Abstract
Neurotrophins (NTs) have recently been found to regulate synaptic transmission in the hippocampus. Whole-cell and single-channel recordings from cultured hippocampal neurons revealed a mechanism responsible for enhanced synaptic strength. Specifically, brain-derived neurotrophic factor augmented glutamate-evoked, but not acetylcholine-evoked, currents 3-fold and increased N-methyl-d-aspartic acid (NMDA) receptor open probability. Activation of trkB NT receptors was critical, as glutamate currents were not affected by nerve growth factor or NT-3, and increased open probability was prevented by the tyrosine kinase inhibitor K-252a. In addition, the NMDA receptor antagonist MK-801 blocked brain-derived neurotrophic factor enhancement of synaptic transmission, further suggesting that NTs modulate synaptic efficacy via changes in NMDA receptor function.
Members of the neurotrophin (NT) gene family play important roles in a wide range of developmental events (1, 2). In addition to promoting neuronal survival and differentiation, we and others have shown that these factors also acutely modulate the efficacy of synaptic transmission (3–9) and play a role in long-term potentiation (LTP; refs. 10–14). The specific targets of NT modulation in the synapse have not been identified. In the hippocampus, activation of the trkB neurotrophin receptor by brain-derived neurotrophic factor (BDNF) or NT-4 rapidly increases the amplitude of postsynaptic currents (4, 15). This effect can be blocked by postsynaptic injection of protein kinase inhibitors, suggesting that the specific synaptic proteins targeted by neurotrophin signaling include postsynaptic neurotransmitter receptors, whose function is modulated by phosphorylation (16–18). In recent studies, we have found that functional trkB receptors are localized to the postsynaptic density (PSD), a synaptic specialization that contains neurotransmitter receptors and second messenger signaling molecules (19). Moreover, we have found that BDNF rapidly and selectively enhances phosphorylation of N-methyl-d-aspartic acid (NMDA) receptor subunits 1 and 2B in isolated hippocampal PSDs (20, 21). In the present report, we investigate neurotrophin modulation of neurotransmitter receptors and identify a mechanism by which BDNF acutely modulates hippocampal synaptic function.
MATERIALS AND METHODS
Cell Culture.
Hippocampal cultures were grown as described (22). Briefly, hippocampi were obtained from embryonic day 18 Sprague–Dawley rats and cells were plated on poly-d-lysine-coated Petri dishes at a final density of 106 cells/35 mm dish. Cultures were maintained in serum-free medium at 37°C in a 95% air/5% CO2 humidified incubator. These cultures contained virtually pure neurons, as judged by neuron-specific enolase immunocytochemistry.
Electrophysiological Recordings.
Voltage clamp recordings were obtained from pyramidal-type cells after 12–16 days in vitro using standard techniques (23). Cells were recorded in voltage clamp mode and held at a resting potential of −40 mV to reduce Mg2+ blockade of NMDA receptors. The external bath solution contained 1.67 mM of Ca, 1 mM of Mg, 5.36 mM of K, 137 mM of Na, 17 mM of glucose, 10 mM of Hepes, 0.001 mM of tetrodotoxin, and 50 mM of sucrose. The pipette solution contained 105 mM of Cs-methanesulfonate, 17.5 mM of Cs-Cl, 10 mM of Hepes-CsOH, 0.2 mM of EGTA, 8 mM of NaCl, 2 mM of Mg-ATP, 2 mM of Na-ATP, 0.3 mM of Na-GTP, 20 mM of phosphocreatinine, and 50 units/ml creatinine phosphokinase. Signals were recorded with an Axopatch 200 amplifier (Axon Instruments), sampled at 2.5 kHz (INDEC IDA15125 interface) and filtered at 5 kHz. Three-barrel micropipettes were used for iontophoretic application of drugs. One barrel, filled with 0.1 M of NaCl, was used for current balancing. Other barrels were filled with either l-glutamate (0.2 M, pH 7.5), acetylcholine-HCl (1.0 M, pH 3.5), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA; 0.02 M, pH 8.0), NMDA (0.1 M, pH 8.0), or saline control. Iontophoretic pulses were applied at 10-s intervals with an ejection time sufficient to achieve stable and consistent responses (typically 10 ms). Data were analyzed by measuring the peak current amplitude for each iontophoretic pulse during the baseline and test periods. Neurotrophins were applied via a microperfusion system. Student’s t test was used for statistical comparisons unless otherwise noted.
