA and kainate types of glutamate receptor subunits. Reverse transcription–PCR analyses of total RNAs were carried out with primers that efficiently amplify the corresponding subunits in whole rat brain tissue samples (Upper). The amplified subunits are indicated above each lane. PCR products are of the predicted size and their nucleotide sequences are identical to those previously cloned in the rat (1). Molecular size standards in base pairs correspond to the φX174 HaeIII digest (lanes L). Numbers aside indicate size in base pairs.
Lesions Caused by Acute Application of Kainate.
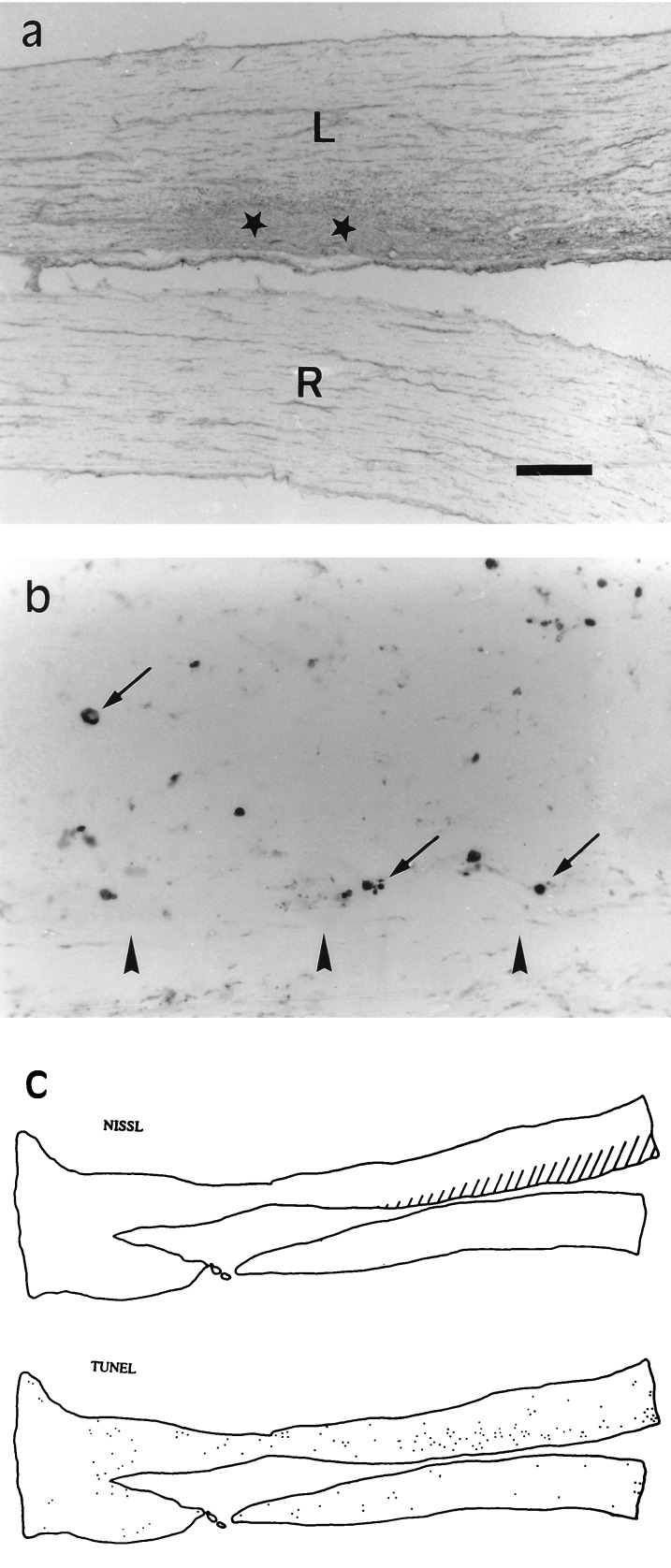
Brief (10 min) infusion of kainate (100 μM) onto the optic nerve surface caused histological damage that was evident, for at least three weeks, in a large area of the nerve, whose extension was evaluated with Nissl staining. Thus, the typical arrangement of interfascicular oligodendrocytes was disrupted in the vicinity of the site where the tip of the cannula was placed (Fig. 2a), indicating that this cell population was severely impaired. This is in accord with the finding that kainate is highly toxic to cultured optic nerve oligodendrocytes (5). Because the drug can diffuse through the subarachnoid space, the lesioned region extended longitudinally along the nerve up to several millimeters from the site of kainate application. In contrast, transversal diffusion into the nerve appeared to be limited by the compactness of the nerve fibers and accordingly, damage was primarily restricted within a distance of 0.5 mm from the nerve surface (Fig. 2a).
