Abstract
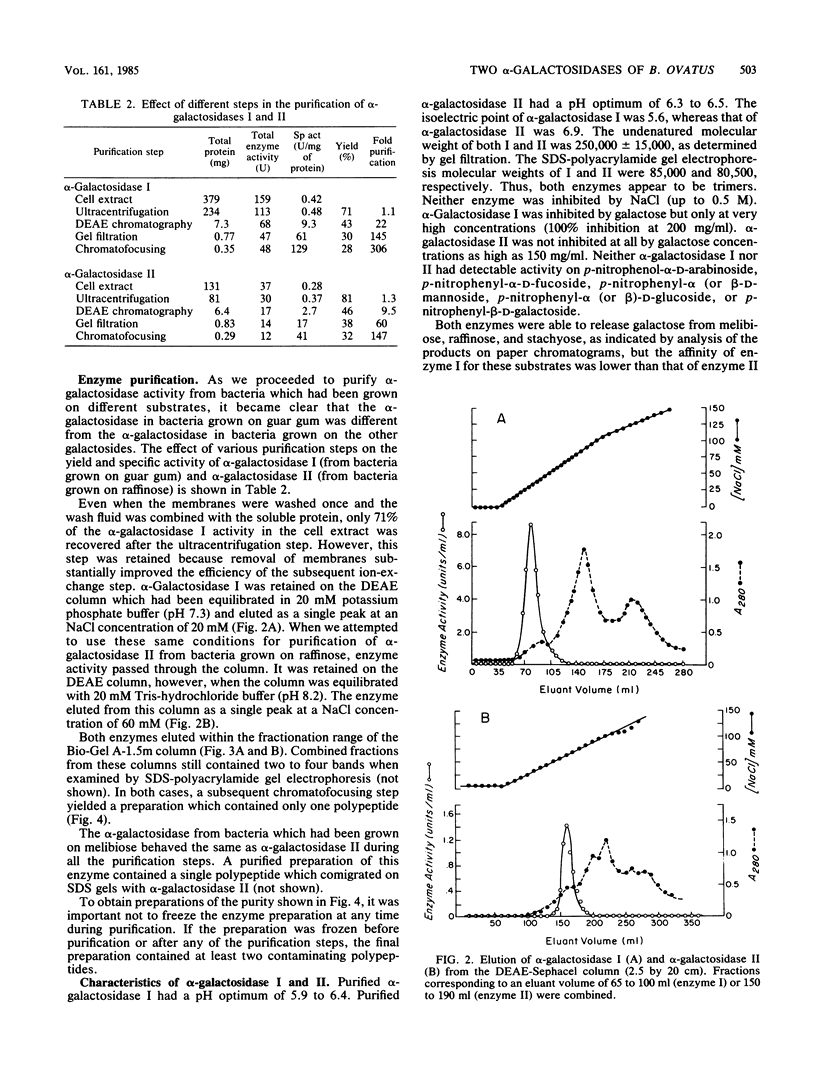
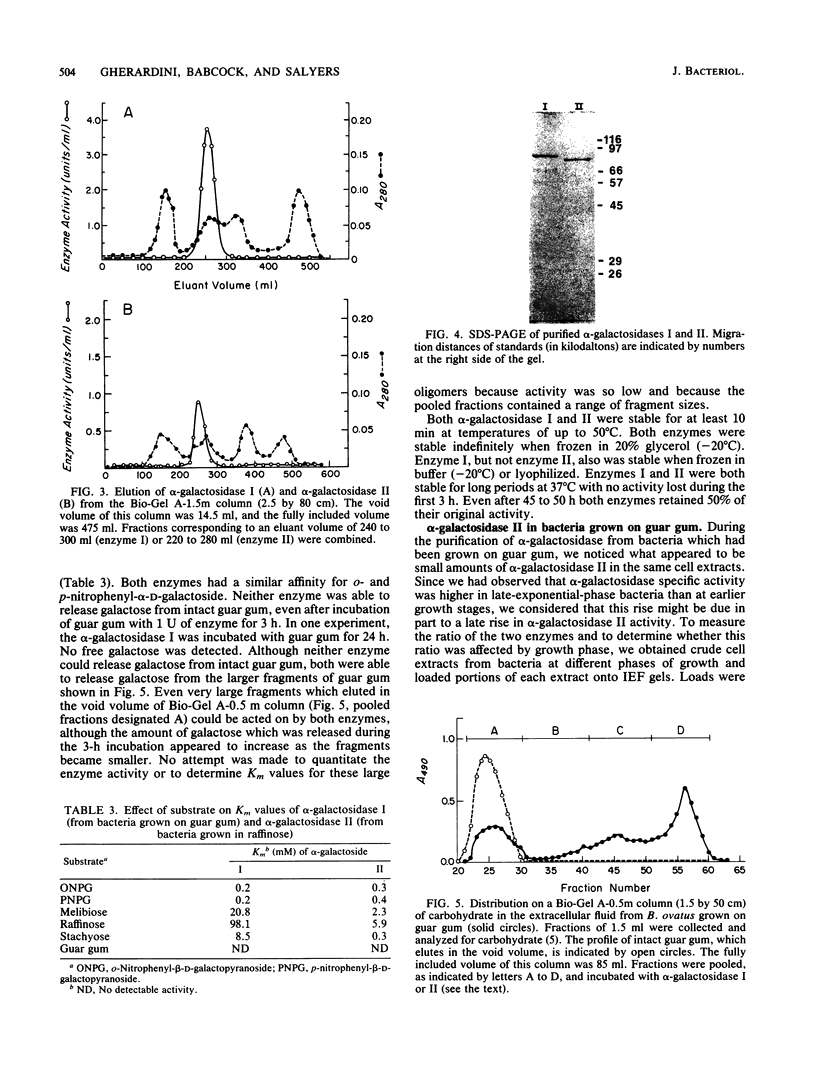
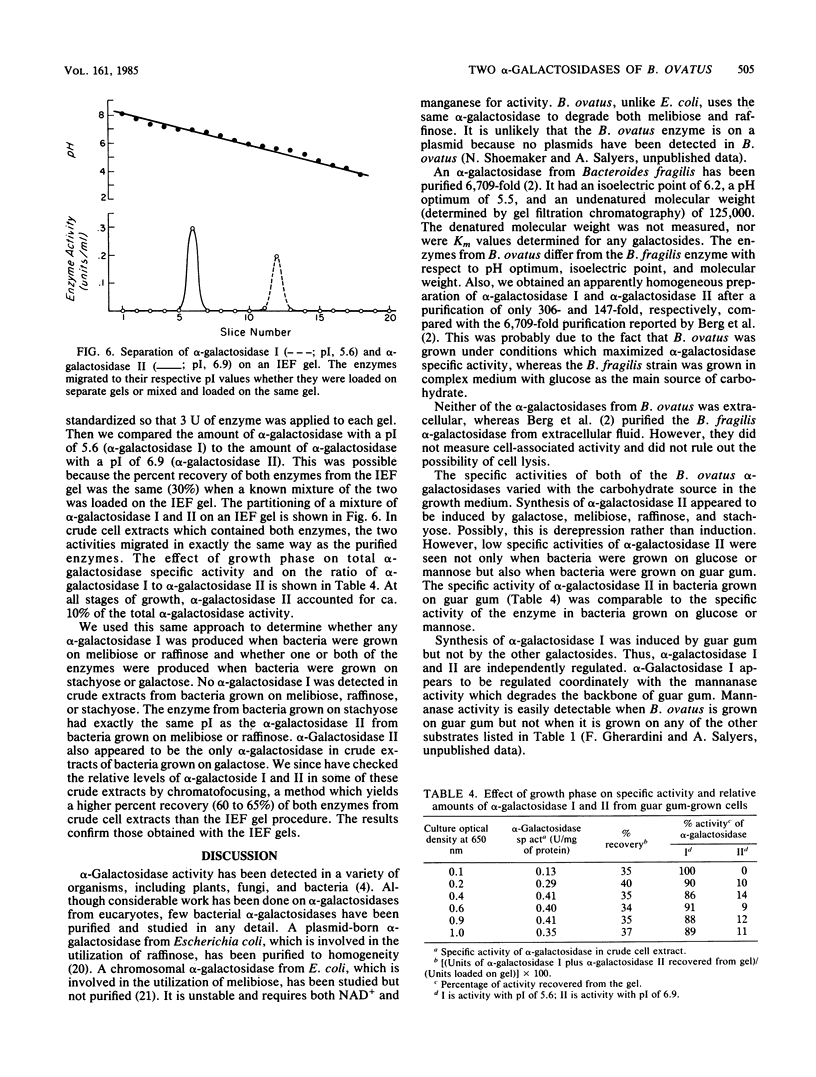
When Bacteroides ovatus is grown on guar gum, a galactomannan, it produces alpha-galactosidase I which is different from alpha-galactosidase II which it produces when grown on galactose, melibiose, raffinose, or stachyose. We have purified both of these enzymes to apparent homogeneity. Both enzymes appear to be trimers and have similar pH optima (5.9 to 6.4 for alpha-galactosidase I, 6.3 to 6.5 for alpha-galactosidase II). However, alpha-galactosidase I has a pI of 5.6 and a monomeric molecular weight of 85,000, whereas alpha-galactosidase II has a pI of 6.9 and a monomeric molecular weight of 80,500. alpha-Galactosidase I has a lower affinity for melibiose, raffinose, and stachyose (Km values of 20.8, 98.1, and 8.5 mM, respectively) than does alpha-galactosidase II (Km values of 2.3, 5.9, and 0.3 mM, respectively). Neither enzyme was able to remove galactose residues from intact guar gum, but both were capable of removing galactose residues from guar gum which had been degraded into large fragments by mannanase. The increase in specific activity of alpha-galactosidase which was associated with growth on guar gum was due to an increase in the specific activity of enzyme I. Low, constitutive levels of enzyme II also were produced. By contrast, enzyme II was the only alpha-galactosidase that was detectable in bacteria which had been grown on galactose, melibiose, raffinose, or stachyose.