Whole-cell recordings of synaptic activity were performed as described above, with the exception of tetrodotoxin, which was omitted from the bath solution. Under these conditions, the majority of synaptic currents reversed between −10 and 0 mV, indicating that they were excitatory; inhibitory currents (presumably chloride-mediated) reversed between −40 and −50 mV. For some recordings, a pipette solution containing 112 mM KCl, 2 mM MgCl2, 0.1 mM CaCl2, 11 mM EGTA, 10 mM Hepes, and 2 mM ATP was used. The holding potential used in these experiments allowed us to select for effects on excitatory currents. Data were analyzed by integrating the synaptic current for each sweep during the baseline and test periods and converting these values to synaptic charge. The charge measurements for all the sweeps in a particular period were averaged and the percent baseline increases for the individual experiments were calculated by dividing the average synaptic charge during the test period by the average synaptic charge from the baseline period.
For cell-attached single-channel recordings, NMDA (10 μM) and glycine (3 μM) were added to a standard pipette solution (10 mM Cs-methanesulfonate/110 mM Na2SO4/25 mM Hepes/33 mM glucose/1.3 mM CaCl2). Cells were placed in an isotonic potassium solution (140 mM potassium gluconate/10 mM Hepes/5 mM EGTA) to set the intracellular potential to ≈0 mV. For recording NMDA activity under steady-state conditions, BDNF (20 ng/ml) was present in both extracellular and pipette solutions. Signals were digitized at 5 kHz and filtered at 1 kHz. The intersweep interval was 2 s. Patches typically contained 1–3 channels as judged by the presence of overlapping openings, and all data were normalized for the number of channels in the patch. Open times and number of openings were obtained from sweeps with nonoverlapping openings by idealization with the half-amplitude crossing criterion and cubic spline interpolation (24). Three multichannel recordings were excluded from this analysis because of a lack of suitable sweeps. Open probability (Po)was measured by dividing open time by total time per sweep. All sweeps, including nulls, were included in the calculation of average Po. NMDA receptor activity was isolated by using a Mg2+-free pipette solution that allowed us to use a holding potential (−80 mV) that was well below the threshold of voltage-gated K+, Na+, and Ca2+ channels. NMDA receptor channel identity was further confirmed by sensitivity to ketamine (50 μM) as well as conductance and reversal potential measurements that were identical to those reported by other laboratories (25, 26).
