Abstract
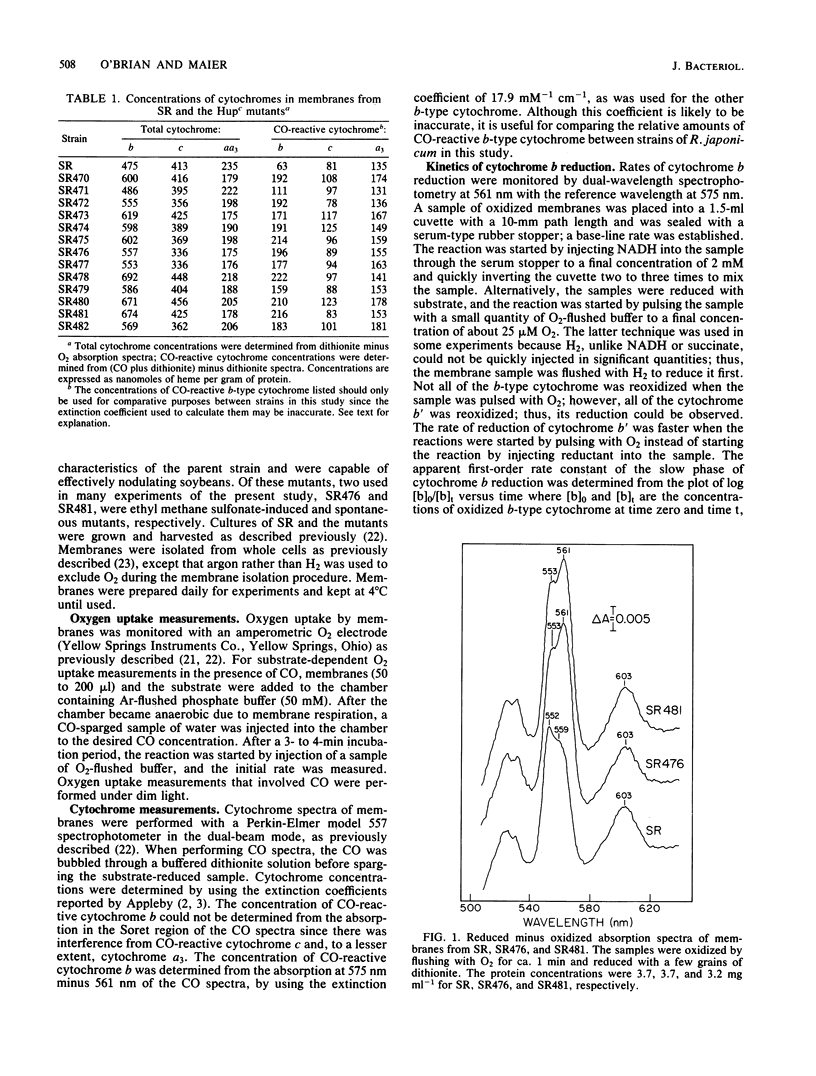
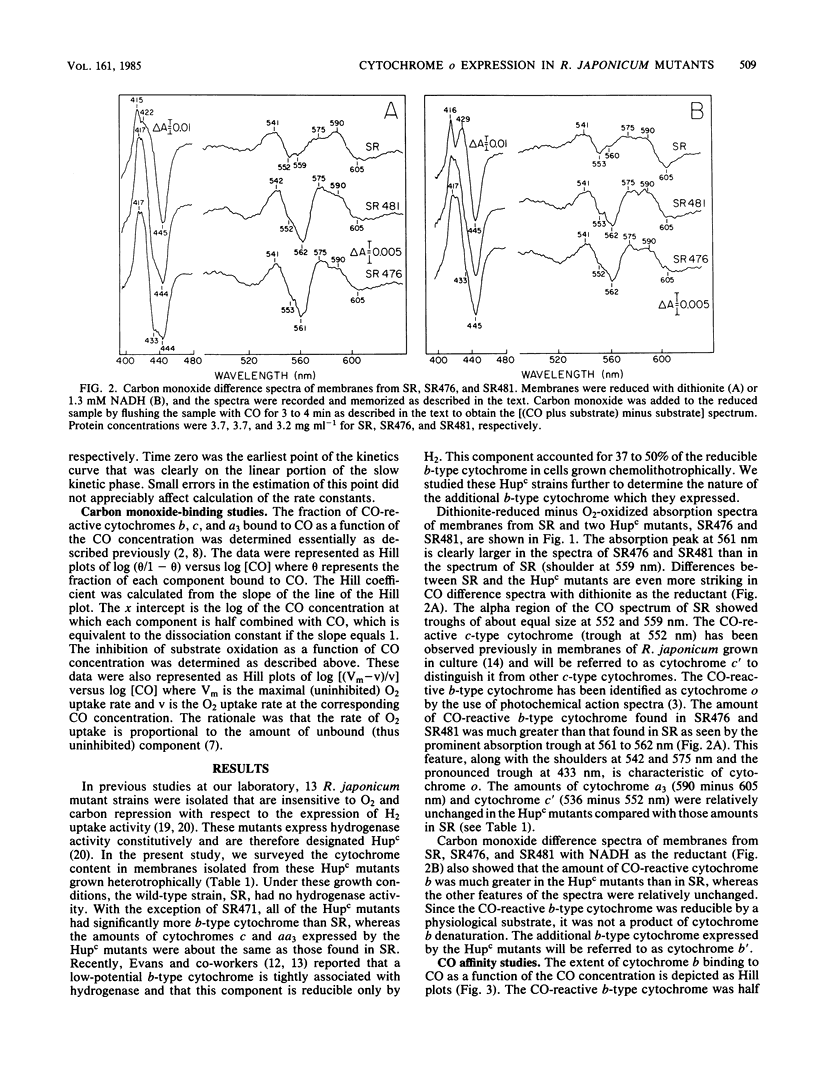
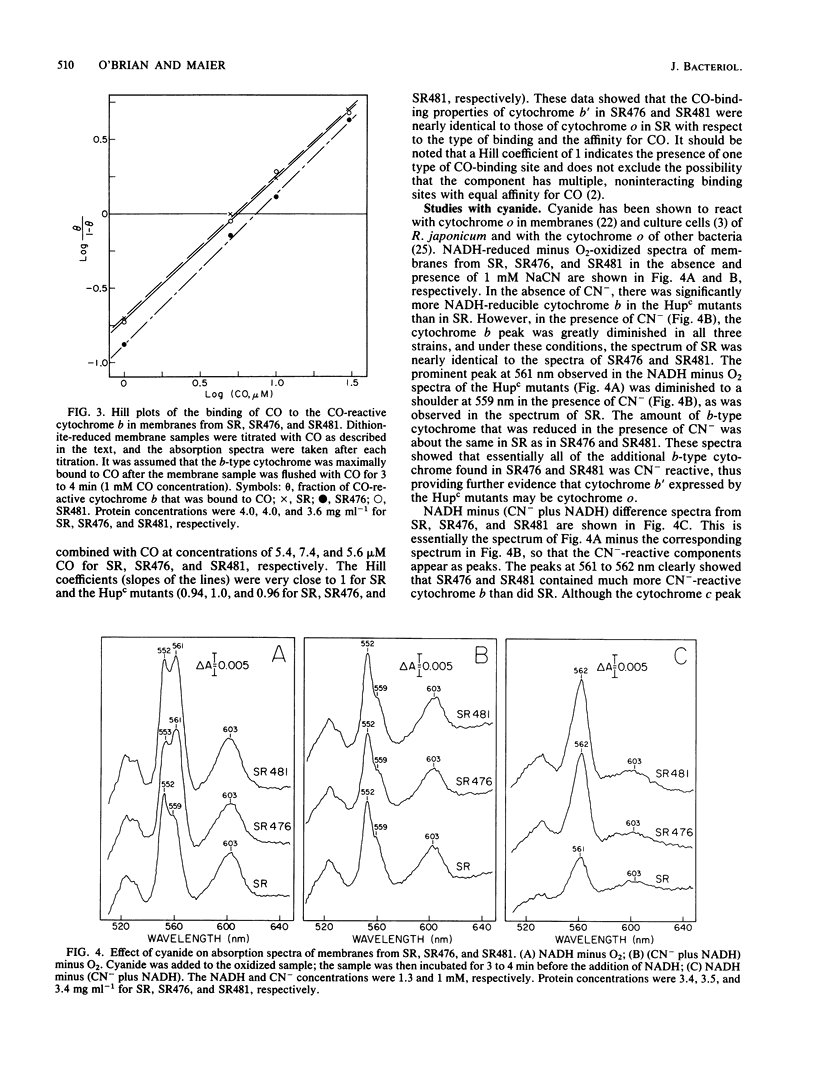
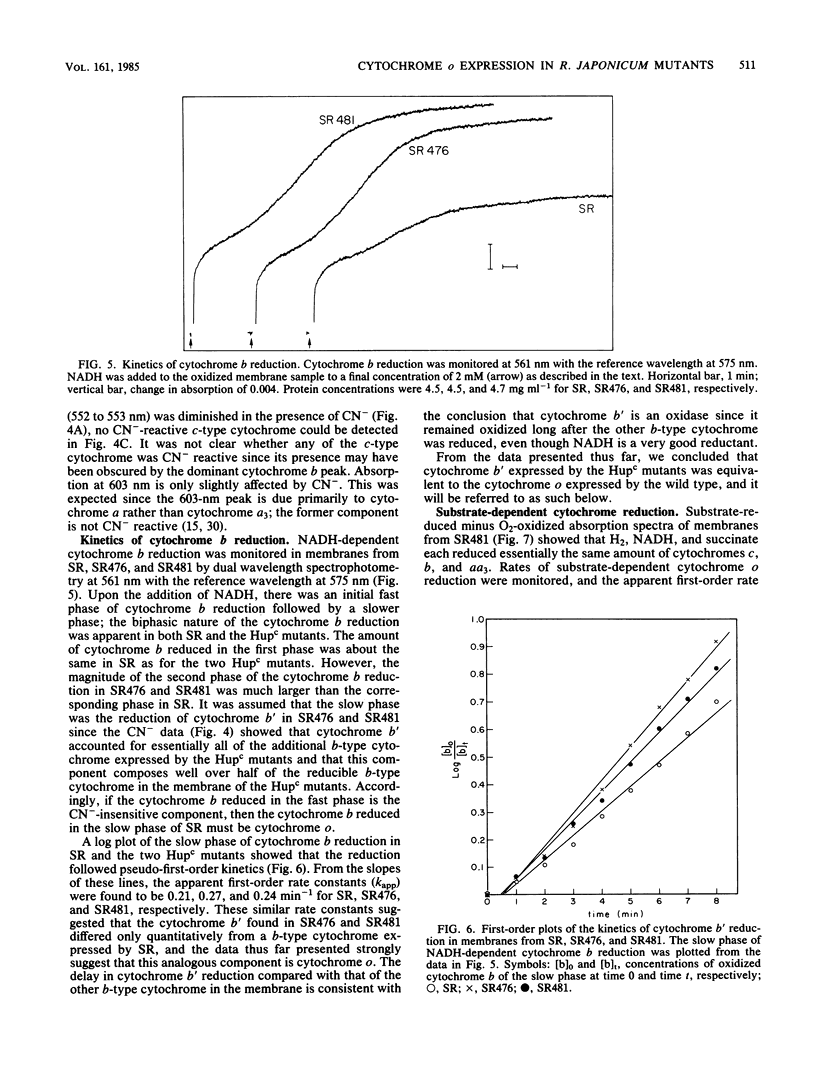
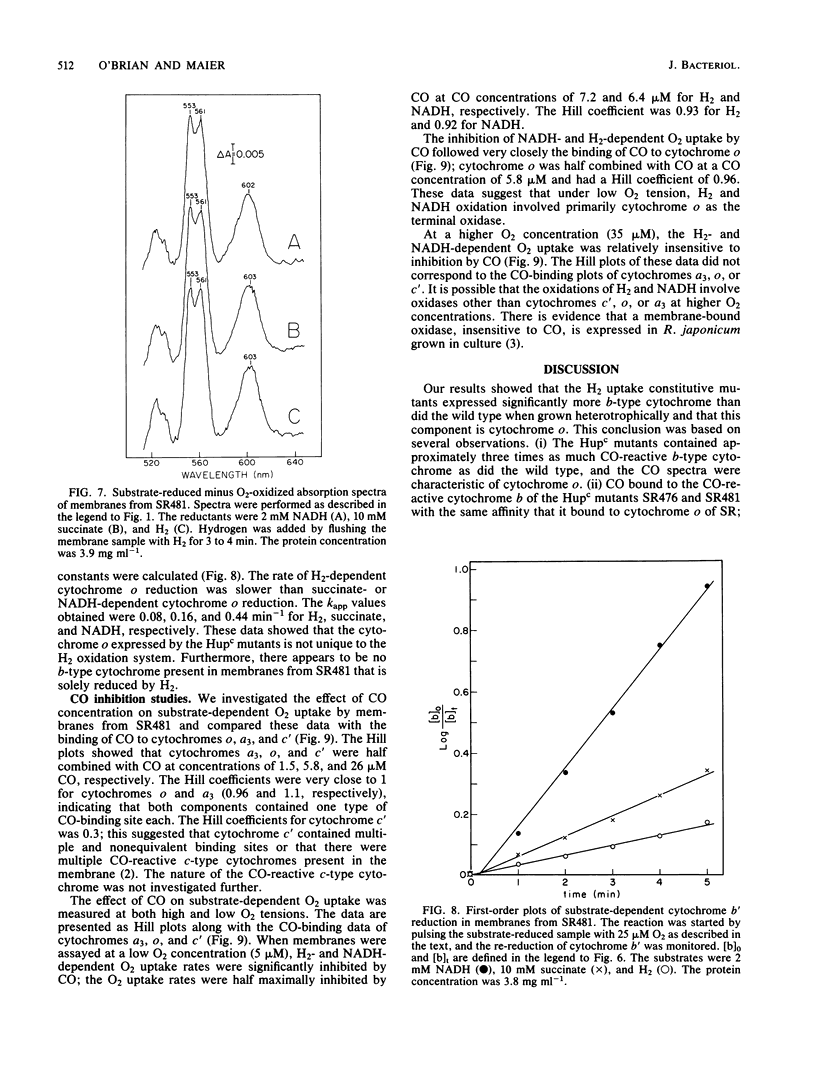
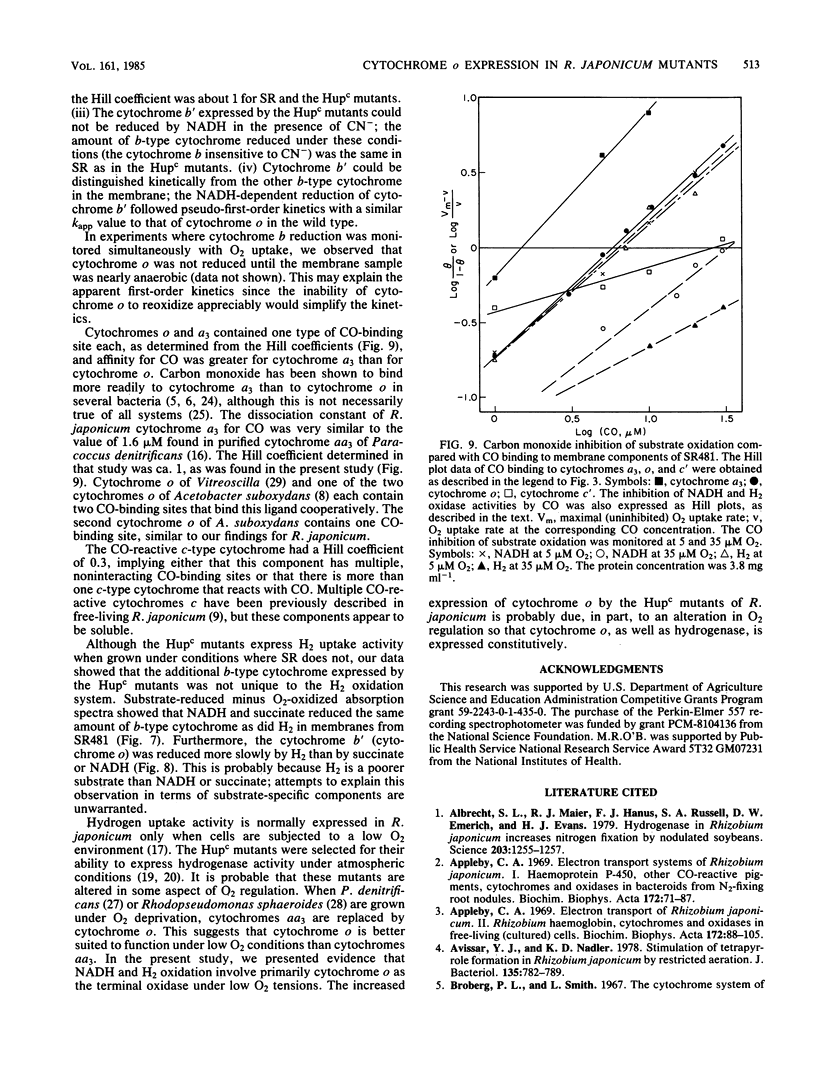
Mutant strains of Rhizobium japonicum constitutive for H2 uptake activity (Hupc) contained significantly more membrane-bound b-type cytochrome than did the wild type when grown heterotrophically. The Hupc strains contained approximately three times more dithionite- and NADH-reducible CO-reactive b-type cytochrome than did the wild type; the absorption features of the CO spectra were characteristic of cytochrome o. This component, designated cytochrome b', was not reduced by NADH in the presence of cyanide. Cytochrome o from the wild type (SR) and cytochrome b' from mutants SR476 and SR481 bound to CO with similar dissociation constants of 5.4, 7.4, and 5.6 microM, respectively. NADH-dependent reduction of cytochrome b' from SR476 and SR481 and the cytochrome o from SR followed pseudo-first-order kinetics with similar rate constants. Based on these spectral, ligand-binding, and kinetic measurements, it was concluded that cytochrome b' expressed by the Hupc mutants