Figure 2.
Acute (10 min) infusion of kainate (100 μM) onto the rabbit optic nerve causes histological damage and apoptotic cell death. (a) Nissl staining of longitudinal sections revealed the location of the lesion (area surrounding stars) in the treated (top) optic nerve that in the illustrated case, corresponded to a large portion of the nasal half. (b) TUNEL staining of a section close to that illustrated in a shows that many cells (arrows) within the damaged area may undergo apoptosis as a consequence of the excitotoxic insult. Arrowheads indicate the nerve nasal surface. (c) Diagram showing the extension of the lesion (dashed area) and the location of TUNEL-labeled cells (dots) in sections nearby those displayed in a and b. The nerves shown here were examined 6 days after operation and are oriented with the eyeball to the right hand side. L and R, left (treated) and right (control) nerves respectively. [Bar = 500 μm (a), 30 μm (b), and 2 mm (c).]
Examination of the nerves with Nissl staining and TUNEL, two conventional procedures to label cells that may be undergoing programmed cell death, revealed a large population of cells whose nuclei were heavily stained and that often formed typical apoptotic bodies (Fig. 2b). The distribution of labeled cell nuclei corresponded well with the area in that damage was obvious in sections counterstained with toluidine blue (Fig. 2c). This suggested that kainate triggered apoptosis in a proportion of cells within the damaged area and that apoptosis may contribute substantially to excitotoxic oligodendroglial death, as described for neurons (2).
Lesions Caused by Chronic Application of Kainate.
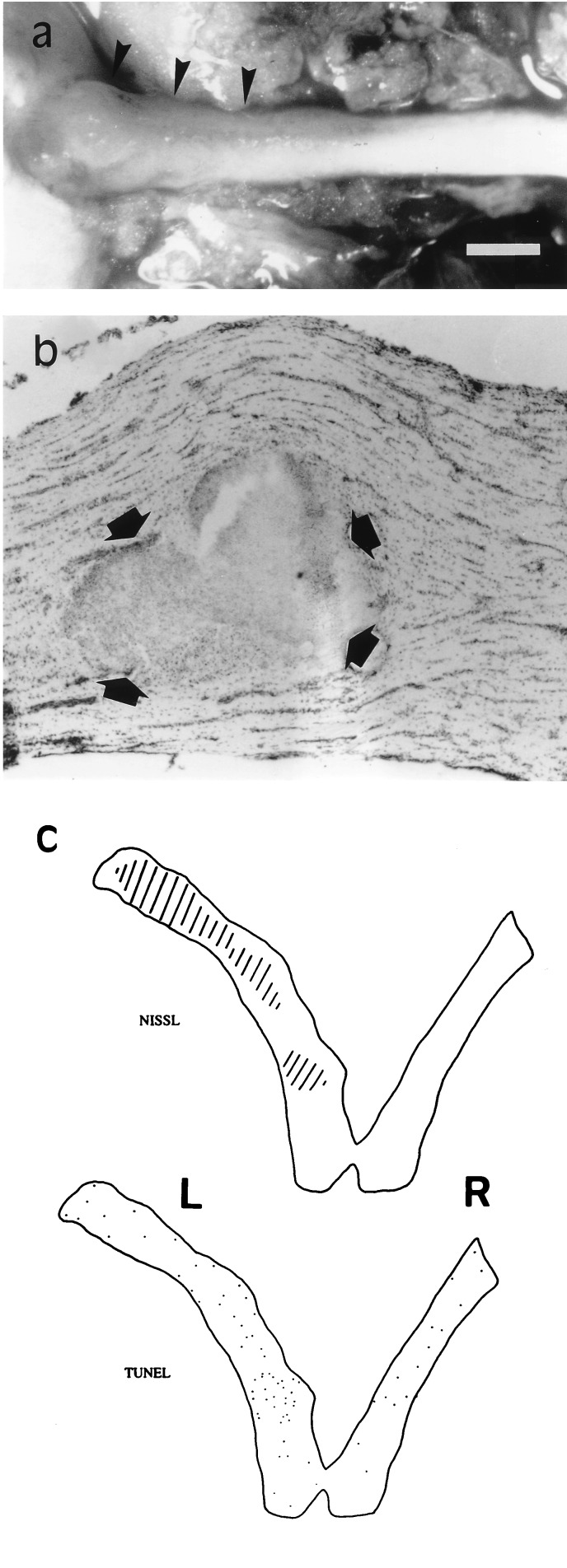
Slow (0.5–1 μl/hr) infusion of kainate (100 μM; 2–6 days) from osmotic pumps onto the nerve surface caused a more extensive damage than that observed in optic nerves exposed briefly to the drug (Fig. 3). In addition, chronic kainate application resulted in severe inflammation that was often macroscopically visible on the nerve surface (Fig. 3a). Profound histological changes were observed with Nissl staining in a relatively large area usually within 4–5 mm from the site of cannula implantation (Fig. 3). These alterations became evident around 4 days after initiating treatment with kainate, whereas the vehicle alone did not produce any histological lesion (data not shown). Interestingly, optic nerve lesions were restricted primarily to areas with a well defined border (Fig. 3b). By the end of the first week of treatment, the size of these areas, or plaques, was usually up to several mm2.