RESULTS
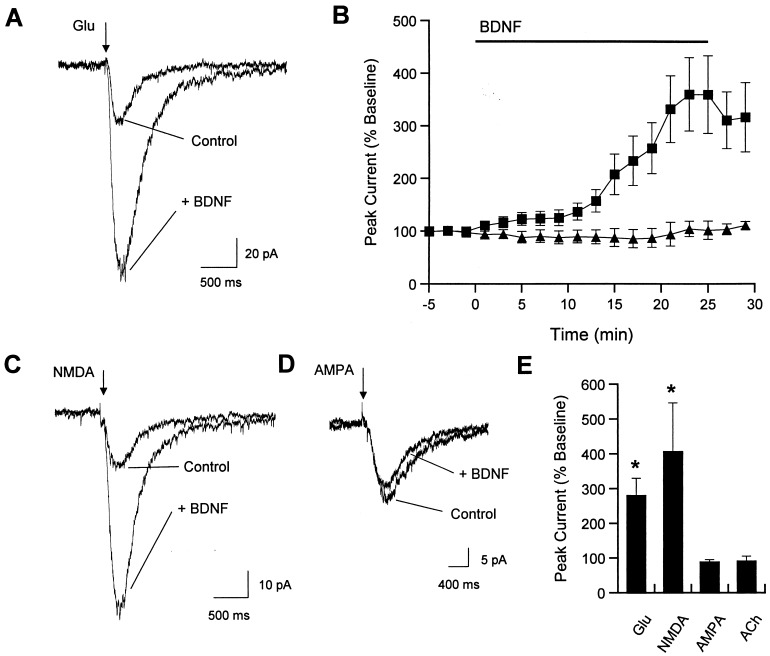
To assess the effects of BDNF and related NTs on postsynaptic transmitter receptors in hippocampal neurons, whole-cell patch clamp recordings were combined with iontophoretic drug application. The sodium channel blocker tetrodotoxin (1 μM) was present in the bath solution throughout these experiments to eliminate action potential-dependent synaptic currents. The amplitude of the glutamate-evoked current was markedly increased by bath application of 20 ng/ml BDNF (Fig. 1A). Enhanced glutamate responses were observed within minutes of exposure to BDNF, with peak increases occurring after 20–25 min (Fig. 1B). Overall, glutamate-evoked responses were increased almost 3-fold after 20 min of BDNF exposure (control response = 55.5 ± 10.4 pA, response during BDNF = 143.7 ± 29.2 pA; mean ± SEM; n = 7 cells; Fig. 1E). BDNF modulation of glutamate sensitivity was selective, because responses to another excitatory transmitter, acetylcholine, were not affected by BDNF exposure (control response = 24.2 ± 5.8 pA, response during BDNF = 23.8 ± 7.3 pA, n = 4; Fig. 1 B and E). In addition, there was no change in glutamate-evoked currents over a similar time interval during application of either heat-inactivated BDNF or the related neurotrophins nerve growth factor (NGF) or NT-3 (see Fig. 3).
Figure 1.
BDNF enhances responsiveness to iontophoretically-applied glutamate or NMDA but not acetylcholine or AMPA. (A) Example traces of control response to glutamate iontophoresis (10-ms pulse; indicated by arrowhead) and response after 20 min of BDNF exposure. Holding potential was −40 mV. The iontophoresis pipette was located in the dendritic region of pyramidal cells. Traces in this and subsequent figures represent the average of five consecutive sweeps. (B) Time course of mean effect of BDNF on glutamate (■) or acetylcholine (▴) responsiveness (mean ± SEM). (C) Example traces of control response to NMDA iontophoresis and response after 20 min of BDNF exposure. (D) Example traces of control response to AMPA iontophoresis and response after 20 min of BDNF exposure. (E) Group data for effects of BDNF on responses to glutamate (P < 0.01; n = 7), NMDA (P < 0.05; n = 7 cells), AMPA (P > 0.4; n = 5), or acetylcholine (P > 0.4; n = 4).
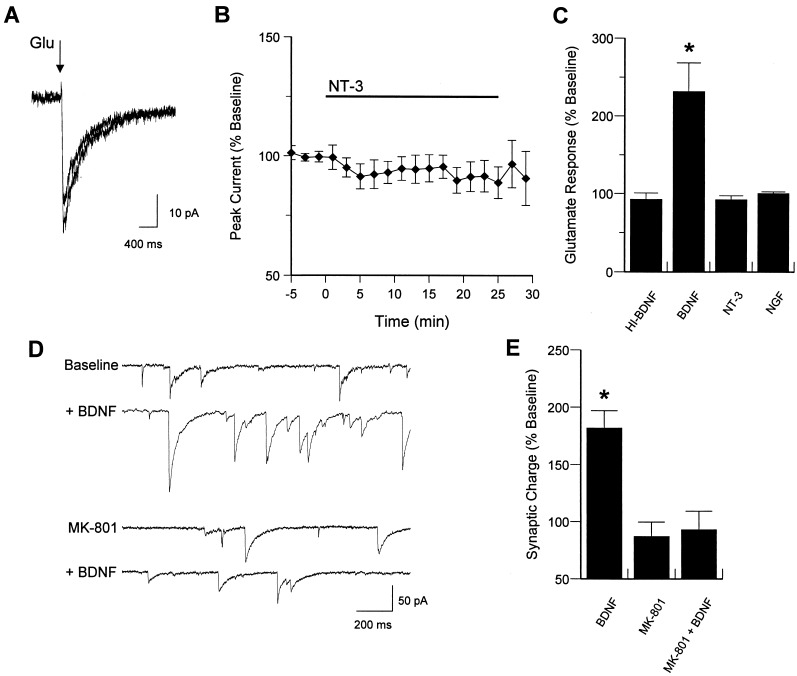
Figure 3.