is equivalent to cytochrome o found in the wild type. H2, NADH, and succinate each reduced the same amount of total b-type cytochrome in membranes from SR481, and the rate of H2-dependent cytochrome o reduction was significantly less than with succinate or NADH as the reductants. It was concluded that neither cytochrome o nor any b-type cytochrome expressed by the Hupc mutants was unique to the H2 oxidation system. At low O2 concentrations, the inhibition of H2 and NADH oxidase activities by CO closely paralleled the binding of CO to cytochrome o rather than cytochromes a3 or c'. This suggested that NADH and H2 oxidation involved primarily cytochrome o as the terminal oxidase at low O2 tensions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht S. L., Maier R. J., Hanus F. J., Russell S. A., Emerich D. W., Evans H. J. Hydrogenase in Rhizobium japonicum Increases Nitrogen Fixation by Nodulated Soybeans. Science. 1979 Mar 23;203(4386):1255–1257. doi: 10.1126/science.203.4386.1255. [DOI] [PubMed] [Google Scholar]
- Appleby C. A. Electron transport systems of Rhizobium japonicum. I. Haemoprotein P-450, other CO-reactive pigments, cytochromes and oxidases in bacteroids from N2-fixing root nodules. Biochim Biophys Acta. 1969 Jan 14;172(1):71–87. doi: 10.1016/0005-2728(69)90093-0. [DOI] [PubMed] [Google Scholar]