Figure 3.
Chronic infusion (2–6 days) of kainate (100 μM) onto the rabbit optic nerve causes inflammation and plaque formation. (a) Dorsal view of a treated nerve including part of the eyeball (left side) 5 weeks after operation. Note zones of inflammation (arrowheads) near the eyeball. (b) Nissl-stained longitudinal section showing a typical lesion (pointed to by arrows) in an optic nerve 4 days after initiating the infusion of kainate. (c) Diagram illustrating the extent of the lesion (dashed area) and the location of TUNEL-labeled cells (dots) in sections proximal to that shown in b. L and R, left (treated) and right (control) nerves, respectively. [Bar = 1.5 mm (a), 300 μm (b), and 2 mm (c).]
Pharmacology of Kainate-Induced Excitotoxicity.
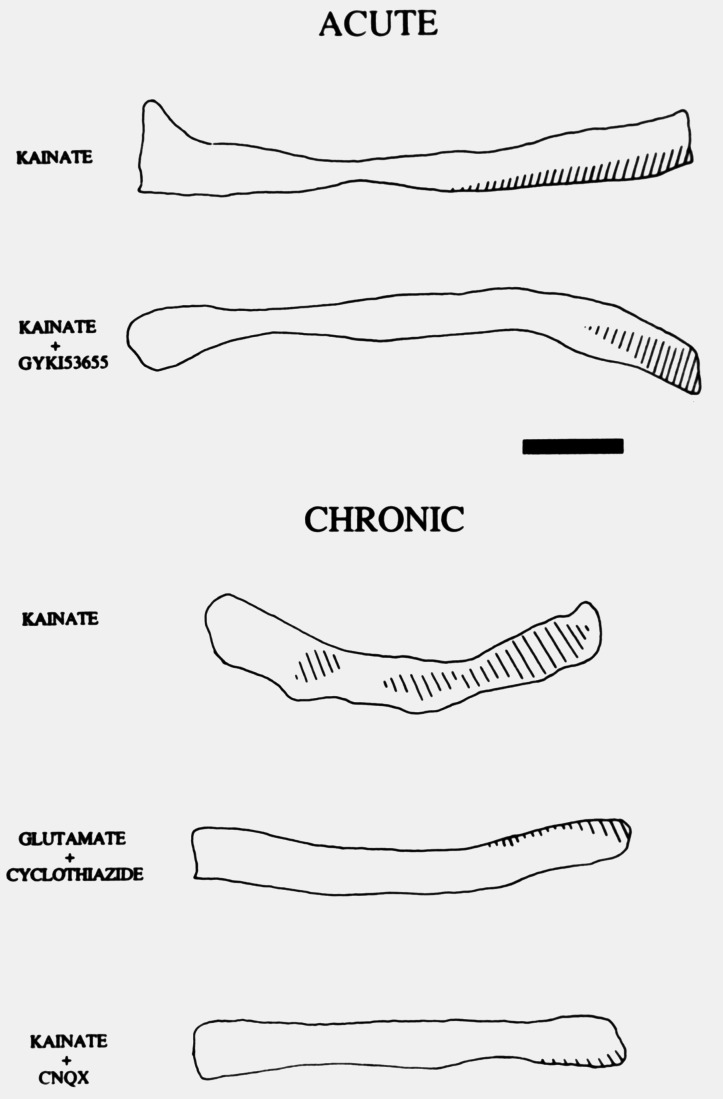
Damage resulting from acute or chronic kainate infusion onto the adult optic nerves was greatly reduced when the drug was applied in conjunction with 6-cyano-7-nitroquinoxaline-2,3-dione (30 μM; Fig. 4), indicating that the lesion was primarily mediated by activation of ionotropic non-NMDA glutamate receptors. Because kainate can activate AMPA- and kainate-preferring receptors (1), I examined their respective contributions to the excitotoxic injury by preferentially activating either receptor subtype. Thus, coapplication of kainate and GYKI53655 at 100 μM, a concentration that selectively blocks AMPA receptors (17), resulted in lesions that were similar in extent to those observed when kainate was applied alone. Moreover, co-infusion of glutamate and cyclothiazide (both at 100 μM), which is a specific allosteric modulator of AMPA receptors and suppresses the desensitization of these receptors thus enhancing their responses (18), only caused lesions restricted to a small area of the nerve located near the cannula tip (Fig. 4). Similar histological damage was observed when AMPA (100 μM) was applied together with cyclothiazide (data not shown). Together, these findings confirm our previous results indicating that kainate excitotoxicity is receptor-mediated (5) and, in addition, strongly suggest that kainate receptors constitute the main receptor subclass involved.
Figure 4.