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg J. O., Lindqvist L., Nord C. E. Purification of glycoside hydrolases from Bacteroides fragilis. Appl Environ Microbiol. 1980 Jul;40(1):40–47. doi: 10.1128/aem.40.1.40-47.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dey P. M., Pridham J. B. Biochemistry of -galactosidases. Adv Enzymol Relat Areas Mol Biol. 1972;36:91–130. doi: 10.1002/9780470122815.ch3. [DOI] [PubMed] [Google Scholar]
- Dygert S., Li L. H., Florida D., Thoma J. A. Determination of reducing sugar with improved precision. Anal Biochem. 1965 Dec;13(3):367–374. doi: 10.1016/0003-2697(65)90327-1. [DOI] [PubMed] [Google Scholar]
- Holt S., Heading R. C., Carter D. C., Prescott L. F., Tothill P. Effect of gel fibre on gastric emptying and absorption of glucose and paracetamol. Lancet. 1979 Mar 24;1(8117):636–639. doi: 10.1016/s0140-6736(79)91079-1. [DOI] [PubMed] [Google Scholar]
- Kasahara M., Anraku Y. Succinate dehydrogenase of Escherichia coli membrane vesicles. Activation and properties of the enzyme. J Biochem. 1974 Nov;76(5):959–966. [PubMed] [Google Scholar]
- Kotarski S. F., Salyers A. A. Isolation and characterization of outer membranes of Bacteroides thetaiotaomicron grown on different carbohydrates. J Bacteriol. 1984 Apr;158(1):102–109. doi: 10.1128/jb.158.1.102-109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz R. G., Gennis R. B. Isoelectric focusing and crossed immunoelectrophoresis of heme proteins in the Escherichia coli cytoplasmic membrane. J Bacteriol. 1982 Apr;150(1):36–45. doi: 10.1128/jb.150.1.36-45.