Glutamate responsiveness is enhanced by BDNF, but not NT-3 or NGF. BDNF-induced increase in synaptic activity is blocked by NMDA receptor antagonism. (A) Example traces of control response to glutamate iontophoresis (10 ms pulse) and response after 20 min of NT-3 exposure. (B) Time course of mean effect of NT-3 on glutamate responses. (C) Group data for effects of heat-inactivated BDNF (HI-BDNF; P > 0.1; n = 3), BDNF (P < 0.01; n = 7), NGF (P > 0.4; n = 10), and NT-3 (P > 0.1; n = 11) on glutamate responsiveness. (D) Example traces of baseline synaptic activity and activity after 20 min of BDNF exposure, in the presence or absence of the NMDA receptor antagonist MK-801 (10 μM). (E) Group data for effect of BDNF on synaptic charge in the absence (P < 0.01; n = 11) or presence (P > 0.3; n = 7) of MK-801.
We next examined responses to specific glutamate receptor agonists to define the receptor subtype(s) affected by BDNF. In particular, we evaluated the NMDA- and AMPA-mediated components of the glutamate-induced current. Specificity of the agonists for their respective receptor subtypes was assessed by using selective antagonists. The non-NMDA antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (10 μM) completely blocked responses to iontophoretically-applied AMPA, while having no effect on NMDA-induced currents; the converse was true of the NMDA receptor antagonist AP-V (100 μM; data not shown). BDNF dramatically enhanced responses to iontophoretically-applied NMDA (control response = 20.6 ± 3.1 pA, response during BDNF = 72.1 ± 22.9 pA; n = 7 cells), but not AMPA (control response = 43.5 ± 24.0 pA, response during BDNF = 43.7 ± 27.1 pA; n = 5), indicating that NMDA receptor responses were selectively modulated by BDNF (Fig. 1 C–E). Heat-inactivated BDNF, used as a control, had no effect on glutamate responses at any time point (n = 3; see Fig. 3C).
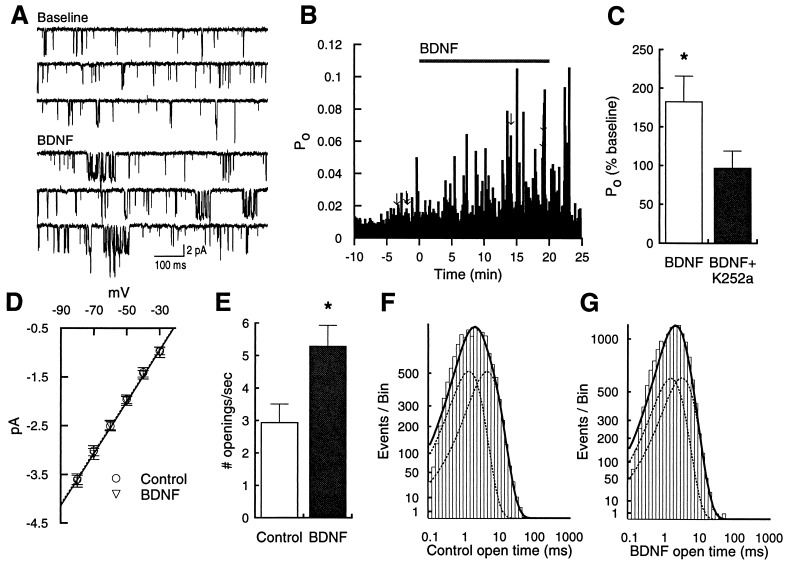
We determined that the effect on the NMDA current resulted at least in part from enhanced activity of individual channels (Fig. 2). Using cell-attached single-channel recordings, acute application of BDNF in the bath solution rapidly increased single-channel Po (Fig. 2 A–C). This effect was mediated by trk receptor activation, and not by direct effects of BDNF on the NMDA receptor itself (27), because the trk tyrosine kinase inhibitor K-252a (200 nM) completely blocked the effect of BDNF on NMDA channel activity (Fig. 2C). To examine steady-state effects, we recorded single-channel activity in control cells and cells pretreated for 15 min with BDNF. Consistent with the within-patch experiments, BDNF-treated cells had a significantly higher NMDA receptor Po compared with controls (0.048 ± 0.006, n = 12, vs. 0.027 ± 0.004, n = 9; P < 0.05). Analysis of current-voltage relationships revealed no change in unitary conductance (53.6 ± 0.002 pS, n = 12, vs. 53.5 ± 0.001 pS, n = 9; P > 0.9; Fig. 2D). To isolate the kinetic parameters responsible for the increased Po, we idealized traces, summed data from all recordings, and measured the average number of openings and mean dwell times in the open state. The open time histograms for control and BDNF-treated cells are shown in Fig. 2 F and G, with the dashed lines representing individual components and the solid line representing the sum. These distributions were not significantly different (P > 0.1, Kolmogorov–Smirnov test). There was, however, a significant increase in channel opening frequency (mean baseline frequency = 2.94 ± 0.57 openings/s, n = 8; frequency for BDNF-treated cells = 5.29 ± 0.64 openings/s, n = 10; Fig. 2E), suggesting that enhanced entry into the open state accounts for BDNF’s effect on Po. These data do not rule out, however, potential BDNF-induced changes in the number of active receptors as well.
