Abstract
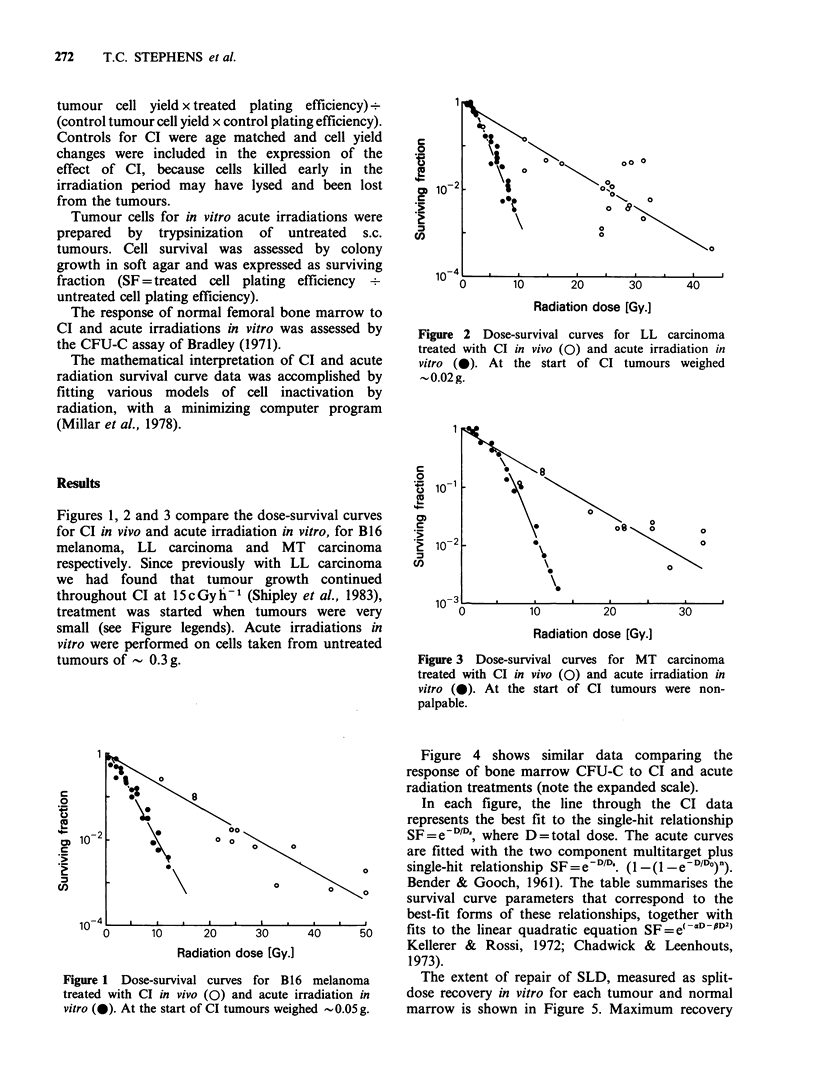
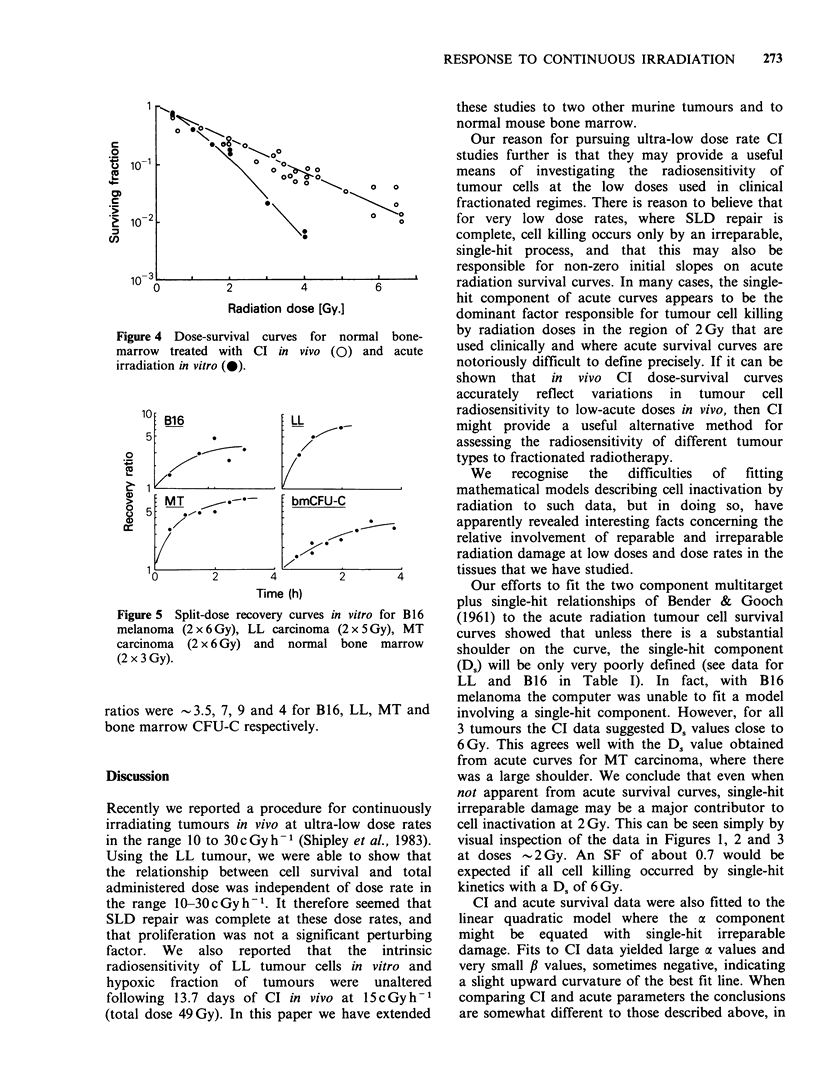
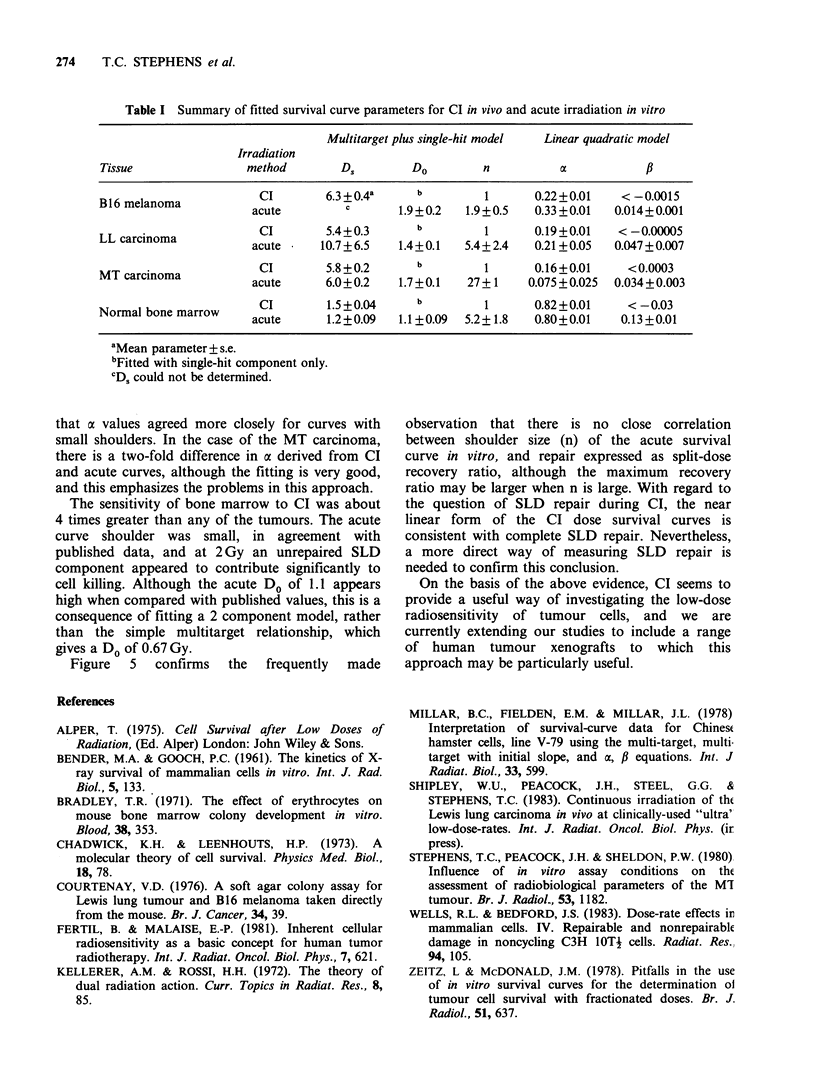
In vivo ultra low dose rate continuous irradiation (CI, 15 cGy h-1) was used in an attempt to accurately quantify low dose radiation response (less than 2 Gy) of both tumour and normal tissue, a parameter which is clinically relevant but difficult to assess using conventional acute radiation survival curves. Dorsally placed tumours were irradiated by housing tumour bearing mice for up to three weeks in a dedicated 137Cs unit taking care to shield the lower body. Response to CI was monitored by an excision cell survival assay. For bone marrow treatment whole body irradiation was used and BM-CFUc were assessed. In all 4 tissues studied (Lewis lung carcinoma, B16 melanoma, carcinoma MT and C57B1/6 bone marrow) cell survival decreased exponentially with dose; the three tumours had similar D0 (approximately 6 Gy), bone marrow was more sensitive (D0 1.5 Gy). Acute in vitro radiation survival curves were analysed using both the multi-target plus single hit and the linear quadratic models of radiation cell kill. Comparison of CI results with acute