Pharmacological profile of excitotoxic damage indicates that it is primarily mediated by kainate, not AMPA, receptors. In both acute and chronic experiments the concentration of applied kainate was 100 μM. Animals were killed 4–7 days after operation and diagrams drawn from Nissl stained longitudinal sections. The dashed areas represent the location of the lesion. GYKI53655 (100 μM), an AMPA-selective antagonist, did not greatly reduce the size of the lesion induced by kainate. Preferential activation of AMPA receptors, by glutamate applied in combination with cyclothiazide (both 100 μM), only caused small lesions. In all instances, kainate lesions were greatly reduced when the agonist was infused together with 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 30 μM). (Bar = 3 mm.)
MS-Like Lesions Caused by Chronic Application of Kainate.
To examine the histological damage caused by chronic exposure to kainate, I carried out immunohistochemical experiments with cell type specific antibody markers. Because oligodendrocytes are highly susceptible to excitotoxic insults (5), I first studied this cell population in optic nerves treated with the toxin, using two oligodendrocyte specific antibody markers. Immunostaining with antibodies to 2′,3′-cyclic nucleotide 3′-phosphodiesterase revealed that the oligodendrocyte population was severely impaired within the regions showing major Nissl alterations (Fig. 5 a and b). In addition, myelin basic protein was also greatly reduced in the areas of extensive damage (Fig. 5c). These findings suggest that kainate causes oligodendrocyte death and this leads to subsequent demyelination of large portions of the optic nerve.
Figure 5.
Chronic, slow, application of kainate (100 μM) causes optic nerve lesions that have the major features of MS. (a and b) Few oligodendrocytes are viewed with antibodies to 2′,3′-cyclic nucleotide 3′-phosphodiesterase within the resulting plaques. Asterisks in a and b denote the center of an area shown at a higher magnification in b. (c) The presence of myelin basic protein is greatly reduced in the damaged regions (star). (d) TUNEL-labeled elements (arrows) located at the edge of a lesioned area (star). (e and f) Axons remaining within the plaques (f) usually have a smaller caliber and present more swellings (arrowheads) as well as dislodgment (arrows) than normal axons (e), as illustrated by neurofilament immunohistochemistry. (g and h) Toluidine blue staining of vessels (arrowheads) in a control nerve (g) and within a damaged area (h), shows evidence of inflammation in the latter in the form of an increased number of large perivascular cells. The nerves illustrated were examined at 4–7 days after initiating the treatment with kainate. [Bar = 140 μm (a and c); 70 μm (d); 35 μm (b, g, and h); and 14 μm (e and f).]