Figure 2.
BDNF increases NMDA receptor Po. (A) Example sweeps from a cell-attached single-channel recording before and during application of BDNF (20 ng/ml). Sweeps came from time points indicated by arrows in B. This patch contained two channels. The holding potential was −80 mV; solutions are described in Methods. (B)Time course for effect of BDNF on channel Po. (C) Acute exposure to BDNF significantly enhanced NMDA receptor channel Po (P < 0.01; n = 9), normalized according to the number of channels in the patch. K-252a (200 nM) completely blocked the effect of BDNF (P > 0.3; n = 6). (D) Current-to-voltage relationships were not different between BDNF-treated cells (n = 12) and control cells (n = 9). Treated cells were preexposed to BDNF for 15 min. (E) The mean number of openings per sec was increased in BDNF-treated cells (P < 0.05). (F) Distribution of open times from summed data of all control cells (n = 8 recordings). Values are plotted on square-root/log coordinates and fitted with the maximum likelihood method (24). Dashed lines represent individual components (1.47 ms and 4.91 ms), solid line represents sum. In these recordings, a briefer time component may be missed due to the relatively long sampling interval used to maximize the total observation time per sweep. (G) Summed distribution from all BDNF-treated cells (n = 10 recordings). Dashed lines represent individual components (1.71 ms and 3.51 ms), solid line represents sum. The shapes of the control and BDNF-treated distributions were not significantly different (P > 0.1, Kolmogorov–Smirnov test), nor were mean open times averaged from individual experiments (control: 1.69 ± 0.28 and 4.69 ± 0.39 ms, BDNF-treated: 1.71 ± 0.29 and 3.98 ± 0.41 ms; P > 0.2).
We have previously shown that the frequency and amplitude of excitatory postsynaptic currents in cultured hippocampal neurons are potentiated by the trkB ligands, BDNF and neurotrophin-4, but not by ligands for other neurotrophin receptors. To determine whether the increased glutamate responsiveness showed a similar specificity, we examined the related neurotrophins NGF and NT-3, which primarily interact with trkA and trkC receptors, respectively. Exposure to NT-3 (20 ng/ml, n = 11 cells), NGF (100 ng/ml; n = 10), or heat-inactivated BDNF (n = 3) had no significant effect (see Fig. 3 A–C for example traces and group data), indicating that BDNF-induced enhancement of glutamate responses is likely to be dependent on trkB receptor activation.
To address the role of increased NMDA responsiveness in BDNF-enhanced excitatory synaptic transmission, we used the specific NMDA receptor antagonist MK-801. BDNF alone increased synaptic charge ≈2-fold after 20 min of exposure (mean change from baseline = 182.3 ± 14.7%; n = 11; Fig. 3 D and E). Pretreatment with MK-801 (10 μM), which caused a small but nonsignificant reduction in baseline synaptic activity (mean change from baseline = 87.4 ± 12.3%; n = 7), completely prevented the BDNF-induced enhancement of synaptic efficacy (Fig. 3 D and E).