- Appleby C. A. Electron transport systems of Rhizobium japonicum. II. Rhizobium haemoglobin, cytochromes and oxidases in free-living (cultured) cells. Biochim Biophys Acta. 1969 Jan 14;172(1):88–105. doi: 10.1016/0005-2728(69)90094-2. [DOI] [PubMed] [Google Scholar]
- Avissar Y. J., Nadler K. D. Stimulation of tetrapyrrole formation in Rhizobium japonicum by restricted aeration. J Bacteriol. 1978 Sep;135(3):782–789. doi: 10.1128/jb.135.3.782-789.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah K. S. Properties of electron transport particles from Halobacterium cutirubrum. The respiratory chain system. Biochim Biophys Acta. 1969 Jun 24;180(2):320–333. doi: 10.1016/0005-2728(69)90117-0. [DOI] [PubMed] [Google Scholar]
- Daniel R. M., Appleby C. A. Anaerobic-nitrate, symbiotic and aerobic growth of Rhizobium japonicum: effects on cytochrome P 450 , other haemoproteins, nitrate and nitrite reductases. Biochim Biophys Acta. 1972 Sep 20;275(3):347–354. doi: 10.1016/0005-2728(72)90215-0. [DOI] [PubMed] [Google Scholar]
- Daniel R. M. The electron transport system of Acetobacter suboxydans with particular reference to cytochrome. Biochim Biophys Acta. 1970 Sep 1;216(2):328–341. doi: 10.1016/0005-2728(70)90224-0. [DOI] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in legume root nodule bacteroids: occurrence and properties. Arch Mikrobiol. 1972;85(3):193–201. doi: 10.1007/BF00408844. [DOI] [PubMed] [Google Scholar]
- Eisbrenner G., Evans H. J. Carriers in electron transport from molecular hydrogen to oxygen in Rhizobium japonicum bacteroids. J Bacteriol. 1982 Mar;149(3):1005–1012. doi: 10.1128/jb.149.3.1005-1012.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisbrenner G., Evans H. J. Spectral evidence for a component involved in hydrogen metabolism of soybean nodule bacteroids. Plant Physiol. 1982 Dec;70(6):1667–1672. doi: 10.1104/pp.70.6.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEMBERG R., PILGER T. B., NEWTON N., CLARKE L. CYTOCHROME OXIDASE AND ITS DERIVATIVES. I. CARBON MONOXIDE AND CYANIDE COMPOUNDS. Proc R Soc Lond B Biol Sci. 1964 Feb 18;159:405–428. doi: 10.1098/rspb.1964.0010. [DOI] [PubMed] [Google Scholar]
- Ludwig B., Gibson Q. H. Reaction of oxygen with cytochrome c oxidase from Paracoccus denitrificans. J Biol Chem. 1981 Oct 10;256(19):10092–10098. [PubMed] [Google Scholar]