Apoptotic bodies and TUNEL-labeled cells were observed in relatively low numbers and they were preferentially located at the edge of, and within, the plaques (Figs. 3c and 5d). Therefore, oligodendroglial cell death after prolonged exposure to kainate appears to be mainly necrotic rather than apoptotic.
Because demyelination may cause changes in axons (7), I analyzed axonal preservation within damaged areas in kainate treated optic nerves. Indeed, neurofilament immunostaining of axons showed that most fine-caliber axons (<1.5 μm) within the majority of plaques were structurally preserved, whereas the density of coarse-caliber axons (>2 μm) was reduced (Fig. 5 e and f). In addition, axons within the plaques had often irregular swellings and an apparent dislodgment (Fig. 5f). Finally, perivascular areas in the damaged areas displayed hypercellularity as compared with normal optic nerves (Fig. 5 g and h), suggestive of an inflammatory process resulting in the invasion of blood cells as occurs in MS (7). In contrast, these alterations were mostly absent in optic nerves exposed to kainate in the presence of 6-cyano-7-nitroquinoxaline-2,3-dione (data not shown).
Long-Term Effects of Acute and Chronic Kainate Toxicity.
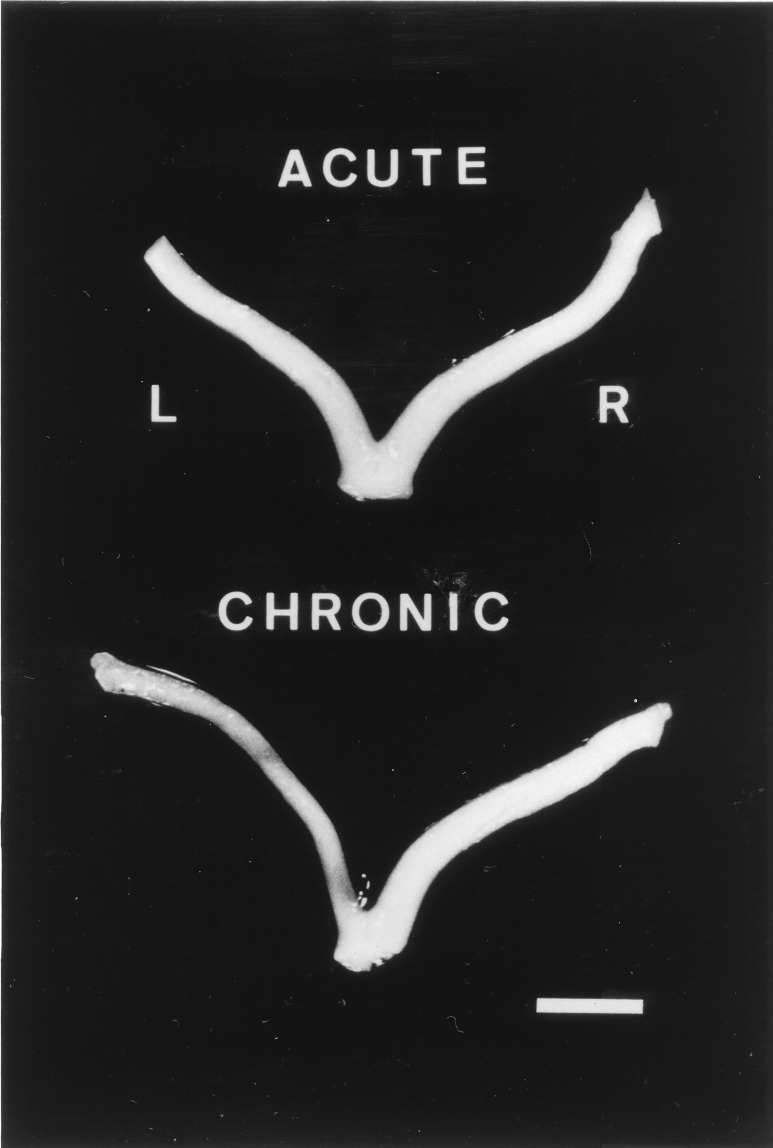
To examine the long-term effects of the kainate excitotoxic injury to the nerve, a group of rabbits (n = 5) was killed 3–5 months after infusion of the glutamate analogue. The damage caused by brief exposure to kainate did not result in long-term macroscopic alterations of the treated nerves (Fig. 6). In contrast, nerves infused for several days with kainate became atrophic and paler indicating that they had undergone permanent and severe cell and myelin loss (Fig. 6). Together, these results strongly suggest that kainate causes irreversible damage to adult optic nerves only if the excitotoxic insult is prolonged.