DISCUSSION
In the present studies, exposure to BDNF, but not NGF or NT-3, increased responsiveness of hippocampal neurons to iontophoretically-applied glutamate. BDNF selectively potentiated the NMDA, but not the AMPA, component of the glutamate response, and had no effect on acetylcholine responses. Single-channel recordings revealed alterations in NMDA channel Po and number of openings, without changes in conductance or mean open time. In addition, the NMDA receptor antagonist MK-801 prevented the BDNF-induced increase in synaptic activity.
Several lines of evidence suggest a role for BDNF in activity-dependent synaptic modifications. For example, tetanizing stimuli that induce long-term potentiation (LTP) increase BDNF mRNA levels (28, 29), and BDNF in turn enhances hippocampal and cortical LTP (10–12). In addition, LTP is impaired in mice with a targeted deletion of the BDNF gene, and can be restored by acute exposure to BDNF (13, 14, 30). Furthermore, BDNF increases tyrosine phosphorylation of NR2B subunits of NMDA receptors in the PSD (21), paralleling the effects of LTP induction (31, 32). Neurotrophins may also play a role in the activity-dependent formation of neuronal circuitry. For example, during development of the visual system, axons from the lateral geniculate nucleus become segregated into eye-specific patches (ocular dominance columns) within their target in the primary visual cortex. This reorganization results from synaptic competition between axons representing the two eyes. The formation of ocular dominance columns is blocked by infusion of BDNF or NT-4, and is also blocked by trkB “receptor bodies” that prevent trkB activation (33, 34).
Our results suggest that in the hippocampus, BDNF may be an endogenous ligand that regulates NMDA receptor-dependent synaptic plasticity. In previous biochemical studies, we found that BDNF increases phosphorylation of the NR1 and NR2B subunits of the NMDA receptor in the isolated PSD. In the present report, we demonstrate that BDNF, acting through the trkB receptor, elicits phosphorylation-dependent enhancement of the functional activity of NMDA channels. It is also known that phosphorylation of NMDA receptors by the tyrosine kinase, src, modulates NMDA channel activity (35), in a manner similar to BDNF. Additionally, a recent study demonstrates that BDNF enhances calcium influx via the NMDA receptor (36). We propose a tentative model in which BDNF stimulates trkB in the PSD, activating a cascade that enhances hippocampal synaptic transmission at least in part by increasing NMDA receptor channel Po.
Acknowledgments
We thank Cephalon, Inc. for the BDNF. We also thank Drs. Robin Davis and Sidney Auerbach for helpful comments on the manuscript. This work was supported by grants from the National Institutes of Health (HD23315 and NS34061) and the Busch Memorial Fund.
ABBREVIATIONS
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- NMDA
N-methyl-d-aspartic acid
- BDNF
brain-derived neurotrophic factor
- PSD
postsynaptic density
- NGF
nerve growth factor
- NT
neurotrophin, Po, open probability
- LTP
long-term potentiation
References
- 1.Thoenen H, Bandtlow C, Heumann R. Rev Physiol Biochem Pharmacol. 1987;109:145–178. doi: 10.1007/BFb0031026. [DOI] [PubMed] [Google Scholar]
- 2.Levi-Montalcini R. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 3.Kang H, Schuman E M. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 4.Levine E S, Dreyfus C F, Black I B, Plummer M R. Proc Natl Acad Sci USA. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lessmann V, Gottmann K, Heumann R. NeuroReport. 1994;6:21–25. doi: 10.1097/00001756-199412300-00007. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka T, Saito H, Matsuki N. J Neurosci. 1997;17:2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H G, Wang T, Olafsson P, Lu B. Proc Natl Acad Sci USA. 1994;91:12341–12345. doi: 10.1073/pnas.91.25.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lohof A M, Ip N Y, Poo M M. Nature (London) 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 9.Wang X H, Poo M M. Neuron. 1997;19:825–835. doi: 10.1016/s0896-6273(00)80964-2. [DOI] [PubMed] [Google Scholar]