- Maier R. J., Campbell N. E., Hanus F. J., Simpson F. B., Russell S. A., Evans H. J. Expression of hydrogenase activity in free-living Rhizobium japonicum. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3258–3262. doi: 10.1073/pnas.75.7.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merberg D., Maier R. J. Mutants of Rhizobium japonicum with Increased Hydrogenase Activity. Science. 1983 Jun 3;220(4601):1064–1065. doi: 10.1126/science.220.4601.1064. [DOI] [PubMed] [Google Scholar]
- Merberg D., O'Hara E. B., Maier R. J. Regulation of hydrogenase in Rhizobium japonicum: analysis of mutants altered in regulation by carbon substrates and oxygen. J Bacteriol. 1983 Dec;156(3):1236–1242. doi: 10.1128/jb.156.3.1236-1242.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Electron transport components involved in hydrogen oxidation in free-living Rhizobium japonicum. J Bacteriol. 1982 Oct;152(1):422–430. doi: 10.1128/jb.152.1.422-430.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Involvement of cytochromes and a flavoprotein in hydrogen oxidation in Rhizobium japonicum bacteroids. J Bacteriol. 1983 Aug;155(2):481–487. doi: 10.1128/jb.155.2.481-487.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. K. Bacterial cytochrome oxidases. A structurally and functionally diverse group of electron-transfer proteins. Biochim Biophys Acta. 1983 Sep 15;726(3):205–243. doi: 10.1016/0304-4173(83)90006-x. [DOI] [PubMed] [Google Scholar]
- Sapshead L. M., Wimpenny J. W. The influence of oxygen and nitrate on the formation of the cytochrome pigments of the aerobic and anaerobic respiratory chain of Micrococcus denitrificans. Biochim Biophys Acta. 1972 May 25;267(2):388–397. doi: 10.1016/0005-2728(72)90126-0. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Motokawa Y., Kikuchi G. Occurrence of both a-type and o-type cytochromes as the functional terminal oxidases in Rhodopseudomonas spheroides. Biochim Biophys Acta. 1970 Mar 3;197(2):284–291. doi: 10.1016/0005-2728(70)90039-3. [DOI] [PubMed] [Google Scholar]
- Tyree B., Webster D. A. The binding of cyanide and carbon monoxide to cytochrome o purified from Vitreoscilla. Evidence for subunit interaction in the reduced protein. J Biol Chem. 1978 Oct 10;253(19):6988–6991. [PubMed] [Google Scholar]
- Vanneste W. H. The stoichiometry and absorption spectra of components a and a-3 in cytochrome c oxidase. Biochemistry. 1966 Mar;5(3):838–848. doi: 10.1021/bi00867a005. [DOI] [PubMed] [Google Scholar]