Figure 6.
Representative examples of the long-term effects (3–5 months) after acute (Upper) or chronic (Lower) kainate application to optic nerves. Notice atrophy after chronic exposure as well as signs of profound dymyelination (paleness), which are not evident in acutely treated nerves. L and R, left (treated) and right (control) nerves, respectively. (Bar = 4 mm.)
DISCUSSION
The results presented here demonstrate that acute and chronic activation of ionotropic non-NMDA glutamate receptors are toxic to cells, primarily oligodendrocytes, in the adult optic nerve. Moreover, the characteristics of kainate-induced lesions depend greatly on whether the glutamate analogue is applied acutely or chronically. In the latter case, the excitotoxic insult causes inflammation, demyelination, and plaque formation, which are typical features of MS.
Kainate Toxicity in Optic Nerves Is Principally Mediated by Kainate, Not AMPA Receptors.
Kainate is a nonselective agonist of AMPA and kainate receptors, which in neurons have respectively an EC50 of 240 and 22 μm for each receptor subtype (19). Likewise, kainate shows a differential affinity for AMPA and kainate receptors in the oligodendroglial cell line CG-4 (EC50 ≈ 265 μM and 5.3 μM, respectively) (20). Because in these experiments kainate (100 μM) was delivered slowly into the subarachnoid space and thereafter diluted in the cerebrospinal fluid, the concentration bathing the nerve surface was certainly significantly lower than that applied. If it is assumed that the diffusion of the drug is similar to that estimated for the hippocampus (21), depending on the distance from the cannula the concentration of kainate reaching optic nerve cells may vary from <100 μM to 1 μM. At such concentrations, the main population of receptors activated will thus belong to the kainate subtype (22). This idea is further supported by the fact that the excitotoxic damage was not significantly reduced when kainate was applied in the presence of the AMPA receptor antagonist GYKI53655. Nonetheless, it cannot be excluded that some of the excitotoxic damage may be caused by activation of AMPA receptors especially near the cannula tip where the kainate concentration is highest.
It seems likely that kainate-induced lesions are principally triggered by direct activation of kainate-preferring receptors at oligodendrocytes, the major type of ionotropic glutamate receptor expressed by this cell type in situ (23, 24). Moreover, pure oligodendrocyte cultures are highly vulnerable to glutamate receptor activation by kainate (5). However, the ability of astrocytes to release glutamate upon ionotropic glutamate receptor activation (25) indicates that these cells might also contribute to the observed excitotoxicity.
Acute and Chronic Exposure of Optic Nerves to Kainate Produce Lesions with Different Characteristics.
The kainate treatments used were chosen to mimic two relevant clinical situations. For instance, the damage resulting from the acute excitotoxic insult may parallel some of the consequences of sudden, transient ischemia, which causes a loss of high energy metabolites with consequent depolarization and a massive release of neurotransmitters, including glutamate (26). Interestingly, acute kainate-induced excitotoxic damage does not result in long-term macroscopic alterations of the optic nerve. It is thus possible that the oligodendrocyte population depleted in acutely damaged areas is replenished by cells generated after activation of the quiescent adult oligodendrocyte progenitors present in the nerve (27), and/or by migrating cells originating at the ventral midline of the third ventricle layer dorsal to the optic chiasm, as occurs during development (28).
In contrast with acute kainate exposure, chronic excitotoxic insults to optic nerves simulate neuropathological conditions of chronic diseases in which oligodendroglia are altered and for which a genetic predisposition exits. Indeed, over-activation of kainate receptors for a few days was sufficient to cause severe inflammation as well as loss of myelin and oligodendrocytes in plaques, alterations that constitute the hallmarks of MS. Importantly, damage after chronic excitotoxicity was irreversible and consequently the nerves became atrophic and demyelinated.
Possible Relevance of Oligodendroglial Excitotoxic Death to Demyelinating Diseases.