- 10.Figurov A, Pozzo-Miller L D, Olafsson P, Wang T, Lu B. Nature (London) 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 11.Akaneya Y, Tsumoto T, Kinoshita S, Hatanaka H. J Neurosci. 1997;17:6707–6716. doi: 10.1523/JNEUROSCI.17-17-06707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang H J, Welcher A A, Shelton D, Schuman E M. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- 13.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Proc Natl Acad Sci USA. 1995;92:8856–8560. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patterson S L, Abel T, Deuel T A, Martin K C, Rose J C, Kandel E R. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 15.Levine E S, Dreyfus C F, Black I B, Plummer M R. Mol Brain Res. 1996;38:300–303. doi: 10.1016/0169-328x(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 16.Roche K W, Tingley W G, Huganir R L. Curr Opin Neurobiol. 1994;4:383–388. doi: 10.1016/0959-4388(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 17.Soderling T R, Tan S E, McGlade-McCulloh E, Yamamoto H, Fukunaga K. J Neurobiol. 1994;25:304–311. doi: 10.1002/neu.480250310. [DOI] [PubMed] [Google Scholar]
- 18.Swope S L, Moss S J, Blackstone C D, Huganir R L. FASEB J. 1992;6:2514–2523. [PubMed] [Google Scholar]
- 19.Wu K, Xu J L, Suen P C, Levine E S, Huang Y Y, Mount H T J, Lin S Y, Black I B. Mol Brain Res. 1996;43:286–290. doi: 10.1016/s0169-328x(96)00211-2. [DOI] [PubMed] [Google Scholar]
- 20.Suen P C, Wu K, Levine E S, Mount H T J, Xu J L, Lin S Y, Black I B. Proc Natl Acad Sci USA. 1997;94:8191–8195. doi: 10.1073/pnas.94.15.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin S Y, Wu K, Levine E S, Mount H T J, Suen P C, Black I B. Mol Brain Res. 1998;55:20–27. doi: 10.1016/s0169-328x(97)00349-5. [DOI] [PubMed] [Google Scholar]
- 22.Lu B, Yokoyama M, Dreyfus C F, Black I B. J Neurosci. 1991;11:318–326. doi: 10.1523/JNEUROSCI.11-02-00318.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 24.Sigworth F J, Sine S M. Biophys J. 1987;52:1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark B A, Farrant M, CullCandy S G. J Neurosci. 1997;17:107–116. doi: 10.1523/JNEUROSCI.17-01-00107.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L Y, Orser B A, Brautigan D L, MacDonald J F. Nature (London) 1994;369:230–232. doi: 10.1038/369230a0. [DOI] [PubMed] [Google Scholar]
- 27.Jarvis C R, Xiong Z G, Plant J R, Churchill D, Lu W Y, MacVicar B A, MacDonald J F. J Neurophysiol. 1997;78:2363–2371. doi: 10.1152/jn.1997.78.5.2363. [DOI] [PubMed] [Google Scholar]
- 28.Patterson S L, Grover L M, Schwartzkroin P A, Bothwell M. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- 29.Castren E, Pitkanen M, Sirvio J, Parsadanian A, Lindholm D, Thoenen H, Riekkinen P J. NeuroReport. 1993;4:895–898. doi: 10.1097/00001756-199307000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T. Proc Natl Acad Sci USA. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rostas J A P, Brent V A, Voss K, Errington M L, Bliss T V P, Gurd J W. Proc Natl Acad Sci USA. 1996;93:10452–10456. doi: 10.1073/pnas.93.19.10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenblum K, Dudai Y, Richterlevin G. Proc Natl Acad Sci USA. 1996;93:10457–10460. doi: 10.1073/pnas.93.19.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cabelli R J, Hohn A, Shatz C J. Science. 1995;267:1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- 34.Cabelli R J, Shelton D L, Segal R A, Shatz C J. Neuron. 1997;19:63–76. doi: 10.1016/s0896-6273(00)80348-7. [DOI] [PubMed] [Google Scholar]
- 35.Yu X M, Askalan R, Keil G J, II, Salter M W. Science. 1997;275:674–678. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]
- 36.Sakai N, Yamada M, Numakawa T, Ogura A, Hatanaka H. Brain Res. 1997;778:318–328. doi: 10.1016/s0006-8993(97)01052-4. [DOI] [PubMed] [Google Scholar]