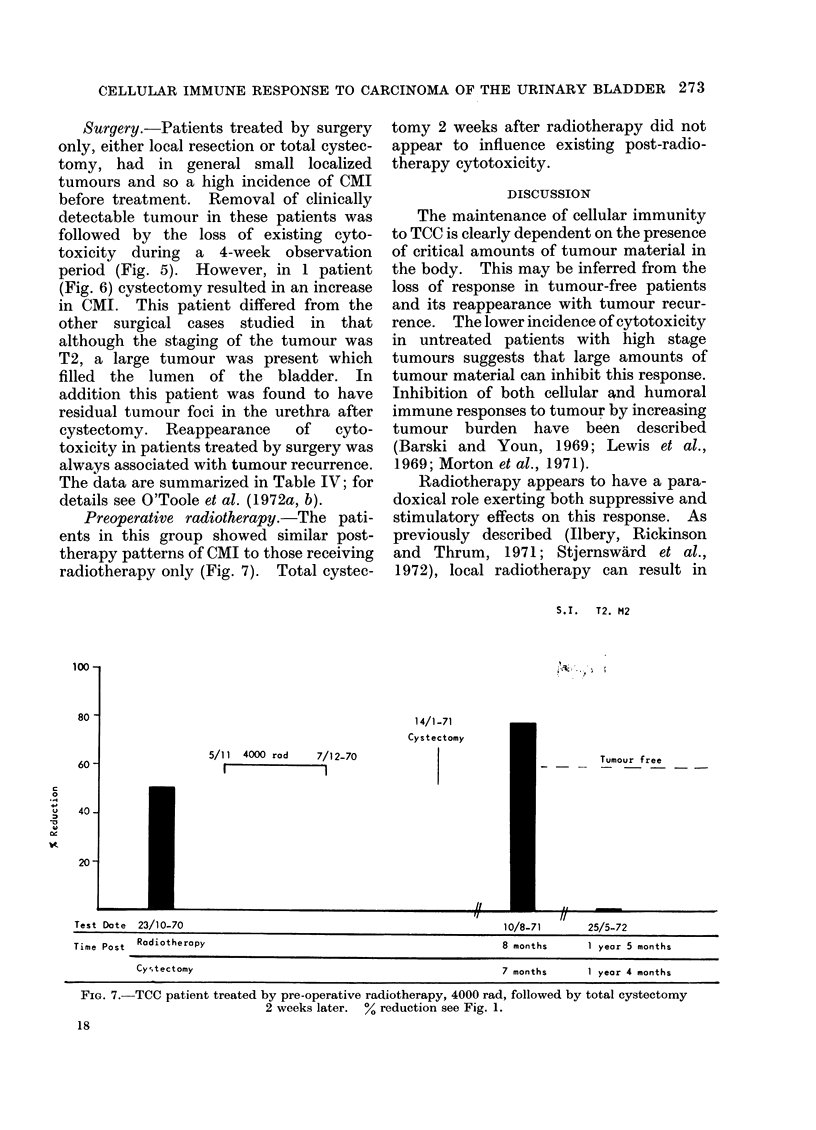
Abstract
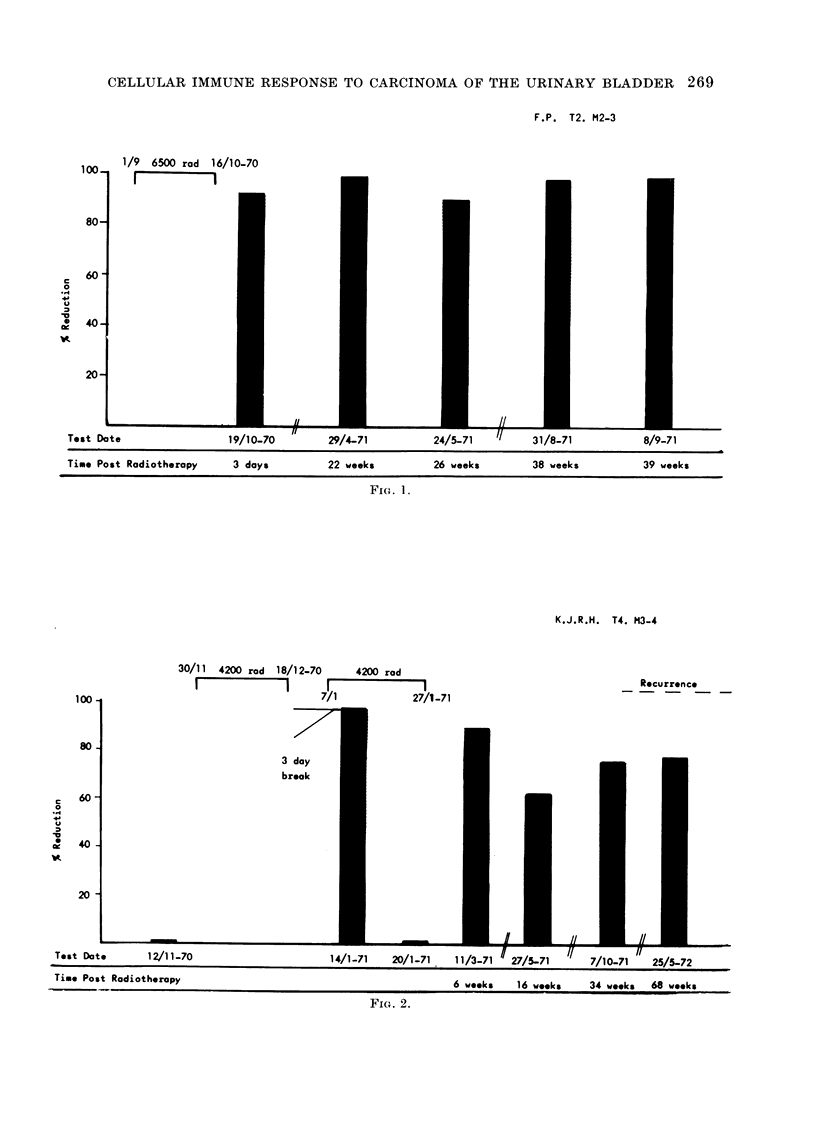
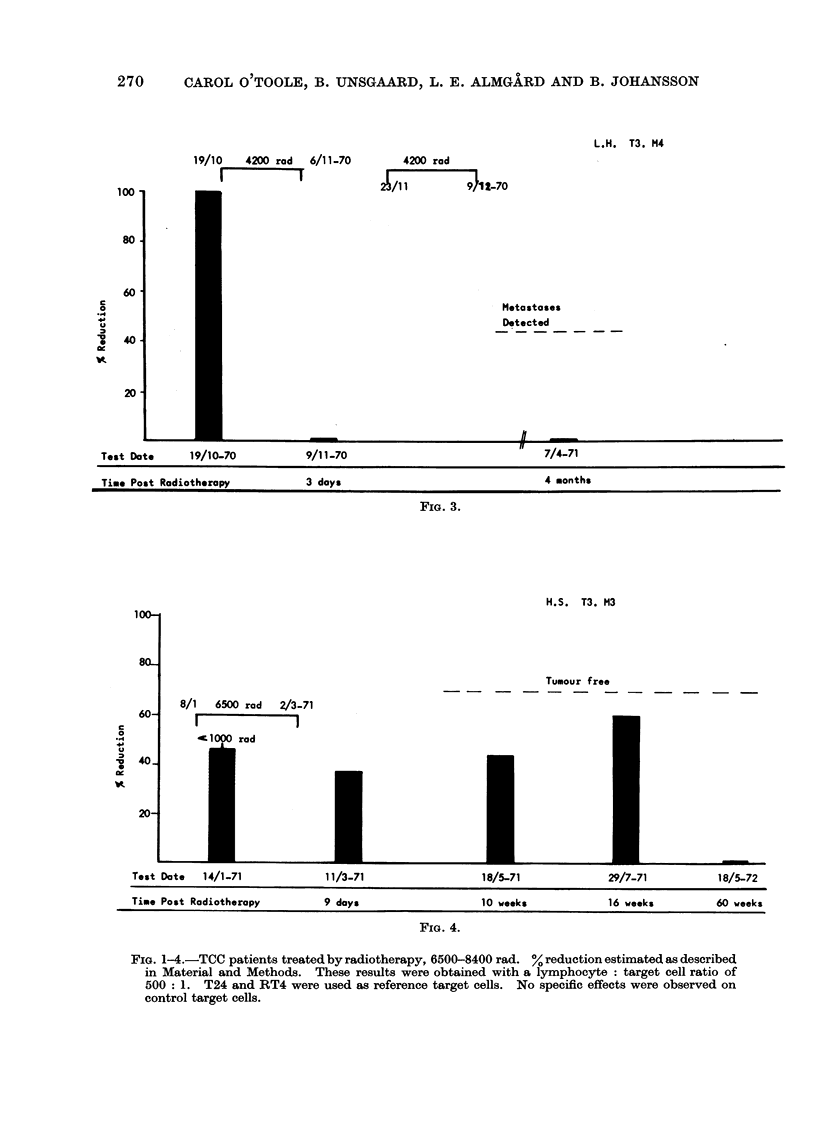
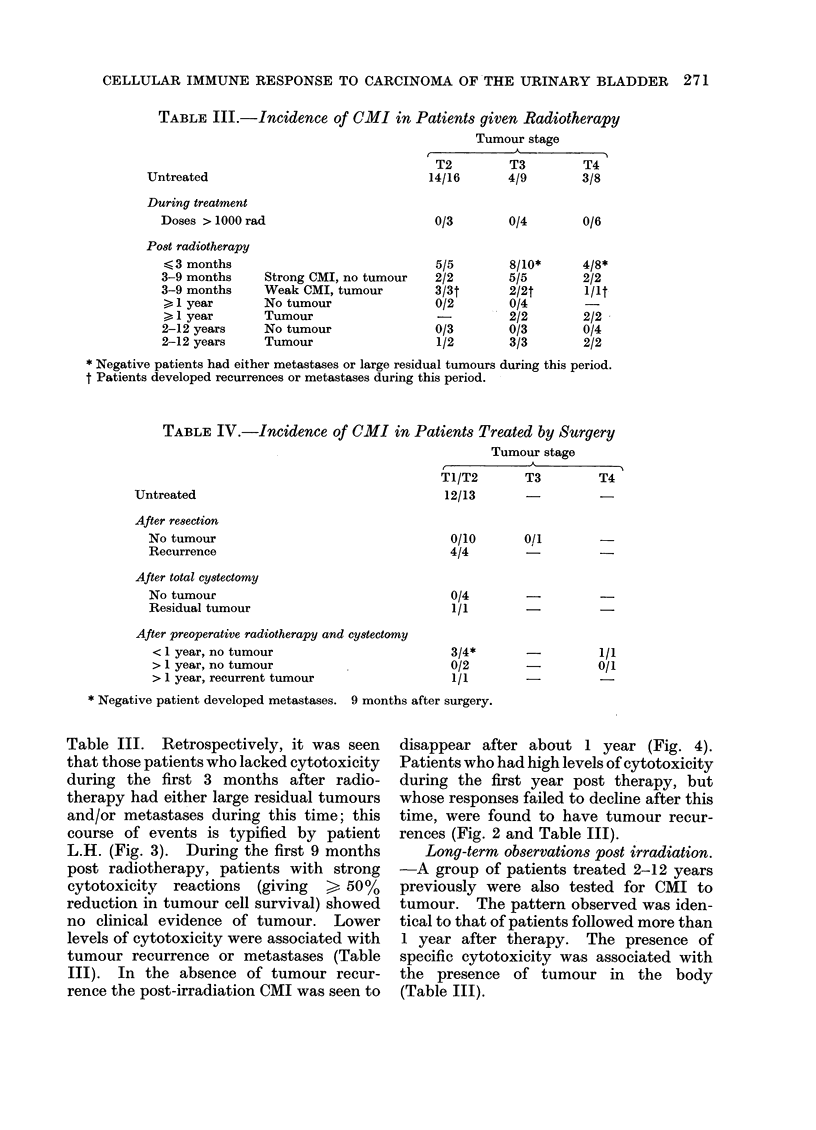
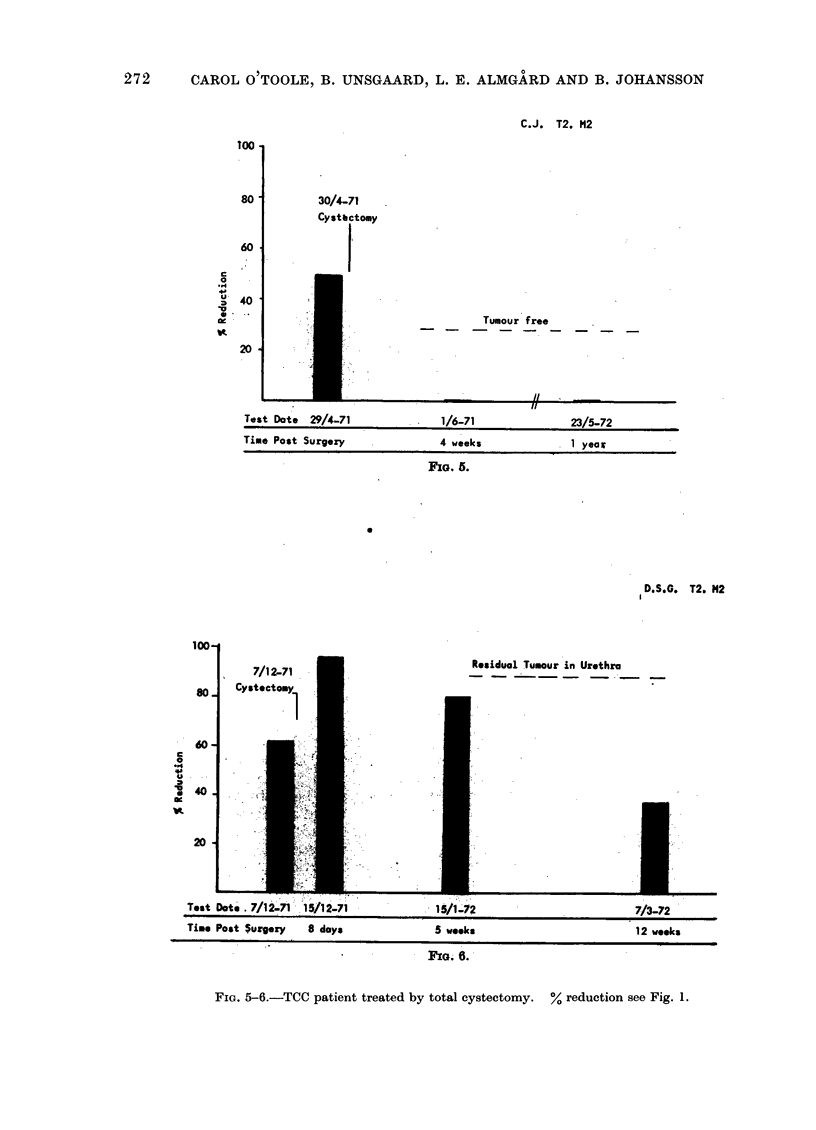
The cell mediated immune response to carcinoma of the urinary bladder in man is influenced significantly by the tumour burden before treatment. Therapy appears to influence this response by causing alterations in the amount of tumour material in the body. Removal of tumour by surgery is seen to result in a loss of detectable CMI. Recurrence of tumour after surgery results in the reappearance of cytotoxicity. Treatment by radiotherapy also results in the eventual loss of CMI after the elimination of tumour material from the body. The loss of activity after radiotherapy, in the absence of tumour recurrence, occurs over a period of about 1 year. Expression of CMI is suppressed during radiotherapy but may return after treatment. Failure to develop lymphocyte cytotoxicity early after radiotherapy is related to the presence of metastases or residual tumour. Low levels of cytotoxicity during the first 9 months after therapy are associated with tumour recurrence. It may be inferred from this that CMI in the early post-irradiation phase has prognostic significance. The absence of CMI at this time reflects the presence of residual viable tumour in the body. The loss of response about 1 year after radiotherapy probably reflects the clearance of tumour-derived material from the body. The persistence or reappearance of cytotoxicity after this time is related to tumour recurrence.