Several biochemical features may render oligodendrocytes more susceptible to excitotoxicity as compared with neurons. (i) Activation of kainate receptors triggers calcium influx via the receptor channel complexes and/or voltage-gated channels (5). Because oligodendrocytes lack an array of calcium-binding proteins that are present in neurons (29), they may be unable to buffer occasional or sustained abnormally high calcium levels. (ii) The glutamate receptor subunit 6, a major component of native kainate receptors (30) is edited to a much lower extent in optic nerve (23) and oligodendrocytes (5), than in the brain (31), a feature that confers to native glutamate receptor subunit 6 containing kainate receptors a much larger conductance (32) and a higher permeability to calcium (33). Finally, glutamate uptake may be less effective in non-synaptic than in synaptic regions, which are virtually surrounded by astroglial processes abundantly endowed with transporters.
Demyelinating diseases are very heterogeneous in nature with respect to their pathological and clinical manifestations (7), and multiple susceptibility loci have been recently proposed (e.g., for MS see ref. 34). It is therefore conceivable that, in the light of the findings reported here, genes encoding molecules that intervene in the glutamatergic system, such as receptors and transporters, might be altered in some of the pathological variants of MS and in other demyelinating diseases. Relevant to this idea is the fact that a type of glutamate transporter, named GLAST, is localized to chromosome 5p14-p12 (35), a recently proposed MS susceptibility locus (34).
Overall, the present results show that after chronic exposure to kainate the optic nerve acquired the main histopathological features of MS, and that excitotoxic insults may serve as a new experimental approach to study MS and other demyelinating diseases. In this model, non-NMDA ionotropic glutamate receptors, primarily of the kainate subtype, expressed by oligodendrocytes are targeted and their sustained activation results in cell death and subsequent demyelination. Most importantly, the findings reported here suggest that a subtle, aberrant enhancement of glutamate action on oligodendrocytes may represent an etiological component in certain leukoencephalites such as MS.
Acknowledgments
I am grateful to Dr. D. Leander (Eli Lilly and Company, Indianapolis, IN) for the generous gift of GYKI53655 and to L. Martínez-Millán for help with the surgery. I also thank J. Lerma, F. Pérez-Cerdá, A. Rodríguez-Antigüedad, and D.J. Fogarty for very valuable comments on the manuscript. This work was supported by grants from the Ministerio de Educación y Cultura (PB94–1377), the Gobierno Vasco (PI96–17), and the Universidad del País Vasco (EC229/97).
ABBREVIATIONS
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- MS
multiple sclerosis
- NMDA
N-methyl-d-aspartate
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling
References
- 1.Hollmann M, Heinemann S. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 2.Michaelis E K. Prog Neurobiol. 1998;54:369–415. doi: 10.1016/s0301-0082(97)00055-5. [DOI] [PubMed] [Google Scholar]
- 3.Glenn-Lin C-L, Bristol L A, Jin L, Dykes-Hoberg M, Crawford T, Clawson L, Rothstein J D. Neuron. 1998;20:589–602. doi: 10.1016/s0896-6273(00)80997-6. [DOI] [PubMed] [Google Scholar]
- 4.Steinhäuser C, Gallo V. Trends Neurosci. 1996;19:339–345. doi: 10.1016/0166-2236(96)10043-6. [DOI] [PubMed] [Google Scholar]
- 5.Matute C, Sánchez-Gómez M V, Martínez-Millán L, Miledi R. Proc Natl Acad Sci USA. 1997;94:8830–8835. doi: 10.1073/pnas.94.16.8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshioka A, Bacskai B, Pleasure D. J Neurosci Res. 1996;46:427–437. doi: 10.1002/(SICI)1097-4547(19961115)46:4<427::AID-JNR4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 7.Prineas J W, McDonald W I. In: Greenfield’s Neuropathology. Graham D I, Lantos P L, editors. Vol. 1. New York: Oxford Univ. Press; 1997. pp. 813–896. [Google Scholar]