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miranda P. M., Horwitz D. L. High-fiber diets in the treatment of diabetes mellitus. Ann Intern Med. 1978 Apr;88(4):482–486. doi: 10.7326/0003-4819-88-4-482. [DOI] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWEIGER A. [Separation of simple sugars on cellulose lavers]. J Chromatogr. 1962 Nov;9:374–376. doi: 10.1016/s0021-9673(00)80803-1. [DOI] [PubMed] [Google Scholar]
- Salyers A. A., Vercellotti J. R., West S. E., Wilkins T. D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977 Feb;33(2):319–322. doi: 10.1128/aem.33.2.319-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., West S. E., Vercellotti J. R., Wilkins T. D. Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. Appl Environ Microbiol. 1977 Nov;34(5):529–533. doi: 10.1128/aem.34.5.529-533.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K., Schmitt R. Raffinose metabolism in Escherichia coli K12. Purification and properties of a new alpha-galactosidase specified by a transmissible plasmid. Eur J Biochem. 1976 Aug 1;67(1):95–104. doi: 10.1111/j.1432-1033.1976.tb10637.x. [DOI] [PubMed] [Google Scholar]
- Schmitt R., Rotman B. Alpha-galactosidase activity in cell-free extracts of Escherichia coli. Biochem Biophys Res Commun. 1966 Mar 8;22(5):473–479. doi: 10.1016/0006-291x(66)90297-x. [DOI] [PubMed] [Google Scholar]
- Varel V. H., Bryant M. P. Nutritional features of Bacteroides fragilis subsp. fragilis. Appl Microbiol. 1974 Aug;28(2):251–257. doi: 10.1128/am.28.2.251-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]