radiation survival curve parameters derived from each model suggest that CI offers a useful means of assessing the low dose radiosensitivity of tumours and may therefore be useful in the prediction of tumour response to clinically relevant fractionated treatments.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENDER M. A., GOOCH P. C. The kinetics of x-ray survival of mammalian cells in vitro. Int J Radiat Biol Relat Stud Phys Chem Med. 1962 May;5:133–145. doi: 10.1080/09553006214550651. [DOI] [PubMed] [Google Scholar]
- Bradley T. R., Telfer P. A., Fry P. The effect of erythrocytes on mouse bone marrow colony development in vitro. Blood. 1971 Sep;38(3):353–359. [PubMed] [Google Scholar]
- Chadwick K. H., Leenhouts H. P. A molecular theory of cell survival. Phys Med Biol. 1973 Jan;18(1):78–87. doi: 10.1088/0031-9155/18/1/007. [DOI] [PubMed] [Google Scholar]
- Courtenay V. D. A soft agar colony assay for Lewis lung tumour and B16 melanoma taken directly from the mouse. Br J Cancer. 1976 Jul;34(1):39–45. doi: 10.1038/bjc.1976.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertil B., Malaise E. P. Inherent cellular radiosensitivity as a basic concept for human tumor radiotherapy. Int J Radiat Oncol Biol Phys. 1981 May;7(5):621–629. doi: 10.1016/0360-3016(81)90377-1. [DOI] [PubMed] [Google Scholar]
- Millar B. C., Fielden E. M., Millar J. L. Interpretation of survival-curve data for Chinese hamster cells, line V-79 using the multi-target, multi-target with initial slope, and alpha, beta equations. Int J Radiat Biol Relat Stud Phys Chem Med. 1978 Jun;33(6):599–603. doi: 10.1080/09553007814550521. [DOI] [PubMed] [Google Scholar]
- Stephens T. C., Peacock J. H., Sheldon P. W. Influence of in vitro assay conditions on the assessment of radiobiological parameters of the MT tumour. Br J Radiol. 1980 Dec;53(636):1182–1187. doi: 10.1259/0007-1285-53-636-1182. [DOI] [PubMed] [Google Scholar]
- Wells R. L., Bedford J. S. Dose-rate effects in mammalian cells. IV. Repairable and nonrepairable damage in noncycling C3H 10T 1/2 cells. Radiat Res. 1983 Apr;94(1):105–134. [PubMed] [Google Scholar]
- Zeitz L., McDonald J. M. Pitfalls in the use of in vitro survival curves for the determination of tumour cell survival with fractionated doses. Br J Radiol. 1978 Aug;51(608):637–639. doi: 10.1259/0007-1285-51-608-637. [DOI] [PubMed] [Google Scholar]