- 8.Ebers G C, Sadovnick A D, Risch N J Canadian Collaborative Study Group. Nature (London) 1995;377:150–153. doi: 10.1038/377150a0. [DOI] [PubMed] [Google Scholar]
- 9.Bradl M, Linington C. Brain Pathol. 1996;6:303–311. doi: 10.1111/j.1750-3639.1996.tb00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller D J, Asekura K, Rodr¡guez M. Brain Pathol. 1996;6:331–344. doi: 10.1111/j.1750-3639.1996.tb00859.x. [DOI] [PubMed] [Google Scholar]
- 11.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 12.Sanger F, Nicklen S, Coulson A R. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodges-Savola C, Rogers S D, Ghilardi J R, Timm D R, Mantyh P W. Glia. 1996;17:52–62. doi: 10.1002/(SICI)1098-1136(199605)17:1<52::AID-GLIA5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg P H. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 15.Matute C, Miledi R. Proc Natl Acad Sci USA. 1993;90:3270–3274. doi: 10.1073/pnas.90.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matute C, Miledi R. Fourth IBRO Congress Neurosci. Abstr. Kyoto: Pergamon; 1995. p. 103. [Google Scholar]
- 17.Paternain A V, Morales M, Lerma J. Neuron. 1995;14:185–189. doi: 10.1016/0896-6273(95)90253-8. [DOI] [PubMed] [Google Scholar]
- 18.Patneau D K, Vickliky L, Jr, Mayer M L. J Neurosci. 1993;13:3496–3509. doi: 10.1523/JNEUROSCI.13-08-03496.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lerma J, Paternain A V, Naranjo J R, Mellström B. Proc Natl Acad Sci USA. 1993;90:11688–11692. doi: 10.1073/pnas.90.24.11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patneau D K, Wright P W, Winters C, Mayer M L, Gallo V. Neuron. 1994;12:357–371. doi: 10.1016/0896-6273(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 21.Herreras O, Largo C, Ibarz J M, Somjen G G, Martín del Río R. J Neurosci. 1994;14:7087–7098. doi: 10.1523/JNEUROSCI.14-11-07087.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodríguez-Moreno A, Herreras O, Lerma J. Neuron. 1997;19:893–901. doi: 10.1016/s0896-6273(00)80970-8. [DOI] [PubMed] [Google Scholar]
- 23.García-Barcina J M, Matute C. Eur J Neurosci. 1996;8:2379–2387. doi: 10.1111/j.1460-9568.1996.tb01201.x. [DOI] [PubMed] [Google Scholar]
- 24.García-Barcina J M, Matute C. Mol Brain Res. 1998;53:270–276. doi: 10.1016/s0169-328x(97)00318-5. [DOI] [PubMed] [Google Scholar]
- 25.Martin D L. Glia. 1992;5:81–92. doi: 10.1002/glia.440050202. [DOI] [PubMed] [Google Scholar]
- 26.Small D L, Buchan A M. In: Primers on Cerebrovascular Diseases. Welch K M A, Caplan L R, Reis D J, Siesjö B K, Weir B, editors. San Diego: Academic; 1997. pp. 244–247. [Google Scholar]
- 27.Wolswijk G, Noble M. In: Neuroglia. Kettenmann H, Ransom B R, editors. New York: Oxford Univ. Press; 1995. pp. 149–161. [Google Scholar]
- 28.Ono K, Yasui Y, Rutishauser U, Miller R H. Neuron. 1997;19:283–292. doi: 10.1016/s0896-6273(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 29.Baimbridge K G, Celio M R, Rogers J H. Trends Neurosci. 1992;15:303–308. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- 30.Wenthold R J, Trumpi V A, Zhu W S, Petralia R S. J. Biol. Chem. 1994;269:1332–1339. [PubMed] [Google Scholar]
- 31.Köhler M, Burnashev N, Sakmann B, Seeburg P H. Neuron. 1993;10:491–500. doi: 10.1016/0896-6273(93)90336-p. [DOI] [PubMed] [Google Scholar]
- 32.Swanson G T, Feldmeyer D, Kaneda M, Cull-Candy S G. J Physiol (London) 1996;469:129–142. doi: 10.1113/jphysiol.1996.sp021295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burnashev N. Curr Opin Neurobiol. 1996;6:311–317. doi: 10.1016/s0959-4388(96)80113-9. [DOI] [PubMed] [Google Scholar]
- 34.Bell J I, Lathrop G M. Nat Genet. 1996;13:377–378. doi: 10.1038/ng0896-377. [DOI] [PubMed] [Google Scholar]
- 35.Stoffel W, Sasse J, Duker M, Muller R, Hofmann K, Fink T, Lichter P. FEBS Lett. 1996;386:189–193. doi: 10.1016/0014-5793(96)00424-3. [DOI] [PubMed] [Google Scholar]