This test is therefore informative as to the presence or absence of tumour after surgery. With regard to radiotherapy, lymphocyte cytotoxicity can be seen to monitor the presence of viable tumour and tumour derived material in the body.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barski G., Youn J. K. Evolution of cell-mediated immunity in mice bearing an antigenic tumor. Influence of tumor growth and surgical removal. J Natl Cancer Inst. 1969 Jul;43(1):111–121. [PubMed] [Google Scholar]
- Bergkvist A., Ljungqvist A., Moberger G. Classification of bladder tumours based on the cellular pattern. Preliminary report of a clinical-pathological study of 300 cases with a minimum follow-up of eight years. Acta Chir Scand. 1965 Oct;130(4):371–378. [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Bubeník J., Perlmann P., Helmstein K., Moberger G. Cellular and humoral immune responses to human urinary bladder carcinomas. Int J Cancer. 1970 May 15;5(3):310–319. doi: 10.1002/ijc.2910050303. [DOI] [PubMed] [Google Scholar]
- Bubeník J., Perlmann P., Helmstein K., Moberger G. Immune response to urinary bladder tumours in man. Int J Cancer. 1970 Jan 15;5(1):39–46. doi: 10.1002/ijc.2910050106. [DOI] [PubMed] [Google Scholar]
- COULSON A. S., CHALMERS D. G. SEPARATION OF VIABLE LYMPHOCYTES FROM HUMAN BLOOD. Lancet. 1964 Feb 29;1(7331):468–469. doi: 10.1016/s0140-6736(64)90799-8. [DOI] [PubMed] [Google Scholar]
- GREENWALT T. J., GAJEWSKI M., McKENNA J. L. A new method for preparing buffy coat-poor blood. Transfusion. 1962 Jul-Aug;2:221–229. doi: 10.1111/j.1537-2995.1962.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Hellström K. E., Hellström I. Cellular immunity against tumor antigens. Adv Cancer Res. 1969;12:167–223. doi: 10.1016/s0065-230x(08)60331-0. [DOI] [PubMed] [Google Scholar]
- Ilbery P. L., Rickinson A. B., Thrum C. E. Blood lymphocyte replicating ability as a measurement of radiation dosage. Br J Radiol. 1971 Nov;44(527):834–840. doi: 10.1259/0007-1285-44-527-834. [DOI] [PubMed] [Google Scholar]
- Lewis M. G., Ikonopisov R. L., Nairn R. C., Phillips T. M., Fairley G. H., Bodenham D. C., Alexander P. Tumour-specific antibodies in human malignant melanoma and their relationship to the extent of the disease. Br Med J. 1969 Sep 6;3(5670):547–552. doi: 10.1136/bmj.3.5670.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton D. L., Eilber F. R., Malmgren R. A. Immune factors in human cancer: malignant melanomas, skeletal and soft tissue sarcomas. Prog Exp Tumor Res. 1971;14:25–42. doi: 10.1159/000392269. [DOI] [PubMed] [Google Scholar]
- O'Toole C., Perlmann P., Unsgaard B., Almgård L. E., Johansson B., Moberger G., Edsmyr F. Cellular immunity to human urinary bladder carcinoma. II. EEffect of surgery and preoperative irradiation. Int J Cancer. 1972 Jul 15;10(1):92–98. doi: 10.1002/ijc.2910100112. [DOI] [PubMed] [Google Scholar]
- O'Toole C., Perlmann P., Unsgaard B., Moberger G., Edsmyr F. Cellular immunity to human urinary bladder carcinoma. I. Correlation to clinical stage and radiotherapy. Int J Cancer. 1972 Jul 15;10(1):77–91. doi: 10.1002/ijc.2910100111. [DOI] [PubMed] [Google Scholar]
- Rigby C. C., Franks L. M. A human tissue culture cell line from a transitional cell tumour of the urinary bladder: growth, chromosone pattern and ultrastructure. Br J Cancer. 1970 Dec;24(4):746–754. doi: 10.1038/bjc.1970.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjernswärd J., Jondal M., Vánky F., Wigzell H., Sealy R. Lymphopenia and change in distribution of human B and T lymphocytes in peripheral blood induced by irradiation for mammary carcinoma. Lancet. 1972 Jun 24;1(7765):1352–1356. doi: 10.1016/s0140-6736(72)91091-4. [DOI] [PubMed] [Google Scholar]
- Takasugi M., Klein E. A microassay for cell-mediated immunity. Transplantation. 1970 Mar;9(3):219–227. doi: 10.1097/00007890-197003000-00005. [DOI] [PubMed] [Google Scholar]



